Abstract
Background
Relationship between live donor renal anatomic asymmetry and post-transplant recipient function has not been studied extensively.
Methods
We analyzed 96 live-kidney donors, who had anatomical asymmetry (>10% renal length and/or volume difference calculated from CT angiograms) and their matching recipients. Split function differences (SFD) were quantified with 99mTc-DMSA renography. Implantation biopsies at time-zero were semi-quantitatively scored. A comprehensive model utilizing donor renal volume adjusted to recipient weight (Vol/Wgt), SFD, and biopsy score was used to predict recipient estimated glomerular filtration rate (eGFR) at one-year. Primary analysis consisted of a logistic regression model of outcome (odds of developing eGFR>60ml/min/1.73 m2 at one-year), a linear regression model of outcome (predicting recipient eGFR at one-year, using the CKD-EPI formula), and a Monte Carlo simulation based on the linear regression model (N=10,000 iterations).
Results
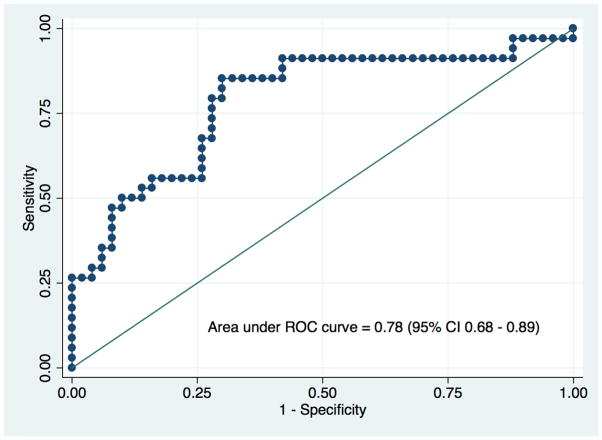
In the study cohort, the mean Vol/Wgt and eGFR at one-year were 2.04 ml/kg and 60.4 ml/min/1.73m2, respectively. Volume and split ratios between two donor kidneys were strongly correlated (r=0.79, p-value<0.001). The biopsy scores among SFD categories (<5%, 5–10%, >10%) were not different (p=0.190). On multivariate models, only Vol/Wgt was significantly associated with higher odds of having eGFR>60ml/min/1.73 m2 (OR=8.94, 95% CI 2.47–32.25, p=0.001) and had a strong discriminatory power in predicting the risk of eGFR<60ml/min/1.73m2 at one-year (ROC curve=0.78, 95% CI 0.68–0.89).
Conclusion
In the presence of donor renal anatomic asymmetry, Vol/Wgt appears to be a major determinant of recipient renal function at one-year post-transplantation. Renography can be replaced with CT volume calculation in estimating split renal function.
Keywords: Kidney size, renal volume, split function, implantation renal biopsy, asymmetrical kidneys, donor nephrectomy, Monte Carlo Simulation
INTRODUCTION
Living donor kidney transplantation is the treatment of choice for advanced renal failure.1 It offers survival benefit and better quality of life when compared to either deceased donor renal transplantation or to dialysis.2 Despite significant improvement in one-year renal allograft survival, most likely due to the use of more potent immunosuppressive drugs, half-lives for grafts originating from living donors have not changed significantly (11.4 years in 1989 to 11.9 years in 2005).3 Although many factors influence late graft attrition, non-immunologic causes, particularly donor kidney volume (as a surrogate marker of transplanted nephron mass), are therefore areas of great interest.4–8 This is particularly true since donated renal volume has been previously demonstrated to be an important factor in subsequent allograft outcomes.9–13
Volumetric imaging based on three-dimensional post-processing data obtained from MR or CT angiograms is a sensitive method for assessment of renal volume due to complex renal anatomy and shape (Supplemental Figure S1).14,15 A volume variation between right and left kidneys has been found to be common (mostly left > right, mean difference 10–15 ml).15 A significant difference in renal size (asymmetry in length > 2 cm and/or volume difference >10%) between the kidneys has previously suggested that a split renal function test (renography as a functional assessment of anatomical asymmetry) should be performed16. In routine clinical practice therefore, additional testing with split renal function has not been unusual and has been indicated in up to 34% of donor evaluations as a guide to selection of one of two kidneys for donor nephrectomy.17,18 In the case of significant asymmetry, this assessment ensures that the better functioning kidney remains in the donor and that the donated kidney provides adequate function for the recipient’s metabolic needs.
Most transplant centers arbitrarily consider anatomical and functional asymmetry of <10% as clinically insignificant (either one of donor kidneys can be removed) and >20% as a relative contraindication for donation due to the concern that chronic histological changes (tubular atrophy, interstitial fibrosis and arteriolosclerosis) may be present in the smaller kidney. However, little is known about the effect of degree of asymmetry in donor kidneys on recipient’s renal function and histologic changes following transplantation.
To study this issue, we designed a retrospective cohort study utilizing living donors at our institution between 2009 and 2013 and subsequently performed a theoretical simulation analysis based on our preliminary findings. In the cohort, we analyzed only living donors who had anatomical asymmetry defined as >10% renal length and/or >10% difference in volume calculated from CT angiograms. The analysis included the donated renal volume adjusted to recipient weight (Vol/Wgt), split function difference (SFD), and semi-quantitative scores of post-implantation renal biopsies. Our goal was to evaluate the impact of renal asymmetry on recipient eGFR at one-year. Since our study was limited by a relatively small sample size, lack of power and risk of type 2 error, we developed a simulation model to determine how changes in predictors (mainly donor eGFR, donated renal volume, split function difference, and renal biopsy score) affect recipient eGFR at one-year after transplantation (one-way sensitivity analysis).
RESULTS
Characteristics of the study cohort
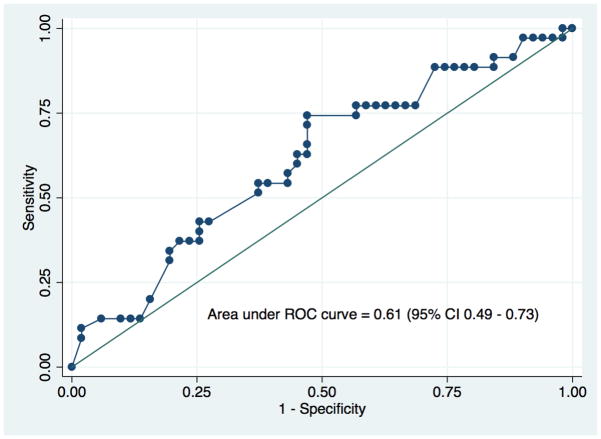
Table 1 lists characteristics of both the 96 donors with renal size asymmetry of >10% and those of the matching recipients, and their transplant outcomes based on SFD categories (<5%, 5–10%, >15%). In our center, approximately 20% of the donors (96 out of 521) who underwent donor nephrectomy between 2009-and 2013-had greater than 10% anatomical asymmetry. More than half of the donors in all categories were women. The mean percent of Vol/Wgt and SFD (|L–R|) were significantly different among the three SFD categories. Compared to their corresponding donors, recipients were older, heavier, and more likely to be male. There was a downward trend in Vol/Wgt with higher SFD categories. The higher SFD categories were also associated with slightly higher total biopsy scores, but that did not reach statistical significance. Recipients in the lowest SFD category had the highest eGFR at one-year and lowest odds of having eGFR<60 ml/min/1.73 m2. Approximately 60% of 96 patients had eGFR<60 ml/min/1.73 m2 at one year in our study cohort. There was a strong correlation(r=0.79, p-value<0.001) between volume and split ratios (L/R) of two donor-kidneys, shown in Figure 1. Discriminatory power of Vol/Wgt (ROC curve=0.78, 95% CI 0.68–0.89; a positive predictive value of 74% at the optimal cut-point of 1.99 ml/kg) to predict risk of eGFR<60ml/min/1.73m2 at one-year was higher compared with SFD (ROC curve=0.61, 95% CI 0.49–0.73), as is shown in Figure 2a and 2b. The Vol/Wgt (r=0.59, p<0.001), the donor eGFR (r=0.35, p<0.001), and the biopsy score (−0.27, p=0.018) were significantly correlated with recipient eGFR at one-year; the SFD (r=−0.19, p=0.079) did not correlate with the above findings (data not shown).
Table 1.
Characteristics of the study cohort and outcomes based on split function difference.
| N=96 | Split Function Difference (%) | |||
|---|---|---|---|---|
| <5% | 5–10% | >10% | P value | |
| N (%) | 47 (49%) | 32 (33%) | 17(18%) | |
| DONOR FACTORS | ||||
| Age (year) | 41.6±11.5 | 46.9±13.5 | 45.3±8.7 | 0.113 |
| Race (AA, %) | 12.8 | 12.5 | 11.8 | 0.994 |
| Gender (female, %) | 53.2 | 56.3 | 70.6 | 0.457 |
| BMI (kg/m2) | 25.4±4.7 | 26.7±4.6 | 25.3±3.6 | 0.368 |
| BSA (m2) | 1.84±0.23 | 1.88±0.28 | 1.80±0.19 | 0.651 |
| Serum creatinine (mg/dl) | 0.83±0.16 | 0.83±0.17 | 0.82±0.16 | 0.982 |
| eGFR (CKD-EPI, ml/min/1.73 m2) | 101±16 | 96±17 | 99±18 | 0.366 |
| Urine albumin/creatinine (mcg/mg) | 6.6±9.3 | 5.6±3.8 | 6.6±5.7 | 0.952 |
| Kidney length difference (|L–R|, %) | 5.1±3.0 | 5.9±4.2 | 5.8±2.9 | 0.724 |
| Kidney volume difference (|L–R|, %) | 6.0±2.8 | 6.1±2.9 | 10.6±6.5 | 0.017 |
| Kidney split function difference (|L–R|, %) | 2.6±1.6 | 7.0±1.2 | 15.8±5.1 | 0.001 |
| RECIPIENT FACTORS | ||||
| Age (year) | 42.2±13.6 | 49.4±15.0 | 48.9±14.2 | 0.049 |
| Race (AA, %) | 10.6 | 21.9 | 6.3 | 0.230 |
| Gender (Female, %) | 36.2 | 31.3 | 31.3 | 0.879 |
| BMI (kg/m2) | 26.7±5.5 | 27.5±5.1 | 28.7±5.5 | 0.415 |
| BSA (m2) | 1.9±0.3 | 2.0±0.2 | 2.0±0.2 | 0.470 |
| Peak PRA (%) | 23±26 | 26±31 | 18±24 | 0.650 |
| Re-transplant (%) | 2.2 | 12.9 | 6.3 | 0.163 |
| Cause of ESRD (Diabetes, %) | 23.4 | 34.4 | 25.0 | 0.547 |
| TRANSPLANT FACTORS | ||||
| HLA mismatch | 3.6±2.0 | 3.6±1.9 | 3.9±1.8 | 0.918 |
| Unadjusted donated renal volume (ml) | 149.0±32.8 | 156.9±38.2 | 147.2±28.4 | 0.516 |
| Donated kidney volume/Recipient weight (ml/kg) | 2.2±0.7 | 2.0±0.6 | 1.7±0.4 | 0.033 |
| Post-perfusion biopsy GS score (0–3) | 0.6±0.7 | 1.1±0.7 | 0.9±0.9 | 0.048 |
| Post-perfusion biopsy IFTA score (0–3) | 0.5±0.5 | 0.5±0.5 | 0.7±0.5 | 0.324 |
| Post-perfusion biopsy AS score (0–3) | 0.8±0.5 | 0.6±0.5 | 1.0±0.6 | 0.090 |
| Post-perfusion biopsy total score (0–9) | 1.9±1.2 | 2.1±1.1 | 2.6±1.3 | 0.190 |
| TRANSPLANT OUTCOMES | ||||
| Delayed allograft function (%) | 2.2 | 0.0 | 6.3 | 0.375 |
| Rejection (%) | 17.0 | 12.9 | 20.0 | 0.806 |
| Tacrolimus level at one year, ng/ml | 7.3±2.5 | 6.9±2.2 | 6.7±1.9 | 0.437 |
| BK viremia (%) | 15.9 | 14.3 | 26.7 | 0.560 |
| Allograft survival at one year (%) | 97.9 | 96.9 | 100 | 0.850 |
| Patient survival at one year (%) | 97.9 | 100 | 100 | 0.932 |
| Recipient serum creatinine at 12 months (CKD-EPI, ml/min/1.73 m2) | 1.37±0.39 | 1.46±0.40 | 1.54±0.40 | 0.242 |
| Recipient eGFR at 12 months (CKD-EPI, ml/min/1.73 m2) | 63.8±19.2 | 57.9±20.1 | 54.6±18.8 | 0.182 |
| Recipient eGFR < 60 ml/min/1.73 m2 at 12 months (%) | 52.3 | 64.3 | 71.4 | 0.360 |
Figure 1.
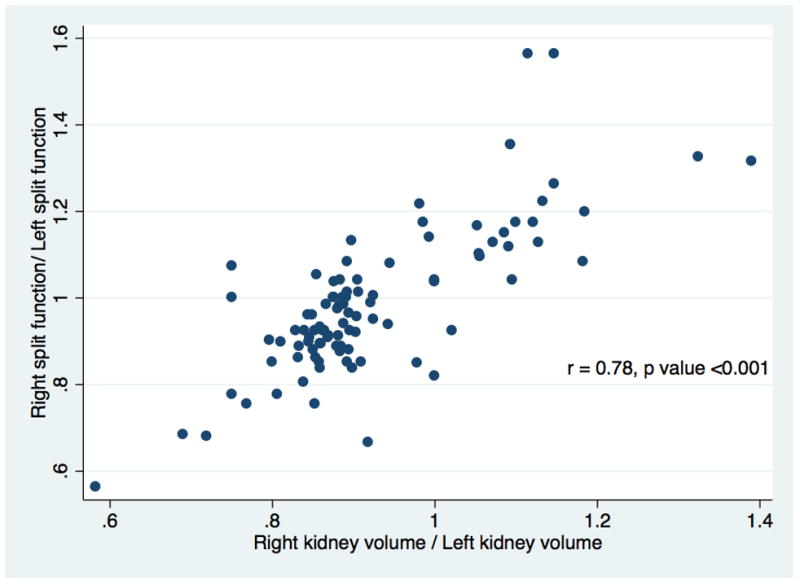
Scatter plot demonstrating relationship between split renal function and renal volume ratios, (L/R).
Figure 2.
Figure 2a. Area under ROC curve for Vol/Wgt to predict risk of recipient eGFR<60 ml/min/1.73 m2 at one-year based on the study cohort.
Figure 2b. Area under ROC for SFD to predict risk of recipient eGFR<60 ml/min/1.73 m2 at one-year based on the study cohort.
Multivariable regression models in the study cohort
In a multivariable linear and logistic regression, only Vol/Wgt was significantly associated with higher recipient eGFR (1 ml/kg increase in adjusted renal volume resulting in 16.815 ml/min/1.73 m2 rise in eGFR, p<0.001) and higher odds of having recipient eGFR>60ml/min/1.73 m2 at one-year (OR=8.94, 95% CI 2.47–32.25, p=0.001), (Table 2). On the other hand, the three categories of SFD and renal biopsy scores did not predict the outcomes in either model.
Table 2.
Multivariable regression models for predicting recipient’s eGFR (linear) and odds of having eGFR > 60 ml/min/1.73 m2 (logistic) at one-year in the study cohort (the models only include the significant variables from univariate analysis and avoid the ones causing multicolinearity).
| LINEAR MODEL | Coefficient | Standard Error | P value | 95% CI | |
|---|---|---|---|---|---|
| Donor eGFR (ml/min/1.73 m2) | 0.1734 | 0.113 | 0.130 | −0.05–0.399 | |
| Weight adjusted donor renal volume (ml/kg) | 16.815 | 3.208 | <0.001 | 10.41–23.21 | |
| Delta split function (%) | −0.203 | 0.340 | 0.552 | −0.88–0.47 | |
| Biopsy score | −2.945 | 1.564 | 0.064 | −6.06–0.17 | |
| Constant | 16.759 | 12.064 | 0.169 | −7.29–40.81 |
| LOGISTIC MODEL | Odds Ratio | Standard Error | P value | 95% CI | |
|---|---|---|---|---|---|
| Donor eGFR (ml/min/1.73 m2) | 1.001 | 0.176 | 0.916 | 0.96–1.03 | |
| Weight adjusted donor renal volume (ml/kg) | 8.938 | 5.853 | 0.001 | 2.47–32.25 | |
| Delta split function | |||||
| 0–5% | 1 | ||||
| 5–10% | 0.775 | 0.489 | 0.687 | 0.22–2.67 | |
| >10% | 1.247 | 0.972 | 0.777 | 0.27–5.75 | |
| Biopsy score | 0.603 | 0.161 | 0.060 | 0.35–1.02 | |
| Constant | 0.019 | 0.038 | 0.050 | 0.00–0.99 | |
| Variance inflation factor (VIF) for the model* | 1.15 |
VIF<10 shows no significant multicollinearity.
Simulation Analysis
The probabilistic model was designed to simulate the outcome (recipient eGFR at one-year) in a hypothetical group of potential living donor (with asymmetric kidneys)-recipient pairs (N=10,000 iterations). Distributions and correlations among the predictors (Vol/Wgt, SDF, biopsy score and donor eGFR) for the model are summarized in Supplemental Table S1 and S2. The model based on coefficients used in the multivariable linear model is shown in Table 2. The mean recipient eGFR at one-year was 60.6 ± 11.9 ml /min /1.73m2 for the simulation cohort (Supplemental Figure S2). The effect of each predictor ranked by its impact on the outcome (one-way sensitivity analysis, Tornado chart) is illustrated in Supplemental Figure S3. The Vol/Wgt had the largest impact on the outcome (range 44.9–82.8 ml/min/1.73 m2); while variation in SFD between 0% and 30% caused modest change in recipient eGFR, range 52.4–67.5 ml/min/1.73m2. An incremental dose-effect response was observed between the Vol/Wgt and the eventual outcome (Figure 3a). There was mild inverse relationship between higher levels of SFD and the outcome (Figure 3b). Vol/Wgt of 2.03 ml/kg and SFD of 6.3% were associated with recipient eGFR of 60.5±12.0 ml/min/1.73 m2 at one-year (Figure 3a and 3b). When the simulation model was restricted to the recipients of kidneys with Vol/Wgt > 2ml/kg (generally considered as adequate renal volume), the recipients in the SFD categories >5%, were less likely to achieve eGFR>60ml/min/1.73m2 at one-year (Supplemental Table S3).
Figure 3.
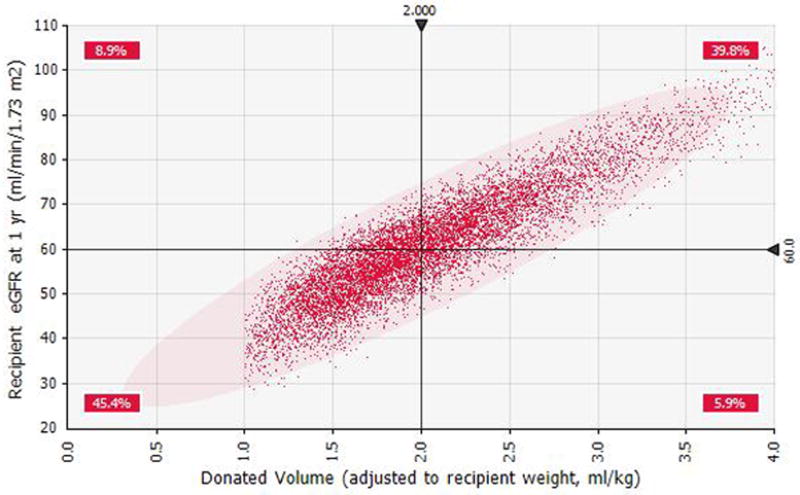
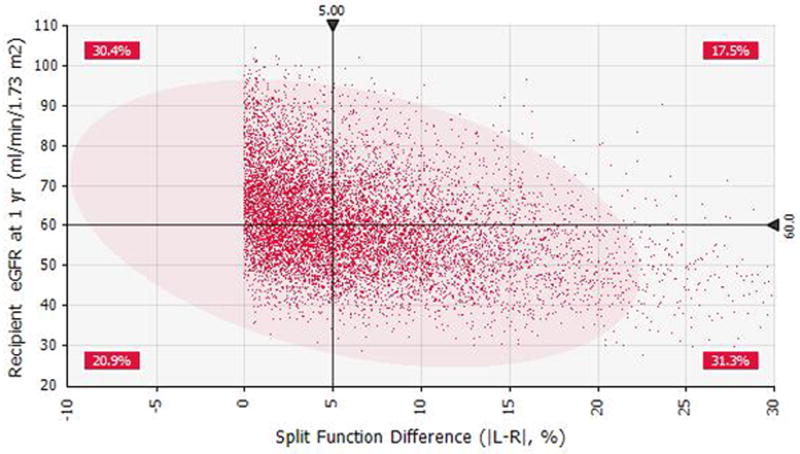
Figure 3a. Scatter plot for weight adjusted renal volume (ml/kg) versus eGFR at one-year based on the Monte Carlo simulation.
Figure 3b. Scatter plot for split renal function difference versus recipient’s eGFR at one-year based on the Monte Carlo simulation.
DISCUSSION
Relationship between living donor renal anatomic asymmetry and post-transplant recipient function has not been studied extensively. This study shows that transplanted renal volume (as a surrogate marker for nephron mass), in the setting of anatomical and functional asymmetry between the two donor kidneys, has the highest impact on allograft function compared with other predictors.11,12,19,20 The simulation data suggest that transplantation of Vol/Wgt >2–2.5 ml/kg generally achieves target recipient eGFR at one-year >60 ml/min/1.73 m2 which is the expected renal function of one healthy kidney. Our study also demonstrates that when SFD is > 10% the smaller donated kidneys with lesser function is significantly associated with neither lower recipient eGFR at one-year nor with increased chronic histological changes (tubular atrophy, interstitial fibrosis, or arteriolosclerosis) in the implantation renal biopsies. However, these findings must be interpreted with caution since 80% of the donors in our study cohort had functional asymmetry of less than 10%.
It is important to focus this discussion on important practical issues and uncertainties in living donor evaluation and medical decision-making. We summarize our views below.
Nonuniform total and split renal function evaluation
The Amsterdam Forum laid down internationally accepted guidelines in the evaluation of potential kidney donors.21,22 However, these guidelines lack specific recommendations regarding methods for GFR estimation and split function assessment. Thus, current practices of pre-transplant living donor evaluations vary considerably among countries and even among transplant centers.23 Halleck et al. conducted a survey among all Eurotransplant centers (7 countries and 61 centers) in 2012.24 They found that majority of the centers use creatinine clearance (64%) in estimating GFR and radioisotopic techniques (82%) in assessing SFD. All German transplant centers (39) measured split function with radioisotopic methods, whereas all Dutch centers (7) utilized CT-based volumetric methods. It appears that CT based volume measurement is becoming the more promising single session method for assessment of donor renal anatomy and function.16,25–27
Do we need split function test to decide on laterality or can CT volumetric measurements replace renography for that purpose?
Significant correlation (r ~ 0.6–0.9) has been reported between CT and renography-based measures of split function.16,25–27 Halleck et al reported a strong correlation (r=0.93, p-value<0.001) between MAG3-based radioisotopic split function estimates and CT-based split renal volume measurements in their center specific cohort of 144 consecutive living donors.24 They also reported that moderate correlation existed between percentage of both split function estimates (MAG3-based split function: r=0.45, p<0.001; CT-measured split cortex volume: r=0.43, p-value<0.001) and the post-transplant eGFR (calculated by using Cockcroft-Gault equation) in the recipient. In our study, we showed that volume and split function ratios between two donor kidneys (r=0.79, p-value<0.001) were strongly correlated. The Vol/Wgt predicted eGFR at 1-year (r=0.59, p<0.001) reasonably well. These findings support the possibility of eliminating the use of split renal function testing in potential living donors with renal asymmetry since renal donor volume estimated by CT-based calculation appears to be an adequate surrogate. Single CT evaluation substituting radioisotopic assessment of split renal function potentially minimizes cost and radiation exposure and is also more convenient for the donors.
Relationship between donated renal volume and post-transplant recipient eGFR
Several studies have previously demonstrated the impact of donated kidney volume on recipient’s short-term outcomes, specifically on GFR. Sikora et al. described a living donor-recipient transplant cohort (N=125 recipients) where the mean donated renal volume of 2.13 ± 0.62 ml/kg (measured by volumetric technique) led in the recipients to a mean eGFR of 63.6 ml/min/1.73m2 at one year after transplantation.12 In the same cohort, a donor volume greater than 2.5 ml/kg was associated with the lowest risk of having eGFR of <60 ml/min/1.73m2. Another study using CT-based prolate ellipsoid formula concluded that a transplanted kidney volume greater than 69.4 ml/m2 predicts a higher eGFR (> 60ml/min/1.73m2) at two-year after transplantation.11 Overall, a minimum weight adjusted renal volume > 2–2.5 ml/kg seems to achieve ideal allograft function in all transplant recipients, as Brenner correctly pointed out two decades ago13. A list of recent references on the subject of the relation of donated renal size and recipient’s renal function is provided in Table 3.
Table 3.
List of the articles focused on the relation between donated renal size and allograft outcome.
| Reference and sample size | Study cohort and design | Follow up (mo.) | Imaging technique | Donated renal size to achieve target GFR of ≥ 60 ml/min | GFR measurement method | Allograft survival benefit | |
|---|---|---|---|---|---|---|---|
| Donated kidney volume (ml) | Sikora et al.13 n=125 |
Living donors, prospective | 12 | CT, volumetric | 2.13 ml/kg | CKD-EPI | Not defined |
| Poggio et al.12 n=119 |
Living donors, prospective | 24 | CT, prolate ellipsoid formula | 69.4 ml/m2 | MDRD | Not defined | |
| Tanriover et al. (current study) n=96 (for MC simulation n=10,000) |
Living donors and retrospective; and MC simulation | 12 | CT, volumetric | 2.04 ml/kg for study cohort; 2.02 ml/kg for the Monte Carlo simulation cohort | CKD-EPI | Not defined | |
| Hugen et al.32 n=114 |
Living donors, retrospective | 12 | CT, volumetric | 72 ml/m2 (for average patient’s eGFR of >55 ml/min/1.73 m2) | Serum creatinine (target <1.5 ml/dL) | Not defined | |
| Donated kidney weight (gm) | Oh et al.29 n=195 |
Living donors, prospective | 1 | N/A | 2 gm/kg (for mean CrCl ≥ 55 ml/min) | 24h urine collection | Not defined |
| Seun Kim et al.31 n=259 |
Living donors, retrospective | 36 | N/A | 3 gm/kg (for mean CrCl ≥ 55) ml/min) | 24h urine collection | No benefit | |
| Giral et al.11 n=914 |
Deceased donor, prospective | 48 | N/A | 2–4 gm/kg | CrCl | No benefit |
Abbreviations: CrCl = Creatinine clearance; MC = Monte Carlo simulation; MDRD = Modification of diet in renal disease; N/A = not applicable.
Role of implantation biopsy in living kidney transplantation
Chronic histologic changes, mostly mild, are commonly detected in renal allografts biopsies obtained intra-operatively at the time of implantation.28–31 Cosio et al. have demonstrated graft interstitial fibrosis (7%), tubular atrophy (25%), and arterial hyalinosis (10%) in biopsies taken from living donor kidneys.32 They reported that interstitial fibrosis correlated well with both function and eventual graft survival. In our study, we observed higher histological scores (2–2.5/9) in kidneys obtained only from older donors (> 40 years), but not in those which had higher split function differences (>10%), (data not shown). Overall, these findings would support the elimination of routine practice of potentially morbid implantation biopsies in LRT since the biopsy findings are mostly benign and variations in biopsy score causes small changes in recipient eGFR at 1-year (52.3–68.9 ml/min/1.73 m2).
To proceed with LRT in case of extreme SFD or to wait for deceased donor renal transplantation (DDRT)?
Based on the results from the simulation model, we conclude that when adequate Vol/Wgt (>2ml/kg) is transplanted, higher SFD categories were associated with only a small incremental risk of developing eGFR<60ml/min/1.73m2 at one-year (an increase from 0–5% to 25–30% in SFD categories led to a decrease in eGFR from 63.9±11.6 ml/min/1.73 m2 to 48.4±9.3 ml/min/1.73 m2) ). In a situation where there is only one living donor available who has extreme SFD/lower eGFR and when preemptive transplantation is a feasible option, the question that needs to be answered is whether to proceed with LRT or wait for a DDRT. In our opinion, the risk of receiving a preemptive LRT with lower eGFR outweighs the benefit of waiting for DDRT with higher eGFR. The reason for this is that preemptive kidney transplantation offers lower mortality (the relative risk [RR] 0.69, 95%CI 0.56–0.85) and lower allograft failure risk (RR 0.73, 95%CI 0.64–0.83) as compared with patients who receive a transplant while on dialysis. 33–35
Strength and limitation of this study
We have comprehensively evaluated in this study the concept of using asymmetrical living donor kidneys and related one year outcomes in the recipients by assessing volumetric renal size measurement, radionuclide renography, and implantation biopsy results as major evaluation criteria. We empowered our findings with a Monte Carlo simulation model using a large hypothetical cohort of patients to overcome our study’s limitation of small sample size, lack of power and risk of causing type 2 errors. We were also able to account for correlations among predictors in the simulation model (@Risk RiskCorrmat function), (Supplemental Table S2). Along with these strengths, our study has several limitations. First, the accuracy of split function measurement with radionuclide renography is subject to variations due to operator dependence, patient habitus, kidney position and depth.16 Second, the follow up period was limited to 12 months despite the fact that GFR at one year predicts long-term allograft survival.36 Third, only a small number of living donors had higher functional asymmetry (SFD>10%; selection bias). Finally, we recognize that assessing renal function with a creatinine-based formula has its limitations due to variability in creatinine generation and its tubular excretion rate.37
Conclusion
Our study supports that the Vol/Wgt estimate seems to be the major determinant of recipient’s renal function at one-year in the presence of renal asymmetry in donor’s kidney and may successfully replace split function testing. A large multicenter study is required to include donors in the higher SFD categories and such a study would more accurately assess the effects of true functional asymmetry in living donors and subsequent recipient renal function.
MATERIAL & METHODS
Study cohort
An approval from the Institutional Review Board at the Columbia University College of Physicians & Surgeons was obtained prior to the research. In our institution, we annually perform approximately 250 renal transplants of which 50% result from living-related donation. This is a retrospective cohort study using living donors with asymmetrical kidneys and their matching recipients who underwent standard evaluation and follow-up care based on our institutional protocol between January 2009 and January 2013. Out of 521 living related donor–recipient pairs, 96 donors (~18 %) met the inclusion criteria (living kidney donors with more than 10% renal length and/or volume mismatch between two kidneys on CT angiogram). All these potential donors underwent renal split function test (99mTc-DMSA radionuclide scintigraphy) to assess functional asymmetry; this was not performed in those donors without asymmetry. The donor selection criteria excluded the donors with significant co-morbidities, such as hypertension, diabetes, BMI>40 or significant social and psychiatric problems. All donors underwent laparoscopic nephrectomy during the study period without major complications, and no conversion to open nephrectomy. In the case of significant asymmetry, better functioning kidney is left for the live donor. An implantation (time-zero) renal biopsy was routinely performed following donor kidney reperfusion in all recipients as a part of our center-specific protocol. The baseline donor-recipient characteristics, laboratory results and radiological data were collected by chart review. The primary outcome measure was recipient’s eGFR at one-year after transplantation that is thought to be predictive of long-term outcome of an allograft.36 Pediatric patients were excluded from the study. In our institution, we use anti-thymocyte globulin induction therapy (6 mg/kg) with an early steroid withdrawal (a total Solumedrol dose of 1000 mg tapered intravenously over four consecutive days and discontinued on day five) followed with maintenance immunosuppression consisting of tacrolimus (0.05 mg/kg twice daily) and mycophenolic acid (720 mg twice daily). Tacrolimus levels are maintained in the range of 10–12 ng/ml for the first 3 months, 8–10 ng/ml in 3–6 months and 6–8 ng/ml thereafter. At one-year follow up, 80% of the patients were still on steroid free regimen and more than 90% were maintained on a tacrolimus based regimen.
Pre-donor nephrectomy kidney function assessment
We assessed GFR for all potential living kidney donors in two steps: 1) initial screening with the CKD-EPI equation targeting 100 ml/min/1.73 m2 or above; 2) 125I-iothalamate plasma clearance (Glofil) if the CKD-EPI was <100 ml/min/1.73 m2.37 Subjects who underwent the Glofil test received 35 millicurries of 125I-iothalamate intravenously into the upper arm. Blood was sampled at 15, 30, 60, 90, 120, and 180 minutes to measure GFR based on plasma disappearance rate of 125I-iothalamate. Our renal function cut-off for donation is a clearance above 80 ml/min/1.73 m2.
Renal volume measurement by CT volumetric technique
Potential donors were evaluated with a 64-row multi-detector CT scanner (LightSpeed VCT XT, GE Health Care, Milwaukee, Wisconsin) with 2.5 mm slice thickness and 0.625 collimation. Triphasic kidney images (unenhanced, arterial phase, and excretory phase) were obtained. All patients received 100 ml contrast agent with 350 mg/mL (Iohexol, Omnipaque 350, GE Healthcare, Princeton, NJ). A 3D advanced postprocessing (Vitrea software, Vital Images Inc., Minnetonka, MN) was performed on an independent workstation by the 3D technologist utilizing volume rendering techniques to better evaluate anatomy and calculate renal volumes based on topographic surface area. Left (L) and right (R) kidney volume percentages were calculated as [L/(L+R)]*100 or vice versa.
Radionuclide scintigraphy
All subjects with renal asymmetry underwent a radionuclide scintigraphy (99mTc-DMSA) to assess functional asymmetry and to guide laterality for donor nephrectomy procedure. The patients were hydrated during the procedure by drinking 1000 ml of fluids. They lay supine on a SPECT/CT. ecoline single head E camera (Siemens, Germany) with a parallel hole collimator interfaced with a digital computer underneath. After intravenous administration of 5 millicuries of 99mTc-DMSA, anterior and posterior planar imaging was performed over both kidneys. Subtraction technique between pre and post-injection was used to calculate net activity. The relative renal uptake was calculated from the following formula: relative right renal uptake (%) = absolute right renal uptake * 100 / (the absolute right + left renal uptake) or vice versa.
Implantation biopsies
Light microscopy sections from implantation biopsies of renal transplants were selectively reviewed by a renal pathologist (ESC). A pathological scoring system was utilized similar to those described by the Banff 97 working classification group and Rule et al.29,38 The percentage of globally sclerotic glomeruli, the percentage of tubular atrophy and of interstitial fibrosis (if any), and the degree of arterio-and/or arteriolosclerosis (if any) were recorded and scored as follows: % of globally sclerotic glomeruli: 0 (0), 1 (1–10%), 2 (11–25%), 3 (26–50%); % of tubular atrophy and interstitial fibrosis: 0 (0), 1 (1–10%), 2 (11–25%), 3 (26–50%); degree of arterio-and arteriolosclerosis: 0 (0), 1 (mild, intimal fibrosis or hyalinosis involving up to 25% of luminal area), 2 (moderate, intimal fibrosis or hyalinosis involving 26–50% of luminal area), 3 (severe, intimal fibrosis or hyalinosis involving greater than 50% of luminal area). Total biopsy score was calculated as the sum of these three categories.
Statistical Analysis
Donor and recipient characteristics were described using mean and standard deviation or frequencies as needed. Comparisons between groups were made using t-test, Kruskal-Wallis, or Chi-square test, as appropriate. Pearson and Rank correlation coefficients were used to examine correlation among predictors of recipient’s GFR at 12-months. Univariate and multivariable linear and logistic regression models were fitted to estimate recipient’s GFR and to predict risk of developing eGFR<60 ml/min/1.73 m2 at 12-months. P-value < 0.05 is considered statistically significant, respectively. Statistical analyses were performed with Stata 13/MP4 (Stata Corp., College Station, TX).
Monte Carlo simulation
Simulation refers to a method whereby the distribution of possible outcome (dependent variable) is generated by a computer using different randomly selected sets of values from the probability distributions of predictors (independent variables) through a formula (a linear regression model in our study). In other words, the computer is trying all valid combinations of the values of predictors to simulate possible outcome. It encompass three steps: 1) Developing a model, defining relationship with dependent and independent variables (the linear regression model) 2) Identifying uncertainty, specifying plausible values of independent variables with probability distributions 3) Analyzing the model with simulation to determine the range and probabilities of all possible outcomes. A sensitivity analysis is carried out with three different analytical techniques: change in outcome statistic, regression analysis and rank correlation calculation. The results of a sensitivity analysis can be displayed as a “tornado” type chart, with longer bars at the top representing the most significant predictors of outcome.
A probabilistic model was developed to simulate the outcome (recipient’s eGFR at one-year) of a hypothetical group of potential living donor (with asymmetrical kidneys >10% renal size difference between two kidneys)-recipient pairs (n = 10,000 iterations) based on the study cohort. Monte Carlo simulation was used to determine how variations in predictors affect the outcome of a transplanted kidney. The predictors were chosen from clinically and/or statistically significant variables found in the univariate and multivariate regression analysis. The main predictors were Vol/Wgt, donor eGFR, renal biopsy score, and split renal function difference. Primary outcome consisted of recipient’s eGFR at one-year after transplantation. We initially defined the distribution of each predictor variable and the correlations among predictors from the study cohort. The final model was fitted based on the coefficients from the multivariable linear regression and adjusted for the correlations among predictors in the model. We utilized @Risk 6.2 software (Palisade Corporation, Ithaca, NY) for simulation task.
Supplementary Material
Acknowledgments
Supported, in part, by NIH T32HL007854-19 (MAH). The authors thank Dr. Robert D. Toto for his critical review of the manuscript.
Abbreviations
- Vol/Wgt
Donated renal volume adjusted to recipient weight
- CI
Confidence interval
- CKD-EPI
Chronic Kidney Disease-Epidemiology Collaboration
- CT
Computerized Tomography
- eGFR
Estimated Glomerular Filtration Rate
- ESRD
End Stage Kidney Disease
- OR
Odds ratio
- SFD
Split function difference
- 99mTc-DMSA
Technetium dimercaptosuccinic acid
Footnotes
Disclosure: The authors of this manuscript have no conflict of interest to disclose as described by the Transplantation.
BT: participated in research design, writing of the paper, and data analysis.
SF: participated in the performance of research.
ESC: participated in the performance of research and writing of the paper.
JHN: participated in the performance of research and writing of the paper.
IO: participated in the performance of research.
PM: participated in the performance of research.
BS: participated in research design, writing of the paper, and data analysis.
JR: participated in research design and writing of the paper.
JJW: participated in the performance of research.
MAC: participated in the performance of research.
SS: participated in the performance of research.
DJC: participated in research design
LER: participated in research design
MAH: participated in research design, writing of the paper, and data analysis.
References
- 1.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. The New England Journal of Medicine. 1995;333:333–6. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 2.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clinical Journal of the American Society of Nephrology : CJASN. 2008;3:471–80. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:450–62. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney International Supplement. 2003:S31–7. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoy WE, Hughson MD, Zimanyi M, et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clinical Nephrology. 2010;74 (Suppl 1):S105–12. doi: 10.5414/cnp74s105. [DOI] [PubMed] [Google Scholar]
- 6.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. The Anatomical Record. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 7.Luyckx VA, Brenner BM. The clinical importance of nephron mass. Journal of the American Society of Nephrology : JASN. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 8.Tan JC, Paik J, Chertow GM, et al. Validity of surrogate measures for functional nephron mass. Transplantation. 2011;92:1335–41. doi: 10.1097/TP.0b013e31823705ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan TV, Bostrom A, Feng S. Optimizing living donor kidney graft function by donor-recipient pair selection. Transplantation. 2006;82:651–6. doi: 10.1097/01.tp.0000229443.98571.10. [DOI] [PubMed] [Google Scholar]
- 10.Giral M, Nguyen JM, Karam G, et al. Impact of graft mass on the clinical outcome of kidney transplants. Journal of the American Society of Nephrology : JASN. 2005;16:261–8. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- 11.Poggio ED, Hila S, Stephany B, et al. Donor kidney volume and outcomes following live donor kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:616–24. doi: 10.1111/j.1600-6143.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Sikora MB, Shaaban A, Beddhu S, et al. Effect of donor kidney volume on recipient outcome: does the “dose” matter? Transplantation. 2012;94:1124–30. doi: 10.1097/TP.0b013e31826f135e. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. Journal of the American Society of Nephrology : JASN. 1992;3:162–9. doi: 10.1681/ASN.V32162. [DOI] [PubMed] [Google Scholar]
- 14.Janoff DM, Davol P, Hazzard J, Lemmers MJ, Paduch DA, Barry JM. Computerized tomography with 3-dimensional reconstruction for the evaluation of renal size and arterial anatomy in the living kidney donor. The Journal of Urology. 2004;171:27–30. doi: 10.1097/01.ju.0000100084.59864.8f. [DOI] [PubMed] [Google Scholar]
- 15.Cheong B, Muthupillai R, Rubin MF, Flamm SD. Normal values for renal length and volume as measured by magnetic resonance imaging. Clinical Journal of the American Society of Nephrology : CJASN. 2007;2:38–45. doi: 10.2215/CJN.00930306. [DOI] [PubMed] [Google Scholar]
- 16.Summerlin AL, Lockhart ME, Strang AM, Kolettis PN, Fineberg NS, Smith JK. Determination of split renal function by 3D reconstruction of CT angiograms: a comparison with gamma camera renography. American Journal of Roentgenology. 2008;191:1552–8. doi: 10.2214/AJR.07.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diez ASC, Powelson J, Goggins W, Taber T, Mujtaba MA, Yakub MS, Mishler D, Perry T, Ping S, Sharfuddin AA. Correlation between volumetric CT measurements and split-renal function and its utility in donor kidney selection [Abstract] Journal of the American Society of Nephrology : JASN. 2012;23:1028. [Google Scholar]
- 18.Shokeir AA, Gad HM, el-Diasty T. Role of radioisotope renal scans in the choice of nephrectomy side in live kidney donors. The Journal of Urology. 2003;170:373–6. doi: 10.1097/01.ju.0000074897.48830.58. [DOI] [PubMed] [Google Scholar]
- 19.Oh CK, Jeon KO, Kim HJ, Kim SI, Kim YS, Pelletier SJ. Metabolic demand and renal mass supply affecting the early graft function after living donor kidney transplantation. Kidney international. 2005;67:744–9. doi: 10.1111/j.1523-1755.2005.67136.x. [DOI] [PubMed] [Google Scholar]
- 20.Hugen CM, Polcari AJ, Farooq AV, Fitzgerald MP, Holt DR, Milner JE. Size does matter: donor renal volume predicts recipient function following live donor renal transplantation. The Journal of Urology. 2011;185:605–9. doi: 10.1016/j.juro.2010.09.098. [DOI] [PubMed] [Google Scholar]
- 21.Delmonico F Council of the Transplantation S. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79:S53–66. [PubMed] [Google Scholar]
- 22.Delmonico FL, Dew MA. Living donor kidney transplantation in a global environment. Kidney International. 2007;71:608–14. doi: 10.1038/sj.ki.5002125. [DOI] [PubMed] [Google Scholar]
- 23.Lennerling A, Loven C, Dor FJ, et al. Living organ donation practices in Europe -results from an online survey. Transplant International. 2013;26:145–53. doi: 10.1111/tri.12012. [DOI] [PubMed] [Google Scholar]
- 24.Halleck F, Diederichs G, Koehlitz T, et al. Volume matters: CT-based renal cortex volume measurement in the evaluation of living kidney donors. Transplant International. 2013;26:1208–16. doi: 10.1111/tri.12195. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson H, Wadstrom J, Andersson LG, Raland H, Magnusson A. Measuring split renal function in renal donors: can computed tomography replace renography? Acta Radiologica. 2004;45:474–80. doi: 10.1080/02841850410005282. [DOI] [PubMed] [Google Scholar]
- 26.el-Diasty TA, Shokeir AA, el-Ghar ME, Gad HM, Refaie AF, el-Din AB. Contrast enhanced spiral computerized tomography in live kidney donors: a single session for anatomical and functional assessment. The Journal of Urology. 2004;171:31–4. doi: 10.1097/01.ju.0000099784.52825.8e. [DOI] [PubMed] [Google Scholar]
- 27.Soga S, Britz-Cunningham S, Kumamaru KK, Malek SK, Tullius SG, Rybicki FJ. Comprehensive comparative study of computed tomography-based estimates of split renal function for potential renal donors: modified ellipsoid method and other CT-based methods. Journal of Computer Assisted Tomography. 2012;36:323–9. doi: 10.1097/RCT.0b013e318251db15. [DOI] [PubMed] [Google Scholar]
- 28.El-Husseini A, Sabry A, Zahran A, Shoker A. Can donor implantation renal biopsy predict long-term renal allograft outcome? American Journal of Nephrology. 2007;27:144–51. doi: 10.1159/000099944. [DOI] [PubMed] [Google Scholar]
- 29.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Annals of Internal Medicine. 2010;152:561–7. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan A, Diwan TS, Franco Palacios CR, et al. Using implantation biopsies as a surrogate to evaluate selection criteria for living kidney donors. Transplantation. 2013;96:975–80. doi: 10.1097/TP.0b013e3182a2b455. [DOI] [PubMed] [Google Scholar]
- 31.Mancilla E, Avila-Casado C, Uribe-Uribe N, et al. Time-zero renal biopsy in living kidney transplantation: a valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation. 2008;86:1684–8. doi: 10.1097/TP.0b013e3181906150. [DOI] [PubMed] [Google Scholar]
- 32.Cosio FG, Grande JP, Larson TS, et al. Kidney allograft fibrosis and atrophy early after living donor transplantation. AmericanJournal of Transplantation. 2005;5:1130–6. doi: 10.1111/j.1600-6143.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 33.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. Journal of the American Society of Nephrology : JASN. 2002;13:1358–64. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 34.Friedewald JJ, Reese PP. The kidney-first initiative: what is the current status of preemptive transplantation? Advances in Chronic Kidney Disease. 2012;19:252–6. doi: 10.1053/j.ackd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney International. 2000;58:1311–7. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 36.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney International. 2002;62:311–8. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney International. 1999;55:713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.