Abstract
Objective
To measure association between hepatic fat and albuminuria (an early marker of renal injury) in individuals without diabetes or hypertension.
Methods
2,281 individuals in the Multi-Ethnic Study of Atherosclerosis without diabetes or hypertension, renal disease, or excess alcohol consumption underwent computed tomography (CT) for assessment of liver attenuation (marker of hepatic lipid content) and urinalysis (for albuminuria) at initial study visit, with assessment of incident and prevalent albuminuria by logistic regression in follow-up.
Results
After adjustment for age, gender, race, smoking, blood pressure, insulin resistance, and body mass index, individuals with less liver fat (higher liver CT attenuation) had a lower probability of having albuminuria at Exam 1 (OR per 10 unit increase in attenuation 0.77, 95 % CI 0.61–0.97, P = 0.02). At median 9.3 years follow-up, albuminuria was identified in 129 individuals were (5.8 %). In fully adjusted models (with age, smoking, body mass index, blood pressure, diabetes and hypertension as time-dependent covariates), lower liver attenuation (greater liver fat) was associated with higher risk of incident albuminuria (OR 0.79, 95 % CI 0.66–0.94, P = 0.008).
Conclusions
Hepatic attenuation is associated with prevalent and incident albuminuria, an early, potent risk factor for renal risk in a population not clearly at risk for future renal failure.
Keywords: Albuminuria, Inflammation, Obesity, Hepatic steatosis
Introduction
Obesity and obesity-related illnesses represent a growing clinical problem worldwide. Among the multiple effects of obesity on health, chronic kidney disease has emerged as a potential consequence and is associated with subsequent poor outcomes. While hypertension and diabetes may explain a significant proportion of obesity-related renal disease, direct toxicity from pro-inflammatory fat—in the absence of hypertension and diabetes—has been recently proposed as a potential mechanism for renal dysfunction, independent of body mass index (BMI) [1–3]. Body mass index may not predict progressive renal failure in patients with type 2 diabetes [4]. Indeed, pro-inflammatory adipokines that characterize hepatic and visceral fat inflammation are associated with albuminuria and chronic kidney disease [5]. While a wealth of data has connected non-alcoholic fatty liver disease—a pro-inflammatory consequence of obesity and dysfunctional adiposity—with renal dysfunction [6–10], longitudinal studies in large multi-racial cohorts without diabetes or hypertension at baseline are less common [11–15]. This is especially relevant given that the risk of albuminuria is reversible with surgical weight loss in small studies, independent of other metabolic risk factors [16].
Despite these promising cross-sectional associations, the long-term prognostic value of measures of pro-inflammatory visceral adiposity on the development of kidney disease are lacking, especially in those individuals without hypertension or diabetes who are generally managed in the clinic. To address this important gap, we studied development of micro- and/or macroalbuminuria over 10 years as a function of initial hepatic fat content—a central, easily measured index of metabolic dysfunction across BMI— among individuals from the Multi-Ethnic Study of Atherosclerosis (MESA). The primary goal of this work was to determine the role of hepatic fat at baseline in predicting future long-term risk of subclinical renal disease in individuals free of hypertension and diabetes.
Methods
Participant population
The MESA study has been described previously [17]. In brief, the MESA cohort consisted of 6,814 men and women of different ethnicities (white, African American, Chinese American, and Hispanic) enrolled from six different national sites and who were free of clinical cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke, or peripheral arterial disease) at the time of study entry. To limit the effects of prevalent hypertension or diabetes on albuminuria, we excluded MESA participants with hypertension (defined as use of any anti-hypertension medication, systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg or self-report) or diabetes (defined as self-reported history of diabetes or use of insulin or oral hypoglycemic therapies, or fasting glucose ≥126 mg/dl). We also excluded subjects with known cancer, cirrhosis, kidney disease or who reported excess alcohol consumption (>14 drinks per week for males; >7 drinks per week for females). Finally, we excluded patients with missing data for body mass index, albuminuria or liver attenuation. All protocols were approved by the Institutional Review Board at each participating institution. All participants provided written informed consent.
Clinical and biomarker assessments
Demographics, medical history, medications, and physical examination (including anthropometric indices, including BMI and waist circumference) were conducted at 5 clinic visits approximately every 2 years (2000–2011) [18]. At baseline, liver attenuation by computed tomography (CT; a marker of hepatic fat content) and coronary artery calcium score was measured in all subjects as reported [19]. Fatty liver was defined as liver tissue with intensity ≤40 Hounsfield units [20–23]. Blood was collected at Exam 1 for insulin, glucose, C-reactive protein, matrix metalloproteinase (MMP)-3 and MMP-9, and plasminogen activator inhibitor-1, as described [24, 25]. Urine albumin and creatinine were assessed as described [26]. Homeostatic model of insulin resistance (HOMA-IR) was calculated as fasting insulin in mU/l × fasting glucose in mg/dl divided by 405 [27].
Urinary albumin and creatinine concentrations were collected as described [28]. In brief, urinary albumin was measured by nephelometry via the Array 360 CE Protein Analyzer (Beckman Instruments), with a lower limit of detection of 0.2 mg/ml. Urinary creatinine was assessed using the Vitros 950IRC instrument (Johnson and Johnson Clinical Diagnostics), with a range of 0.05–16.5 mg/dl and a coefficient of variation 2.5–2.9 %. A random spot urine albumin to creatinine ratio was calculated. These measures were obtained from Exams 1, 2, 3, and 5. “Microalbuminuria” was defined by spot urinary albumin-to-creatinine ratio as 30–300 mg urinary albumin per gram of urinary creatinine, and “macroalbuminuria” was defined as urinary albumin to creatinine ratio >300 mg. For the purposes of analysis, “prevalent albuminuria” was defined as any albuminuria at Exam 1, and “incident albuminuria” was defined as the subsequent development of albuminuria in individuals without albuminuria at Exam 1.
Statistical analysis
Covariates were examined for normality and parametric or non-parametric tests were selected as appropriate to compare clinical and biochemical characteristics, stratified by prevalent (baseline) and incident albuminuria. We used logistic regression to explore the relationship between hepatic steatosis and prevalent albuminuria, including after adjustment for relevant confounders. In addition to age, gender, and race, further adjustments were performed for metabolic risk factors that (1) are well-known to influence the development of renal dysfunction (diabetes, IFG, HOMA-IR, BMI, systolic blood pressure) and (2) are associated with obesity and insulin resistance (IFG, HOMAIR, diabetes). We then used discrete-time logistic regression to model the relationship between hepatic steatosis and incident albuminuria, including after adjustment for relevant clinical and metabolic risk factors. Given the importance of hypertension and diabetes in risk of albuminuria, we adjusted for the development of either condition as a time-dependent covariate. Direct adjusted survival curves from the final survival models visualized survival free of albuminuria during follow-up [29]. SAS (version 9.4, SAS Institute, Cary, NC, USA) and R (version 3.1.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/) were used for all analyses. Two-sided p-values less than 0.05 were considered statistically significant.
Results
After exclusions (listed above), the final cohort was comprised of 2,281 individuals (Table 1). In general, MESA participants without albuminuria at any time during the study period were younger with a more salutatory metabolic profile, signified by a slightly lower BMI, systolic blood pressure, HOMA-IR, waist circumference, and serum IL-6 concentration. Relative to those with incident or baseline albuminuria, individuals without albuminuria also had evidence of less hepatic fat content (measured as a slightly higher liver attenuation). Of note, neither overall weight nor estimated glomerular filtration rate at baseline was different between those with and without albuminuria. A higher coronary artery calcium score (a marker of subclinical cardiovascular disease) was associated with presence of albuminuria, as has been reported in MESA [26].
Table 1. Baseline characteristics of the study population, stratified by prevalent (baseline) and incident albuminuria.
| All subjects (n = 2,281) | No albuminuria (n = 2,083) | Incident albuminuria (n = 129) | Baseline albuminuria (n = 69) | P value | |
|---|---|---|---|---|---|
| Age (years) | 56.0 [50.0–65.0] | 56.0 [50.0–65.0] | 61.0 [51.0–70.0] | 65.0 [51.0–72.0] | <0.0001 |
| Male gender, n (%) | 1,048 (45.9) | 954 (45.8) | 62 (48.1) | 32 (46.4) | 0.73 |
| Race/ethnicity, n (%) | 0.65 | ||||
| White | 960 (42.1) | 881 (42.3) | 57 (44.2) | 22 (31.9) | |
| Chinese | 314 (13.8) | 288 (13.8) | 17 (13.2) | 9 (13.0) | |
| Black | 491 (21.5) | 447 (21.5) | 25 (19.4) | 19 (27.5) | |
| Hispanic | 516 (22.6) | 467 (22.4) | 30 (23.3) | 19 (27.5) | |
| Smoking, n (%) | <0.01 | ||||
| Never smoker | 1,197 (52.6) | 1,110 (53.4) | 51 (39.5) | 36 (52.2) | |
| Former smoker | 768 (33.7) | 696 (33.5) | 54 (41.9) | 18 (26.1) | |
| Current smoker | 312 (13.7) | 273 (13.1) | 24 (18.6) | 15 (21.7) | |
| Weight (pounds) | 162.4 [140.3–189.3] | 162.2 [140.5–189.3] | 166.5 [137.5–191.0] | 165.0 [142.0–197.2] | 0.19 |
| Body mass index (kg/m2) | 26.4 [23.6–29.8] | 26.4 [23.6–29.7] | 26.3 [23.4–29.9] | 27.6 [24.9–31.4] | <0.01 |
| Waist circumference (cm) | 93.3 [84.3–101.7] | 93.0 [84.1–101.5] | 94.0 [86.8–105.0] | 97.2 [89.0–107.5] | <0.0001 |
| Systolic blood pressure (mmHg) | 113.5 [104.5–124.0] | 113.0 [104.0–123.0] | 116.0 [107.5–129.0] | 122.5 [114.0–129.5] | <0.0001 |
| Diastolic blood pressure (mmHg) | 69.0 [63.0–75.0] | 69.0 [63.0–75.0] | 70.0 [64.5–76.5] | 70.5 [63.5–75.0] | 0.28 |
| Glucose (mg/dl) | 85.0 [80.0–91.0] | 85.0 [80.0–90.0] | 85.0 [81.0–91.0] | 89.0 [83.0–94.0] | <0.01 |
| Insulin (mU/l) | 7.2 [5.4–10.1] | 7.1 [5.4–9.9] | 7.5 [5.5–11.0] | 9.1 [6.4–13.3] | <0.01 |
| Homeostatic model of insulin resistance (HOMA-IR) | 1.5 [1.1–2.2] | 1.5 [1.1–2.1] | 1.6 [1.1–2.4] | 2.1 [1.3–2.9] | <0.001 |
| Estimated glomerular filtration rate (ml/min) | 81.0 [71.6–92.3] | 81.0 [71.9–92.3] | 81.6 [70.4–92.3] | 80.4 [71.0–94.6] | 0.73 |
| C-reactive protein (mg/1) | 1.5 [0.7–3.4] | 1.4 [0.6–3.3] | 1.7 [0.6–4.0] | 2.5 [1.0–5.0] | 0.17 |
| Plasminogen activator inhibitor-1 (ng/ml) | 16.0 [8.0–28.0] | 15.0 [8.0–27.5] | 18.5 [15.0–49.0] | 23.5 [18.0–36.0] | 0.13 |
| Intercellular adhesion molecule-1 (ng/ml) | 263.2 [225.8–304.1] | 262.8 [225.8–304.1] | 257.8 [226.8–300.7] | 299.8 [216.0–349.8] | 0.16 |
| Interleukin-2 (pg/ml) | 849.0 [688.0–1,051.0] | 849.0 [690.0–1,049.0] | 751.5 [614.0–1,047.5] | 939.0 [812.0–1,189.0] | 0.03 |
| Interleukin-6 (pg/ml) | 1.0 [0.7–1.6] | 1.0 [0.6–1.6] | 1.1 [0.7–1.9] | 1.5 [0.9–2.2] | <0.001 |
| Matrix metalloproteinase-3 (ng/ml) | 11.9 [8.4–17.9] | 11.8 [8.3–17.8] | 11.2 [7.8–20.8] | 15.1 [9.6–18.5] | 0.74 |
| Matrix metalloproteinase-9 (ng/ml) | 197.9 [149.3–292.0] | 197.7 [148.5–291.9] | 207.4 [164.5–279.4] | 207.7 [147.7–317.9] | 0.29 |
| Soluble tumor necrosis alpha-α receptor (pg/ml) | 1,211.0 [1,054.0–1,421.0] | 1,206.0 [1,049.0–1,409.0] | 1,271.5 [1,090.5–1,513.5] | 1,423.0 [1,193.0–1,669.0] | <0.0001 |
| Exercise (metabolic equivalents METS minutes/week) | 912.5 [210.0–2,205.0] | 941.3 [210.0–2,205.0] | 900.0 [210.0–2,400.0] | 630.0 [0.0–1,605.0] | 0.25 |
| Coronary artery calcium score (CAC) | 0.0 [0.0–20.6] | 0.0 [0.0–16.1] | 0.0 [0.0–66.8] | 0.0 [0.0–74.2] | <0.0001 |
| CAC category | <0.01 | ||||
| 0 | 1,459 (64.0) | 1,353 (65.0) | 66 (51.2) | 40 (58.0) | |
| 1–100 | 516 (22.6) | 466 (22.4) | 33 (25.6) | 17 (24.6) | |
| >100 | 306 (13.4) | 264 (12.7) | 30 (23.3) | 12 (17.4) | |
| Microalbuminuria | 67 (2.9) | 0 (0.0) | 0 (0.0) | 67 (97.1) | <0.0001 |
| Macroalbuminuria | 2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (2.9) | <0.0001 |
| Liver attenuation (Hounsfield units HU) | 63.0 [57.5–67.5] | 63.0 [58.0–67.5] | 61.5 [53.5–66.5] | 61.5 [54.0–65.5] | <0.0001 |
| Fatty liver (HU ≤40) | 90 (3.9) | 72 (3.5) | 10 (7.8) | 8 (11.6) | <0.0001 |
Entries are expressed as median (interquartile range) or number of subjects (percentage of total). P values refer to three-way comparison between no albuminuria, incident, prevalent albuminuria
A higher liver attenuation by CT (less hepatic fat) was associated with lower odds of albuminuria at baseline (unadjusted odds ratio OR for every 10 Hounsfield unit increase in attenuation 0.74, 95 % confidence interval CI 0.61–0.90, P = 0.002; Table 2). After adjustment for age, gender, race, smoking, baseline systolic blood pressure, HOMA-IR, and body mass index, the relationship between liver attenuation and albuminuria persisted (adjusted OR 0.77, 95 % CI 0.61–0.97, P = 0.02). Notably, individuals with a frank fatty liver had a nearly 2.96-fold higher odds of prevalent albuminuria after adjustment (Table 2). There was no evidence of heterogeneity in this association by age, gender, race or obesity status (BMI > or <30 kg/m2). IL-6, C-reactive protein, and estimated GFR were not significant in models for prevalent albuminuria.
Table 2. Logistic regression models for prevalent (baseline) albuminuria.
| Variable | Adjustments | Odds ratio (95 % confidence interval) | P value | Total subjects (n) | Subjects with baseline albuminuria (n) |
|---|---|---|---|---|---|
| Liver attenuation (per 10 HU) | None | 0.74 (0.61–0.90) | <0.01 | 2,281 | 69 |
| Age, gender, race, smoking, BMI, SBP, fasting glucose | 0.74 (0.60–0.93) | 0.008 | 2,277 | 69 | |
| Age, gender, race, smoking, waist circumference, SBP, fasting glucose | 0.74 (0.59–0.92) | 0.006 | 2,277 | 69 | |
| Age, gender, race, smoking, BMI, SBP, HOMA-IR | 0.77 (0.61–0.97) | 0.02 | 2,273 | 69 | |
| Fatty liver (HU ≤40) | None | 3.41 (1.58–7.35) | <0.01 | 2,281 | 69 |
| Age, gender, race, smoking, BMI, SBP, fasting glucose | 2.96 (1.26–6.97) | 0.01 | 2,277 | 69 | |
| Age, gender, race, smoking, waist circumference, SBP, fasting glucose | 3.11 (1.33–7.24) | 0.009 | 2,277 | 69 | |
| Age, gender, race, smoking, BMI, SBP, HOMA-IR | 2.60 (1.06–6.39) | 0.04 | 2,273 | 69 |
SBP systolic blood pressure, BMI body mass index, HOMA-IR homeostatic model of insulin resistance, HU Hounsfield units
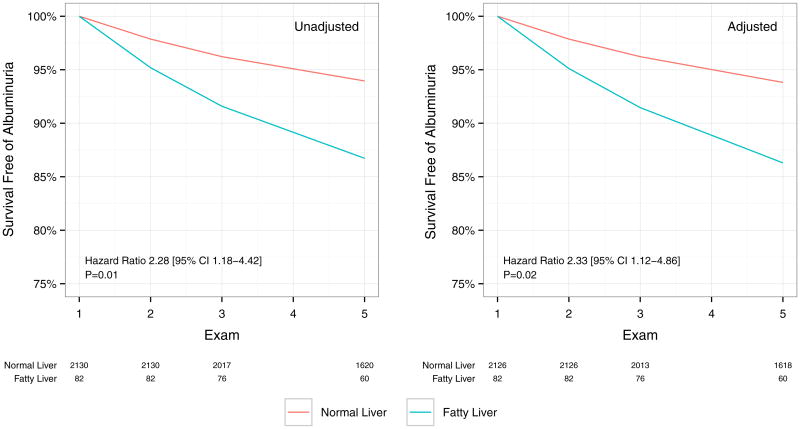
At a median of 9.3 years (IQR 8.5–9.7) of follow-up, 129 individuals developed albuminuria (129/2,281; 5.8 %). A fatty liver was associated with a greater risk of incident albuminuria, with 5.6 % incident albuminuria (119 cases/2,191 individuals) in individuals with a normal liver versus 12.2 % in individuals with frank fatty liver (10 cases/90 individuals; P = 0.03). Every 10 unit increase in liver attenuation was associated with a lower odds of incident albuminuria in unadjusted models (OR 0.81, 95 % CI 0.70–0.95, P = 0.009). In fully adjusted models (with smoking, age, BMI, systolic blood pressure, HOMA-IR, diabetes status and hypertension as time-dependent covariates), the association between increased liver attenuation and albuminuria was not significantly altered (multivariable OR 0.75; 95 % CI 0.63–0.89, P = 0.001; Table 3; Fig. 1). Results were similar with fatty liver (multivariable OR 2.13, 95 % CI 1.02–4.34, P = 0.04). Further adjustment by IL-6 (which was associated with incident albuminuria) did not significantly change the effect size for liver attenuation. Results were similar for both incident and prevalent albuminuria when waist circumference was used instead of BMI.
Table 3. Discrete time logistic regression models for incident albuminuria in long-term follow-up in MESA.
| Variable | Adjustments | Hazard ratio (95 % confidence interval) | P value | Total subjects | Subjects with incident albuminuria |
|---|---|---|---|---|---|
| Liver attenuation (per 10 HU) | None | 0.81 (0.70–0.95) | 0.009 | 2,212 | 129 |
| Age, gender, race, smoking, BMI, SBP, fasting glucose | 0.75 (0.63–0.89) | 0.001 | 2,208 | 129 | |
| Age, gender, race, smoking, waist circumference, SBP, fasting glucose | 0.77 (0.65–0.92) | 0.003 | 2,208 | 129 | |
| Agea, gender, race, smokinga, BMIa, SBPa, fasting glucosea, diabetesa, IFGa, hypertensiona | 0.79 (0.67–0.95) | 0.01 | 2,208 | 129 | |
| Age, gender, race, smoking, BMI, SBP, HOMA-IR | 0.76 (0.63–0.91) | 0.003 | 2,204 | 129 | |
| Agea, gender, race, smokinga, BMIa, SBPa, HOMA-IRa, diabetesa, IFGa, hypertensiona | 0.79 (0.66–0.94) | 0.008 | 2,212 | 129 | |
| Fatty liver (HU ≤40) | None | 2.28 (1.18–4.42) | 0.01 | 2,212 | 129 |
| Age, gender, race, smoking, BMI, SBP, fasting glucose | 2.59 (1.28–5.27) | 0.009 | 2,208 | 129 | |
| Age, gender, race, smoking, waist circumference, SBP, fasting glucose | 2.59 (1.27–5.28) | 0.009 | 2,208 | 129 | |
| Agea, gender, race, smokinga, BMIa, SBPa, fasting glucosea, diabetesa, IFGa, hypertensiona | 2.10 (1.02–4.34) | 0.045 | 2,208 | 129 | |
| Age, gender, race, smoking, BMI, SBP, HOMA-IR | 2.41 (1.14–5.05) | 0.02 | 2,204 | 129 | |
| Agea, gender, race, smokinga, BMIa, SBPa, HOMA-IRa, diabetesa, IFGa, hypertensiona | 2.13 (1.02–4.34) | 0.04 | 2,208 | 129 |
BMI body mass index, SBP systolic blood pressure, HOMA-IR homeostatic model of insulin resistance, IFG impaired fasting glucose
Adjustments were as time-dependent covariates
Fig. 1.
Survival free of albuminuria (macro- or micro-) was computed with discrete time logistic regression across Exams 1, 2, 3 and 5. Albuminuria was not assessed at Exam 4. Unadjusted data are shown at left and data adjusted for age, gender, race, BMI, systolic blood pressure and HOMA-IR are shown at right. Numbers at risk are shown below the graphs
Discussion
In a study of 2,281 participants in MESA without diabetes or hypertension at study entry, more hepatic fat (as reflected in lower CT attenuation) was associated with greater risk for albuminuria at the index study visit. In addition, greater hepatic fat content (by CT) identified individuals at higher risk for subsequent development of albuminuria over 9 years. These associations were independent of age, gender, race, or body mass index, as well as systolic blood pressure and insulin resistance (by HOMA-IR), suggesting that liver fat measures by CT may be a robust, independent early marker of metabolic disease before the onset of frank diabetes or hypertension. Given the ease of measurement of liver attenuation in any non-contrast CT and the central pathophysiologic importance of the liver as a marker of dysfunctional adiposity, these results suggest that non-invasive assessment of hepatic steatosis may provides a window into early metabolic changes that predate established renal and vascular dysfunction.
Though data studying the long-term impact of direct markers of hepatic steatosis—a central index of visceral obesity and metabolic dysfunction—on renal dysfunction have been mixed, the preponderance of evidence suggests a link between hepatic fat and renal damage. This topic has been extensively reviewed recently [30]. In a recent report from the Edinburgh Type 2 Diabetes Study, Jenks and colleagues defined hepatic steatosis by ultrasonographic criteria in 933 patients with T2D (530 with steatosis; 57 %), reporting no difference in decline in glomerular filtration rate or incident albuminuria to 4 years' follow-up [31]. Separate data using ultrasound to detect steatosis in the National Health and Nutrition Examination Survey (1988–1994) also demonstrated no association with chronic kidney disease or albuminuria [32]. Nevertheless, investigations in several other studies have consistently demonstrated an association between hepatic steatosis and incident kidney disease (defined by glomerular filtration rate or proteinuria) [11–14]. Each of these studies provides extensive adjustments for cardiac and metabolic risk, implying that hepatic fat accumulation may impact renal disease independent of effects on diabetes or hypertension alone. In addition, studies in type 1 diabetes using ultrasound-defined hepatic steatosis have shown the opposite result, with non-alcoholic fatty liver disease associated with greater risk of decline in renal function or microalbuminuria [6, 8]. Microalbuminuria, in turn, has been associated with greater liver fibrosis in patients without diabetes with fatty liver disease [33], suggesting an important cross-talk between liver tissue architecture, hepatic steatosis, and renal dysfunction.
One of the important limitations of several published studies in this field is the inclusion of patients with established hypertension or diabetes, significant contributors to chronic kidney disease worldwide. In contrast, our findings extend these prior results in a pre-diabetic and diabetic population to an even earlier stage, thereby providing additional evidence for a robust association between hepatic fat accumulation by CT and prevalent albuminuria in the community, independent of age, race, gender, or obesity status. In contrast to prior work utilizing less sensitive ultrasound measures, MESA utilized CT measures of liver attenuation as an index of hepatic fat content, which is tightly associated with lipid content in phantom studies and in patients [34, 35] and identifies individuals with significant hepatic steatosis (with a liver attenuation <40 HU associated with >30 % hepatic steatosis) [20–23]. Furthermore, we specifically limited our population to individuals without diabetes or hypertension at index examination to target a population not readily recognized as having significant renal risk. Indeed, the demonstration that a single index of hepatic fat content is independently associated with incident albuminuria in this population (even after adjustment for incident diabetes or hypertension) is a testament to the importance of the liver not only in metabolic, but also renal, risk. In a large pooled study of 13,324 individuals from the Atherosclerosis Risk in Communities and Cardiovascular Health Study (mean BMI 27.2 kg/m2) over 9.3 years, Elsayed and colleagues [36] found that changes in waist-to-hip ratio were associated with an increased hazard of chronic kidney disease (0.4 mg/dl increase in serum creatinine or 15 ml/min/1.73 m2 decrement in glomerular filtration rate) while BMI was not associated with renal dysfunction in this study.
The results of this study should be viewed in the context of its design. One striking finding in the study was the relatively low prevalence of “fatty liver” as defined by Hounsfield unit threshold (<40 HU). On first blush, this observation suggests that our population may not have been at highest metabolic risk; however, our observation that liver attenuation (treated as a continuous variable) is strongly associated with renal outcomes is a testament to the nature of attenuation as a continuous marker of prognostically important hepatic steatosis. Gold standard indices of hepatic lipid content (e.g., proton magnetic resonance spectroscopy) were not available within MESA; however, CT attenuation is closely associated with spectroscopic results [34, 35]. While the event rate in our population supported the extent of adjustment in multivariable models, additional follow-up for more events may add power to the modeling results. Finally, examining more sensitive measures of renal dysfunction (e.g., cystatin C, neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, or interleukin-18) may be more informative for different renal injury mechanisms. Albuminuria, however, remains a powerful marker of future cardiac, vascular, and renal risk that is used clinically as a criterion for early renal dysfunction.
In conclusion, these results from MESA demonstrate the association between hepatic attenuation, an easily accessible, marker of inflammation and metabolic disease, with prevalent and incident albuminuria, which is an early and potent risk factor for renal risk and all-cause mortality in a population not clearly at risk for future renal failure. Using hepatic fat content as a potential metric to identify at-risk individuals may identify those at future risk of renal dysfunction and its downstream health consequences.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. Dr. Shah is supported by a Fellow-to-Faculty Award from the American Heart Association. Dr. Allison was supported by funding for the MESA Abdominal Body Composition Ancillary study from the National Heart, Lung, and Blood Institute (R01-HL088451). MESA is supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI). Jingzhong Ding and Matthew J. Budoff are supported by R01-HL-085323 and R01-HL-071739, respectively, from NHLBI.
Footnotes
Conflict of interest Dr. Murthy has minor stock in General Electric. There are no other disclosures for any other authors relevant to the content of this manuscript.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
All other authors have no financial disclosures relevant to the content of this manuscript.
Contributor Information
Ravi V. Shah, Email: rshah1@bidmc.harvard.edu, Department of Cardiology and Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, 185 Pilgrim Road, Suite 454-East, Boston, MA 02215, USA.
Matthew A. Allison, Department of Family and Preventative Medicine, University of California-San Diego, San Diego, CA, USA
Joao A. C. Lima, Cardiology Division, Johns Hopkins Medical Institute, Baltimore, MD, USA
Siddique A. Abbasi, Department of Cardiology and Medicine, Brown University, Providence, RI, USA
Morgana Mongraw-Chaffin, Department of Family and Preventative Medicine, University of California-San Diego, San Diego, CA, USA.
Michael Jerosch-Herold, Non-Invasive Cardiovascular Imaging, Brigham and Women's Hospital, Boston, MA, USA.
Jingzhong Ding, Department of Medicine, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA.
Matthew J. Budoff, Department of Cardiology and Medicine, University of California-Los Angeles, Los Angeles, CA, USA
Venkatesh L. Murthy, Email: vlmurthy@med.umich.edu, Cardiovascular Medicine Division, Department of Medicine, Nuclear Medicine and Cardiothoracic Imaging Divisions, Department of Radiology, University of Michigan, 1338 Cardiovascular Center, Ann Arbor, MI, USA.
References
- 1.ChandieShaw PK, Berger SP, Mallat M, Frolich M, Dekker FW, Rabelink TJ. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care. 2007;30(7):1840–1844. doi: 10.2337/dc07-0028. [DOI] [PubMed] [Google Scholar]
- 2.Lin WY, Pi-Sunyer FX, Liu CS, Li CI, Davidson LE, Li TC, et al. Central obesity and albuminuria: both cross-sectional and longitudinal studies in Chinese. PLoS ONE. 2012;7(12):e47960. doi: 10.1371/journal.pone.0047960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam GE, Han K, Park YG, Kim YH, Lee KS, Cho KH, et al. Abdominal obesity is associated with albuminuria in women: the 2011 Korea National Health and Nutrition Examination Survey. J Womens Health (Larchmt) 2014;23(3):267–274. doi: 10.1089/jwh.2013.4497. [DOI] [PubMed] [Google Scholar]
- 4.Mohsen A, Brown R, Hoefield R, Kalra PA, O'Donoghue D, Middleton R, et al. Body mass index has no effect on rate of progression of chronic kidney disease in subjects with type 2 diabetes mellitus. J Nephrol. 2012;25(3):384–393. doi: 10.5301/jn.5000062. [DOI] [PubMed] [Google Scholar]
- 5.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305(12):F1629–F1636. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of chronic kidney disease in patients with Type 1 diabetes and non-alcoholic fatty liver. Diabet Med. 2012;29(2):220–226. doi: 10.1111/j.1464-5491.2011.03427.x. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Chonchol M, Zoppini G, Abaterusso C, Bonora E. Risk of chronic kidney disease in patients with nonalcoholic fatty liver disease: is there a link? J Hepatol. 2011;54(5):1020–1029. doi: 10.1016/j.jhep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53(7):1341–1348. doi: 10.1007/s00125-010-1720-1. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444–450. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 10.Targher G. Visceral adipose tissue may mediate the link between non-alcoholic fatty liver disease and endocrine abnormalities. J Hepatol. 2006;45(3):454. doi: 10.1016/j.jhep.2006.06.010. author reply-6. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol. 2008;19(8):1564–1570. doi: 10.1681/ASN.2007101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, et al. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism. 2008;57(4):569–576. doi: 10.1016/j.metabol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, et al. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern Med. 2011;50(10):1081–1087. doi: 10.2169/internalmedicine.50.5043. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Mantovani A, Pichiri I, Mingolla L, Cavalieri V, Mantovani W, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37(6):1729–1736. doi: 10.2337/dc13-2704. [DOI] [PubMed] [Google Scholar]
- 15.Foster MC, Hwang SJ, Massaro JM, Hoffmann U, DeBoer IH, Robins SJ, et al. Association of subcutaneous and visceral adiposity with albuminuria: the Framingham Heart Study. Obesity (Silver Spring) 2011;19(6):1284–1289. doi: 10.1038/oby.2010.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson LM, Romeo S, Jacobson P, Burza MA, Maglio C, Sjoholm K, et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish obese subjects (SOS): a prospective controlled intervention trial. Int J Obes (Lond) 2015;39:169–175. doi: 10.1038/ijo.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58(2):140–146. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeb I, Katz R, Nasir K, Ding J, Rezaeian P, Budoff MJ. Relation of nonalcoholic fatty liver disease to the metabolic syndrome: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2013;7(5):311–318. doi: 10.1016/j.jcct.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244(2):479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol. 2012;22(5):1075–1082. doi: 10.1007/s00330-011-2349-2. [DOI] [PubMed] [Google Scholar]
- 22.Maruzzelli L, Parr AJ, Miraglia R, Tuzzolino F, Luca A. Quantification of hepatic steatosis: a comparison of computed tomography and magnetic resonance indices in candidates for living liver donation. Acad Radiol. 2014;21(4):507–513. doi: 10.1016/j.acra.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 24.Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, et al. Relation of leptin to left ventricular hypertrophy (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112(5):726–730. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah RV, Abbasi S, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013;61:1698–1706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3(6):595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tison GH, Blaha MJ, Budoff MJ, Katz R, Rivera JJ, Bertoni AG, et al. The relationship of insulin resistance and extracoronary calcification in the multi-ethnic study of atherosclerosis. Atherosclerosis. 2011;218(2):507–510. doi: 10.1016/j.atherosclerosis.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garimella PS, Ix JH, Katz R, Shlipak MG, Criqui MH, Siscovick DS, et al. Association of albumin-creatinine ratio and cystatin C with change in ankle-brachial index: the multi-ethnic study of atherosclerosis (MESA) Am J Kidney Dis. 2015;65:33–40. doi: 10.1053/j.ajkd.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143(10):1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64(4):638–652. doi: 10.1053/j.ajkd.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Jenks SJ, Conway BR, Hor TJ, Williamson RM, McLachlan S, Robertson C, et al. Hepatic steatosis and non-alcoholic fatty liver disease are not associated with decline in renal function in people with Type 2 diabetes. Diabet Med. 2014;31(9):1039–1046. doi: 10.1111/dme.12456. [DOI] [PubMed] [Google Scholar]
- 32.Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol. 2012;36(5):466–471. doi: 10.1159/000343885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59(9):1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27(1):108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 35.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230(1):276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 36.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]