Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) and heart failure (HF) are obesity-related conditions with high cardiovascular mortality. Whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction among the general population is unknown.
Methods
We performed a cross-sectional analysis of 2,713 participants from the multicenter, community-based Coronary Artery Risk Development in Young Adults (CARDIA) study who underwent concurrent computed tomography (CT) quantification of liver fat and comprehensive echocardiography with myocardial strain measured by speckle tracking during the Year-25 examination (age 43-55 years, 58.8% women, 48.0% black). NAFLD was defined as liver attenuation ≤ 40 Hounsfield units after excluding other causes of liver fat. Subclinical left ventricular (LV) systolic dysfunction was defined using values of absolute peak global longitudinal strain (GLS). Diastolic dysfunction was defined using Doppler and tissue Doppler imaging markers.
Results
The prevalence of NAFLD was 10.0%. Participants with NAFLD had lower early diastolic relaxation (e’) velocity (10.8±2.6 vs. 11.9±2.8 cm/s), higher LV filling pressure (E/e’ ratio, 7.7±2.6 vs. 7.0±2.3) and worse absolute GLS (14.2±2.4% vs. 15.2±2.4%) than non-NAFLD (p<0.0001 for all). When adjusted for HF risk factors or body mass index, NAFLD remained associated with subclinical myocardial remodeling and dysfunction (p<0.01). The association of NAFLD with e’ velocity (β= -0.36[SE=0.15] cm/s, p=0.02), E/e’ ratio (β= 0.35[0.16], p=0.03) and GLS (β= -0.42[0.18]%, p=0.02) was attenuated after controlling for visceral adipose tissue. Effect modification by race and sex was not observed.
Conclusions
NAFLD is independently associated with subclinical myocardial remodeling and dysfunction, and provides further insight into a possible link between NAFLD and heart failure.
Keywords: heart failure, epidemiology, echocardiography, NAFLD, obesity, CARDIA
Both heart failure (HF) and non-alcoholic fatty liver disease (NAFLD) are obesity-related conditions with high cardiovascular morbidity and mortality that have reached epidemic proportions(1-3). Growing evidence suggests that NAFLD is an independent risk factor for cardiovascular disease and is associated with impaired endothelial function(4), a higher prevalence of vulnerable coronary plaques(5, 6) and with unfavorable levels of markers of subclinical atherosclerotic disease, including increased carotid intima media thickness(7) and coronary artery calcification (CAC)(8-10). In fact, patients with NAFLD are more likely to die from complications of cardiovascular disease than from liver-related death(11). Small studies of selected patients, primarily in adolescents, have found that NAFLD is also associated with myocardial insulin resistance(12), altered cardiac energy metabolism(13), abnormal left ventricular (LV) structure, and impaired diastolic function(14-16). However, whether these associations between NAFLD and abnormalities in myocardial structure and function apply to the general adult population is unknown.
Visceral adipose tissue (VAT) is an endocrine organ that secretes factors contributing to vascular inflammation and insulin resistance, and may be a risk factor for NAFLD or a marker of NAFLD severity(17). Since NAFLD and metabolic syndrome features often coexist, any relationship between NAFLD and cardiac remodeling and dysfunction may be moderated by VAT volume, obesity, or other cardiometabolic risk factors. To date, it remains unclear whether the associations between NAFLD and subclinical abnormalities in myocardial structure and function are independent of these factors. Improved knowledge of the mechanisms underlying any observed associations may provide pathophysiologic insight into a possible link between NAFLD and clinical HF.
We therefore sought to examine the associations between NAFLD and early changes in LV structure and function in a population-based study and whether the strengths of these associations were influenced by cardiometabolic risk factors, including other markers of adiposity. We hypothesized that NAFLD is significantly associated with subclinical abnormalities in cardiac structure and function even after the adjustment for body-mass index (BMI) and VAT.
METHODS
Study Sample
CARDIA is a multicenter community-based longitudinal cohort study of the development and determinants of cardiovascular disease in black and white young adults recruited from 1985-1986 at 18–30 years of age across 4 U.S. cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). The study design has been published previously(18). Eight examinations have been completed to date, which were approved by institutional review boards at all sites and informed consent obtained at every examination. The present study includes participants who underwent both comprehensive echocardiography (including tissue Doppler imaging and speckle-tracking analysis) and computed tomography (CT) scanning of both the thorax and abdomen from June 2010 to August 2011 as part of the 25-year follow-up examination.
There were 3,498 participants (45.5% men, 50.5% black) that attended the CARDIA Year-25 exam. Participants were excluded from the CT exam if they were pregnant, weighed more than 450 lbs. or were unable to fit within the CT gantry (n=28). We also excluded those missing measurements for liver fat (n=304), those without an echocardiogram or with poor speckle-tracked images (n=27), 62 participants with a medically verified history of acute myocardial infarction, angina or HF, those with a self-reported history of hepatitis C or cirrhosis (n=30) and those with a risk factor for chronic liver disease (e.g. intravenous drug use) or with a potential cause of secondary hepatic steatosis (n=338): alcohol consumption ≥ 20 g/day in women and ≥ 30 g/day in men(19) (n=179), self-reported human immunodeficiency virus (HIV) (n=22), prior intravenous drug use (n=93), and medications known to cause hepatic steatosis (e.g. valproic acid, methotrexate, tamoxifen and/or amiodarone) (n=44). The remaining 2,713 participants formed the sample population (Figure 1).
Figure 1.
Study sample—Abbreviations: CT, computed tomography; HIV, human immunodeficiency virus
*Alcohol use was defined as ≥ 20 g/day in women, ≥ 30 g/day in men. †Medications = valproic acid, methotrexate, tamoxifen and amiodarone.
Measurements
Standardized protocols for data collection were used across study centers and measurements have previously been described(18).
Computed Tomography
The CT protocol included the heart and abdomen using a non-contrast CT scan performed using GE (GE 750HD 64 and GE LightSpeed VCT 64 Birmingham and Oakland Centers, respectively; GE Healthcare, Waukesha, Wisconsin) or Siemens (Sensation 64, Chicago and Minneapolis Centers; Siemens Medical Solutions, Erlangen, Germany) multidetector CT scanners and has been described previously(10). Quality control and image analysis was performed at a core reading center (Wake Forest University Health Sciences, Winston-Salem, North Carolina). In the current study, NAFLD was defined as liver attenuation ≤ 40 HU after exclusion of other causes of liver fat (Figure 1)(20). Measurement of liver attenuation was performed in the right lobe of the liver using CT slices through the upper abdomen and was reported as the average of nine measurements on three slices using circular regions of interest of 2.6 cm2. The interclass correlation coefficient between different readers on a random selected sample of 156 participants was 0.975 for liver attenuation, indicating high reproducibility of CT measured liver attenuation in this study. The methods for assessment of adiposity within the CARDIA study have also been described previously(10). The VAT volumes were the sum of fat voxels within 10 mm set of slices centered at the L4-5 disk within the intra-abdominal cavity. The interclass correlation coefficient for inter-reader comparisons was 0.989 for VAT, and intra- and inter-reader error were 2.4% and 6.7%, respectively, in 156 scans that were blinded and reevaluated.
Echocardiography
Comprehensive echocardiography, including Doppler and tissue Doppler imaging, was performed using an Artida cardiac ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) by trained sonographers using a standardized protocol across all field centers. Experienced sonographers made measurements from digitized images using a standard software offline image analysis system (Digisonics, TX, USA). The echocardiography protocol at Year-25 has been previously published and followed existing American Society of Echocardiography guidelines for study acquisition and measurement(21, 22). Quality control and image analysis was performed at a core reading center (Johns Hopkins University, Baltimore, Maryland). Abnormal LV relaxation was defined as lateral tissue Doppler e’ velocity < 10 cm/s(23). Increased LV filling pressure was defined as E/e’ ratio ≥ 12 alone or the combination of E/e’ ratio 8-12 and left atrial (LA) volume index ≥ 34 ml/m2(23). LV mass was indexed to height2.7 and LA volume indexed to height. The speckle tracking echocardiography images for myocardial strain and strain rate measurements were analyzed in a 16-segment basis for LV mid-wall layer, using Wall Motion 2-dimensional Tracking software (Toshiba Medical Systems). Three cardiac cycles from each view were recorded for offline analyses. Strain was calculated as the change in segment length relative to its enddiastolic length, and the peak systolic value was recorded.
Statistical Analysis
Linear regression models were used to quantify cross-sectional associations between the exposure (continuous liver attenuation or presence of NAFLD), and the outcome variables (echocardiographic parameters). Multinomial logistic regression models and logistic regression models were then used to assess the association of presence of NAFLD on categories of subclinical LV systolic and diastolic dysfunction, respectively. Covariates in the multivariable model were chosen a priori for clinical importance. Potential confounders included age, race, sex, study center, socioeconomic level, alcohol intake, physical activity score, and HF risk factors (e.g. diabetes status, systolic blood pressure, total cholesterol, high density lipoprotein, and lipid and antihypertensive medication use). Potential effect modifiers were BMI and VAT. Pearson correlation coefficients were computed between obesity measures and liver attenuation. Six models were fitted: Model 1 (base model): age, race, sex, study center, educational level, income level, alcohol intake, smoking status and physical activity score; Model 2: Base model + HF risk factors; Model 3: Base model + BMI; Model 4: Base model + VAT; Model 5: Base model + HF risk factors + BMI; Model 6: Base model + HF risk factors + VAT. Interaction terms were generated between NAFLD and race, sex, age, diabetes and hypertension status and levels of VAT volume or BMI in terms of e’ velocity, E/e’ ratio or GLS. A P value < 0.05 was considered statistically significant. Analyses were performed using SAS 9.4 (SAS institute, Cary, NC).
RESULTS
Clinical, Fat Distribution, and Metabolic Characteristics
Clinical, demographic, metabolic, and laboratory characteristics of the 2,713 participants (58.8% women, 48.0% black), stratified by presence of NAFLD, are summarized in Table 1. NAFLD was present in 271 participants (prevalence=10.0%). NAFLD participants were of similar age compared to non-NAFLD, but more likely to be male, white, and have the metabolic syndrome (Table 1). NAFLD participants were also more likely to be obese and have increased waist circumference, waist-to-hip ratio and higher levels of CT-measured VAT volume (Table 1). When compared to non-NAFLD, NAFLD participants exhibited a higher prevalence of insulin resistance, as demonstrated by higher fasting insulin, glucose, and homeostatic model assessment of insulin resistance (HOMA-IR) scores, hypertrigylceridemia and increased high sensitivity c-reactive protein levels (Table 1).
Table 1.
Characteristics of the Overall Study Sample and Participants With and Without NAFLD, the CARDIA Study, Year 25 Examination (2010-2011)
| Overall Sample (n=2713) | No NAFLD (n=2442) | NAFLD (n=271) | P valuea | |
|---|---|---|---|---|
| Age, years | 50.1 ± 3.6 | 50.1 ± 3.6 | 50.5 ± 3.7 | 0.05 |
| Women | 1595 (58.8) | 1472 (60.3) | 123 (45.4) | <0.0001 |
| Menopause status - yes | 657 (41.6) | 599 (41.1) | 58 (47.9) | 0.34 |
| - Not sure | 247 (15.7) | 230 (15.8) | 17 (14.1) | |
| Black | 1302 (48.0) | 1192 (48.8) | 110 (40.6) | 0.01 |
| Grade of School completed | 15.1 ± 2.7 | 15.1 ± 2.6 | 15.1 ± 2.7 | 0.87 |
| Income < $50,000/year | 907 (33.9) | 805 (33.5) | 102 (37.8) | 0.16 |
| Smokers | ||||
| Never | 1723 (64.3) | 1558 (64.7) | 165 (61.6) | 0.29 |
| Former | 570 (21.3) | 503 (20.9) | 67 (35.0) | |
| Alcohol drinkers | 2089 (77.8) | 1882 (77.8) | 207 (77.5) | 0.92 |
| Average alcohol use (drinks/week) | 3.1 ± 4.5 | 3.1 ± 4.5 | 3.0 ± 4.6 | 0.72 |
| Physical activity (exercise units/week) | 335.3 ± 276.2 | 340.8 ± 279.1 | 285.8 ± 243.2 | 0.0006 |
| Comorbidities | ||||
| Hyperlipidemia | 1375 (50.8) | 1222 (50.2) | 153 (56.7) | 0.04 |
| Hypertension | 875 (32.3) | 729 (29.9) | 146 (53.9) | <0.0001 |
| Chronic kidney disease | 153 (5.7) | 134 (5.5) | 19 (7.1) | 0.29 |
| Diabetes Mellitus | 383 (14.2) | 268 (11.0) | 115 (42.4) | <0.0001 |
| Metabolic syndromeb | 558 (20.6) | 396 (16.2) | 162 (59.8) | <0.0001 |
| Systolic blood pressure (mmHg) | 118.5 ± 15.4 | 117.9 ± 15.4 | 123.5 ± 15.0 | <0.0001 |
| Diastolic blood pressure (mmHg) | 73.9 ± 10.9 | 73.4 ± 10.8 | 78.7 ± 10.1 | <0.0001 |
| BMI (kg/m2) | 30.4 ± 7.2 | 29.7 ± 6.9 | 36.2 ± 7.5 | <0.0001 |
| BMI ≥ 30 | 1213 (44.8) | 996 (40.9) | 217 (80.1) | <0.0001 |
| Weight (lbs) | 193.5 ± 48.5 | 188.7 ± 45.7 | 236.8 ± 51.5 | <0.0001 |
| Height (cm) | 170.1 ± 9.5 | 169.8 ± 9.4 | 172.4 ± 9.2 | <0.0001 |
| Waist circumference (cm) | 94.5 ± 16.0 | 92.5 ± 14.9 | 111.8 ± 15.4 | <0.0001 |
| Waist-to-hip ratio | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | <0.0001 |
| Body surface area (m2) | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.3 ± 0.3 | <0.0001 |
| CT fat measures | ||||
| Total Abdominal Fat Volume (cm3) | 489.3 ± 218.5 | 468.4 ± 210.8 | 679.2 ± 195.0 | <0.0001 |
| SAT (cm3) | 341.1 ± 171.1 | 331.2 ± 169.9 | 431.0 ± 155.8 | <0.0001 |
| VAT (cm3) | 130.1 ± 73.1 | 120.0 ± 64.3 | 221.6 ± 83.9 | <0.0001 |
| Liver Attenuation (HU) | 55.6 ± 11.8 | 58.5 ± 7.4 | 28.9 ± 9.8 | <0.0001 |
| Metabolic variables | ||||
| Fasting insulin (μU/mL), mean ± SD | 11.3 ± 9.8 | 10.3 ± 9.0 | 20.8 ± 11.6 | <0.0001 |
| Fasting glucose (mg/dL) | 99.1 ± 27.7 | 96.6 ± 23.6 | 121.5 ± 46.0 | <0.0001 |
| Hemoglobin A1c (%) | 5.7 ± 1.0 | 5.6 ± .9 | 6.4 ± 1.5 | <0.0001 |
| HOMA-IR score, mean | 2.9 ± 3.0 | 2.6 ± 2.5 | 6.3 ± 4.3 | <0.0001 |
| Total Cholesterol (mg/dL) | 193.0 ± 36.5 | 193.1 ± 36.4 | 191.6 ± 37.7 | 0.50 |
| LDL cholesterol (mg/dL) | 113.0 ± 32.6 | 113.2 ± 32.6 | 110.5 ± 31.9 | 0.21 |
| HDL cholesterol (mg/dL) | 57.9 ± 17.7 | 59.0 ± 17.6 | 47.9 ± 15.3 | <0.0001 |
| VLDL cholesterol (mg/dL) | 21.6 ± 11.7 | 20.6 ± 11.0 | 31.0 ± 13.8 | <0.0001 |
| Triglycerides (mg/dL) | 112.7 ± 85.9 | 104.6 ± 65.7 | 185.5 ± 170.6 | <0.0001 |
| Creatinine (mg/dL) | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.2 | 0.43 |
| Glomerular Filtration rate (mL/minute/1.73m2) | 96.2 ± 20.7 | 96.0 ± 20.6 | 97.9 ± 20.8 | 0.15 |
| C-reactive protein (mg/L) | 3.4 ± 6.6 | 3.2 ± 6.6 | 5.1 ± 5.6 | <0.0001 |
| log hsCRP | 0.4 ± 1.2 | 0.4 ± 1.2 | 1.2 ± 1.0 | <0.0001 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; CARDIA, Coronary Artery Risk Development in Young Adults; BMI, body mass index; CT, computed tomography; VAT, visceral adipose tissue; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; hsCRP, high sensitivity c-reactive protein NAFLD = liver attenuation ≤ 40 HU
Results are expressed as mean ± standard deviation or number (%); t-test for continuous variables, chi-square or Fischer exact for categorical variables for the difference between NAFLD and no NAFLD
Defined using ATPIII criteria
Association of NAFLD with Cardiac Structural and Functional Abnormalities: Unadjusted Analyses
Compared to those without NAFLD, participants with fatty liver disease exhibited cardiac remodeling, manifested by higher LV mass index, LV relative wall thickness, LV end-diastolic volume and LA volume index (Table 2). There was no significant difference in LV end-systolic volume. Among the systolic function parameters, circumferential strain and GLS, but not ejection fraction (EF), were significantly worse in NAFLD participants. Several diastolic function parameters were worse in NAFLD, including lower E/A ratio and e’ velocity, and a higher E/e’ ratio (Table 2). Cardiac output was also higher in the NAFLD participants though this effect was attenuated after accounting for body surface area.
Table 2.
Echocardiographic Characteristics of CARDIA Participants With and Without Nonalcoholic Fatty Liver Disease (NAFLD), The CARDIA Study, Year 25 Examination (2010-2011)
| No NAFLD (N=2442) | NAFLD (N=271) | P valuea | |
|---|---|---|---|
| Cardiac dimensions | |||
| LV mass (g) | 164.1 ± 49.1 | 196.5 ± 57.0 | <0.0001 |
| LV mass index (g/m2.7)b | 39.3 ± 11.0 | 45.1 ± 12.2 | <0.0001 |
| LV end-diastolic volume (mL) | 110.3 ± 29.0 | 115.8 ± 31.8 | 0.01 |
| LV end-systolic volume (mL) | 42.9 ± 16.6 | 44.8 ± 18.7 | 0.14 |
| LV relative wall thickness | 0.35 ± 0.07 | 0.37 ± 0.07 | <0.0001 |
| Left atrial volume (mL) | 49.0 ± 15.8 | 53.0 ± 15.9 | 0.0001 |
| Left atrial volume index (ml/m)c | 28.8 ± 9.0 | 30.7 ± 8.8 | 0.002 |
| LV systolic function | |||
| Absolute global longitudinal strain (%) | 15.2 ± 2.4 | 14.2 ± 2.4 | <0.0001 |
| Absolute global circumferential strain (%) | 15.4 ± 2.8 | 15.0 ± 3.0 | 0.05 |
| Left ventricular ejection fraction (%) | 61.6 ± 7.0 | 62.0 ± 7.7 | 0.42 |
| Abnormal ejection fraction < 55% | 344 (14.1) | 37 (15.8) | 0.76 |
| LV diastolic function | |||
| E/A ratio | 1.3 ± 0.4 | 1.2 ± 0.3 | <0.0001 |
| Isovolumic relaxation time (ms) | 73.4 ± 9.3 | 74.0 ± 8.8 | 0.32 |
| E deceleration time (ms) | 176.7 ± 39.2 | 180.9 ± 39.9 | 0.11 |
| Lateral tissue Doppler e’ velocity (cm/s) | 11.9 ± 2.8 | 10.8 ± 2.6 | <0.0001 |
| E/e’ ratio | 7.0 ± 2.3 | 7.7 ± 2.6 | <0.0001 |
| Hemodynamic variables | |||
| Cardiac output (L/min) | 5.6 ± 1.5 | 6.5 ± 1.6 | <0.0001 |
| Cardiac index (L/min/m2) | 2.8 ± 0.71 | 2.9 ± 0.7 | 0.06 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; LV, left ventricular
NAFLD = liver attenuation ≤ 40 HU
Results are expressed as mean ± standard deviation for continuous variable and n(%) for categorical variables; t-test for continuous variables, Chi-square for categorical variables
Indexed to height^2.7
Indexed to height
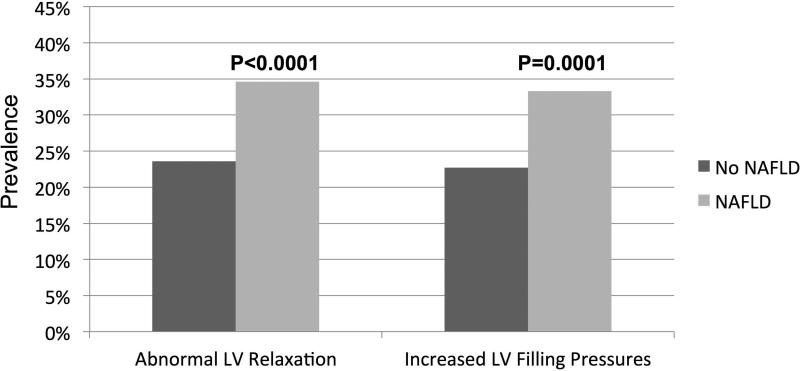
Utilizing quartiles of GLS, we assessed differences in the severity of subclinical systolic dysfunction between NAFLD and non-NAFLD participants (see Figure 2). Despite a normal EF in both groups (Table 2), NAFLD participants were more likely to have significant subclinical systolic impairment on speckle tracked imaging than non-NAFLD (P<0.001 for trend). Similarly, compared to non-NAFLD, NAFLD participants were more likely to have subclinical diastolic dysfunction demonstrated by abnormal LV relaxation (34.6% vs. 23.6%, P<0.0001) and increased LV filling pressures (33.3% vs. 23.7%, P<0.001; see Figure 3).
Figure 2.
Quartiles of global longitudinal strain in NAFLD participants compared to non-NAFLD—NAFLD was associated with worse global longitudinal strain compared to non-NAFLD (P<0.0001 for trend).
Figure 3.
Markers of subclinical diastolic dysfunction in NAFLD participants compared to non-NAFLD—Abnormal left ventricular (LV) relaxation = lateral tissue Doppler e’ velocity < 10 cm/s. Increased LV filling pressures = lateral E/e’ ratio ≥ 12 or left atrial volume index ≥ 34 ml/m2. NAFLD was associated with both abnormal LV relaxation (P<0.0001) and increased LV filling pressures (P=0.0001) compared to non-NAFLD.
Multivariable Analyses
In multivariable linear regression analyses adjusted for demographics and health behaviors, the presence of NAFLD remained associated with worse GLS (Table 3, P<0.0001). This association remained significant when adjusted for HF risk factors or measures of adiposity, including either BMI or VAT. However, the association between NAFLD and GLS was attenuated in the fully adjusted models with both HF risk factors and measures of adiposity. Likewise, NAFLD remained independently associated with significant impairment (quartile 1) in GLS when adjusted for demographics and health behaviors (Odds Ratio: 3.40; 95% confidence interval: 2.10,5.48); the association persisted even after adjusting for traditional HF risk factors or measures of adiposity, including VAT. However, this associated was attenuated when adiposity measures were added to the HF risk factor model (Table 4). NAFLD was also associated with several markers of diastolic dysfunction, including e’ velocity, E/A and E/e’ ratio (a marker of LV filling pressure) when adjusted for demographics and health behaviors (Table 3 and Table 4). These associations persisted in subsequent models after adjustment for BMI (Table 3 and Table 4). However, only e’ velocity and E/e’ ratio remained significant after adjustment for VAT. In fully adjusted models for markers of adiposity, there was a trend towards a significant association only between NAFLD and e’ velocity, which is a marker of impaired cardiac relaxation (p=0.05, Table 3). NAFLD was also independently associated with increased LV mass index, LV end-diastolic volume and LA volume index when adjusted for demographics and health behaviors, however these associations were attenuated after adjustment for HF risk factors. NAFLD was independently associated with cardiac output when adjusted for HF risk factors or BMI, though VAT attenuated this effect (Table 3). No significant interactions between NAFLD and sex, race, diabetes or hypertension status in association with markers of subclinical myocardial dysfunction were observed. There was, however, an interaction between NAFLD and age wherein younger (age range 42-50 years old) NAFLD participants were more likely to have impaired GLS (β=-0.76, SE=0.26, P=0.003) and lower e’ velocity (β=-0.57, SE=0.22, P=0.01) than older (age 51-59 years) NAFLD participants. While the interaction term for NAFLD and age was statistically significant, stratified analysis of the association between NAFLD and measures of subclinical myocardial dysfunction in younger and older participants indicated that younger NAFLD participants had more severely impaired GLS and lower e’ velocity than older participants, but the direction of the association was the same regardless of age. Not surprisingly there was an inverse correlation between BMI and liver attenuation (r = -0.39, P<0.0001), and VAT and liver attenuation (r = -0.54, P<0.0001). However, the variance inflation factors were < 2 for all model covariates, suggesting that multicollinearity did not interfere with model fit. In addition, there was no significant interaction between NAFLD and levels of VAT volume or BMI in terms of e’ velocity, E/e’ ratio or GLS. In sensitivity analyses, all findings remained consistent even when the cut-point for the definition of NAFLD was increased to ≤ 51 HU (consistent with a liver/spleen ratio < 1.0, data not shown) and when continuous liver attenuation was used (Supplemental Table 1).
Table 3.
Linear Regression Analysis for the Association of NAFLD with Continuous Markers of Subclinical Myocardial Dysfunction, The CARDIA Study, 2010-2011
| Model 1: Base modela | Model 2: Base + HF risk factorsb | Model 3: Base + BMI | Model 4: Base + VAT | Model 5: Base + HF risk factors + BMI | Model 6: Base + HF risk factors + VAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | |
| Cardiac Dimensions | ||||||||||||
| LV mass indexc | 5.8 (0.8) | <0.0001 | 2.8 (0.8) | 0.0004 | 0.8 (0.7) | 0.24 | 1.4 (0.8) | 0.08 | 0.09 (0.72) | 0.90 | 0.42 (0.80) | 0.60 |
| LV end-diastolic volume | −1.9 (1.8) | 0.03 | −2.2 (1.9) | 0.24 | −7.1 (1.8) | <0.0001 | −4.0 (2.0) | 0.04 | −8.1 (1.83) | <0.0001 | −5.5 (2.0) | 0.006 |
| Left atrial volume indexd | 1.9 (0.6) | 0.001 | 0.6 (0.6) | 0.34 | −1.6 (0.6) | 0.004 | −0.7 (0.6) | 0.28 | −1.6 (0.60) | 0.005 | −0.9 (0.63) | 0.15 |
| LV systolic myocardial function | ||||||||||||
| Absolute GLS | −0.93 (0.16) | <0.0001 | −0.58 (0.17) | 0.0005 | −0.59 (0.17) | 0.0005 | −0.42 (0.18) | 0.02 | −0.34 (0.17) | 0.04 | −0.27 (0.18) | 0.05 |
| LV diastolic myocardial function | ||||||||||||
| E/A ratio | −0.13 (0.02) | <0.0001 | −0.06 (.02) | 0.008 | −0.07 (0.02) | 0.003 | −0.03 (0.03) | 0.26 | −0.04 (0.02) | 0.14 | −0.008 (0.03) | 0.75 |
| E/e’ ratio | 0.80 (0.15) | <0.0001 | 0.34 (.15) | 0.03 | 0.35 (0.15) | 0.004 | 0.35 (0.16) | 0.03 | 0.20 (0.15) | 0.20 | 0.16 (0.16) | 0.31 |
| Lateral tissue Doppler e’ velocity | −0.77 (0.14) | <0.0001 | −0.34 (.14) | 0.02 | −0.32 (0.15) | 0.0004 | −0.36 (0.15) | 0.02 | −0.28 (0.15) | 0.05 | −0.22 (0.15) | 0.05 |
| Hemodynamic variables | ||||||||||||
| Cardiac output | .78 (.11) | <0.0001 | 0.41 (0.11) | 0.0002 | 0.27 (0.11) | 0.01 | 0.22 (0.11) | 0.06 | 0.12 (0.11) | 0.27 | 0.08 (0.11) | 0.47 |
Abbreviations: GLS, global longitudinal strain; HF, heart failure; BMI, body mass index; VAT, visceral adipose tissue; LV, left ventricle
NAFLD is defined as CT liver attenuation ≤ 40 HU
Base model: center, age, race, sex, education, income level, alcohol intake (drinks/week), smoking status (current/former vs. never), physical activity score
HF risk factors: systolic blood pressure, antihypertensive medication use, anti-hyperlipidemic medication use, total cholesterol, HDL cholesterol, diabetes status, GFR
Indexed to height2.7
Indexed to height
Table 4.
Odds Ratios for the Association of NAFLDa With Markers of Systolic and Diastolic Subclinical Myocardial Dysfunction, The CARDIA Study, 2010-2011
| Unadjusteda | Model 1: Base modelb | Model 2: Base + HF risk factorsc | Model 3: Base + BMI | Model 4: Base + VAT | Model 5: Base + HF risk factors + BMI | Model 6: Base + HF risk factors + VAT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Quartiles of Absolute GLS, % (range) | ||||||||||||||
| Q1 (16.7 - 23.8) | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||||
| Q2 (15.1 - 16.7) | 2.03 | 1.26-3.29 | 1.92 | 1.17-3.15 | 1.48 | 0.88-2.48 | 1.73 | 1.04-2.88 | 1.48 | 0.87-2.52 | 1.47 | 0.86-2.50 | 1.39 | 0.80-2.40 |
| Q3 (13.6 - 15.1) | 2.25 | 1.40-3.61 | 2.04 | 1.25-3.35 | 1.43 | 0.85-2.40 | 1.60 | 0.96-2.66 | 1.28 | 0.75-2.17 | 1.32 | 0.78-2.23 | 1.19 | 0.69-2.05 |
| Q4 (6.6 - 13.6) | 3.59 | 2.29-5.65 | 3.40 | 2.10-5.48 | 1.95 | 1.18-3.24 | 2.28 | 1.39-3.73 | 1.82 | 1.08-3.05 | 1.66 | 1.00-2.77 | 1.52 | 0.89-2.59 |
| Abnormal LV relaxation | 1.77 | 1.34-2.33 | 1.78 | 1.34-2.38 | 1.18 | 0.86-1.62 | 1.42 | 1.05-1.93 | 1.15 | 0.83-1.58 | 1.11 | 0.80-1.53 | 0.98 | 0.70-1.38 |
| Increased LV filling pressures | 1.76 | 1.33-2.32 | 1.98 | 1.47-2.66 | 1.36 | 0.99-1.88 | 1.36 | 1.00-1.86 | 1.20 | 0.87-1.68 | 1.13 | 0.81-1.57 | 1.07 | 0.76-1.51 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; CARDIA, Coronary Artery Risk Development in Young Adults; GLS, global longitudinal strain; LV, left ventricle; HF, heart failure; BMI, body mass index; VAT, visceral adipose tissue; OR, odds ratio; 95%CI, 95% confidence interval
NAFLD is defined as CT liver attenuation ≤ 40 HU
adjusted for center only
base model: adjusted for model 1 + age, race, sex, study center, education, income level, alcohol intake (drinks/week), smoking status (current/former vs. never), physical activity score
HF risk factors: systolic blood pressure, antihypertensive medication use, anti-hyperlipidemic medication use, total cholesterol, HDL cholesterol, diabetes status, GFR Abnormal diastolic relaxation was defined as lateral tissue doppler e’ velocity < 10. Abnormal diastolic filling pressures were defined as 8 < E/e’ ratio < 12 and left atrial volume index ≥ 34 or E/e’ ratio ≥ 12
DISCUSSION
In a large, population-based, cross-sectional study of both black and white middle-aged adults with NAFLD, we have shown that NAFLD is associated with both subclinical cardiac remodeling and systolic and diastolic function independent of traditional HF risk factors or markers of adiposity. In addition, to the best of our knowledge, this is the first study to also consider the effect of VAT, a potential confounder of the association between NAFLD and myocardial remodeling/dysfunction, on these observations.
Left Ventricular Dysfunction in NAFLD
Consistent with prior research(14, 15, 24, 25), participants with NAFLD were characterized by higher LV mass, LV end-diastolic volume, and LV relative wall thickness. In addition, on a population level we confirmed that NAFLD is associated with lower early diastolic relaxation (e’) tissue velocity, lower E/A ratio and higher estimated LV filling pressures (E/e’ ratio), thus implying the presence of underlying subclinical diastolic dysfunction(14-16, 26-28). Moreover, using speckle-tracking echocardiography, we also found that participants with NAFLD had reduced longitudinal LV systolic function, despite having a normal EF. A major finding of our study is that NAFLD is associated with the aforementioned echocardiographic features of subclinical LV remodeling and early LV diastolic and systolic dysfunction independent of several metabolic variables, including traditional HF risk factors and obesity (as measured by BMI). It is well established that obesity (e.g. BMI) is closely related to HF risk factors. Therefore, when BMI is added to the model with HF risk factors, we hypothesize that the attenuated findings are likely due to over-fitting and over-adjustment for the effects of BMI, which may in fact lie in the causal pathway between NAFLD and subclinical myocardial dysfunction. In addition, the beta coefficients in the models with BMI alone or with heart failure risk factors alone are very similar suggesting that obesity may account for a significant proportion of the observed association between NAFLD and subclinical myocardial dysfunction. Although there was some attenuation of associations by VAT, many of the associations between NAFLD and echocardiographic findings were still significant, suggesting that the correlation between NAFLD and VAT also does not fully explain our findings.
The current study adds to the available literature in that it used modern speckle tracking techniques to assess early markers of systolic dysfunction in NAFLD participants. To date, only two small prior studies(25, 27) with inconsistent findings have examined subclinical systolic dysfunction in NAFLD using speckle-tracking. Our study is unique in that we used a population-based sample of otherwise healthy adults to analyze longitudinal strain among those with NAFLD. Myocardial strain is an important predictor of both morbidity(29) and mortality(30); therefore, identification of impaired early LV myocardial function via these techniques may help to identify NAFLD patients at increased cardiovascular risk.
Another novel finding in the current study is that NAFLD is independently associated with LA volume, even when adjusted for traditional HF risk factors and BMI. LA volume is an indicator of both the severity and duration of LV diastolic dysfunction(31) and LA size has proven to be a powerful predictor of outcome in several disease entities, including myocardial infarction(32), severe aortic valve stenosis(33), HF(34), and type 2 diabetes(35). LA volume may also be a better predictor of future symptomatic HF than the presence of diastolic dysfunction(35), and thus, the presence of increased LA volume in NAFLD may serve as a marker of future HF events.
Potential Pathophysiologic Mechanisms
Several potential pathophysiologic mechanisms may explain the association between NAFLD and subclinical myocardial dysfunction (Figure 4). We have demonstrated that NAFLD is related to increased body surface area and an increase in cardiac output and LV filling pressures, which we hypothesize that over time may lead to the development of clinical HF. In addition, hepatic steatosis is associated with insulin resistance(12), myocardial lipid toxicity(36, 37), and the systemic release of several inflammatory mediators(38) that can impair cardiac function(39, 40). Prior studies have demonstrated that increased epicardial and intramyocardial fat content is associated with impaired cardiac metabolism(13, 41). Although we did not analyze for cardiac ectopic fat in the current study, the attenuation of many of the observed effects by other metabolically active ectopic fat deposits (e.g. VAT) suggests that abnormal ectopic fat storage may be a marker of the cumulative effects of NAFLD and insulin resistance in the setting of pathologic adiposity(42, 43). We have shown that markers of obesity (especially VAT) and traditional HF risk factors attenuate, but do not completely account for the association between NAFLD and subclinical myocardial dysfunction (Figure 4). Ultimately, these mechanisms paired with an increase in cardiac output may lead to volume overload of a stiff LV and may result in the development of future clinical HF as LV filling pressures rise. Therefore, identification of hepatic steatosis in middle age during which time heart failure risk factors (stage B) often transition to clinical (stage C) HF, may provide additional information about the pathogenesis of clinical HF development. Interestingly, several large population-based cohort studies have demonstrated that moderately elevated levels of serum γ-glutamyltransferase (GGT), which are possible markers of underlying NAFLD and atherosclerosis, are independently associated with an increased risk of incident heart failure(44-46). Thus, NAFLD may be an important future target in order to prevent and treat the increasing HF epidemic. Further prospective evaluations of these cross-sectional observed associations are needed.
Figure 4.
Proposed pathophysiologic mechanisms for the relationship between NAFLD and subclinical myocardial dysfunction—NAFLD is related to increased body surface area with a resultant increase in cardiac output (CO) and left ventricular (LV) filling pressures, which leads to the development of clinical heart failure (HF). NAFLD is also associated with insulin resistance, myocardial lipid toxicity and systemic inflammation that can impair cardiac function. Both obesity and traditional HF risk factors attenuate, but do not completely account for the association between NAFLD and subclinical myocardial dysfunction, which when coupled with an increase in CO in the setting of a stiff LV leads to clinical HF. Note: Dotted lines indicate that the direction of association is not clearly established. Abbreviations: CAD, coronary artery disease; HTN, hypertension; LV, left ventricular; NAFLD, nonalcoholic fatty liver disease; VAT, visceral adipose tissue.
Strengths and Limitations
The strengths of the present study include our large, well-characterized population-based cohort of both whites and blacks, a NAFLD prevalence that is consistent with published population estimates(19), the use of tissue Doppler imaging and speckle-tracking analysis to assess subclinical myocardial dysfunction, and the measurement of a comprehensive set of metabolic covariates, particularly VAT. Thus, our findings are more generalizable to the U.S. population compared to previously published findings in small, selected samples of NAFLD patients. Importantly, we excluded participants with known chronic liver disease and those with other potential reasons for increased hepatic fat, and thus were able to isolate those with the highest likelihood of having NAFLD in a subclinical state.
Some limitations warrant mention. Our findings are cross-sectional; therefore, neither temporal nor causal relationships can be inferred. CT is a relatively insensitive measure of hepatic fat compared to hepatic triglyceride content measured by proton magnetic resonance spectroscope (MR spectroscopy)(20, 47), which may bias our results toward the null and underestimate the strength of the association between NAFLD and subclinical myocardial remodeling/dysfunction. Liver biopsy, the gold standard for diagnosis of NAFLD(19), is not feasible in epidemiologic studies given the risks associated with the procedure. In addition, because contemporaneous laboratory data on hepatic function was not available to us in the present study, we excluded participants at high risk of chronic liver disease. Finally, there is no laboratory test for NAFLD; thus documenting steatosis on imaging in the presence of risk factors after exclusion of other liver diseases makes the diagnosis. Therefore, the NAFLD definition used in this study is similar to what is used in clinical practice. Furthermore, serum aminotransferases are often normal despite the presence of liver injury in NAFLD(48). Thus, we doubt that hepatic function variables would have improved the classification of the NAFLD phenotype. We also acknowledge that several of the observed differences in echocardiographic parameters (e.g. E/A ratio 1.2 vs. 1.3), though statistically significant, may not represent a clinically significant difference. In addition, given the high correlation between markers of obesity and NAFLD we chose to show the differential effects of traditional heart failure risk factors and markers of obesity on associations in separate models. Thus, our findings illuminate potential pathophysiologic mechanisms of the association between NAFLD and clinical heart failure, and do not demonstrate a direct link to clinical HF. Future prospective study to determine the impact of NAFLD on the future development clinical HF is needed.
Conclusion
In conclusion, NAFLD is independently associated with subclinical myocardial remodeling and dysfunction independent of established HF risk factors including obesity, dyslipidemia, hypertension and diabetes. Other ectopic fat deposits, such as visceral adipose tissue, likely moderate some of this observed association. NAFLD may play an important role in the development of HF, especially HF with preserved ejection fraction, and the association between NAFLD and subclinical myocardial dysfunction provides pathophysiologic insight into the potential link between NAFLD and HF.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the participants of the CARDIA study for their long-term commitment and important contributions to the study.
Financial Support:
1. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging. This manuscript has been reviewed by CARDIA for scientific content.
2. L.B.V. is supported by the National Institutes of Health (1 F32 HL116151-01), the American Liver Foundation (New York, NY) and the American Association for the Study of Liver Diseases Foundation (Alexandria, VA).
3. S.J.S. is supported by National Institutes of Health R01 HL107577.
4. J.E.W. is supported by the American Heart Association (13 POST 16820054)
Abbreviations (in order of appearance)
- NAFLD
non-alcoholic fatty liver disease
- HF
heart failure
- CT
computed tomography
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- LV
left ventricle
- GLS
global longitudinal strain
- e’
early diastolic tissue velocity
- SE
standard error
- CAC
coronary artery calcification
- VAT
visceral adipose tissue
- BMI
body mass index
- HIV
human immunodeficiency virus
- HU
Hounsfield units
- LA
Left atrium
- A
late (atrial) transmitral velocity
- E
early transmitral velocity
- HOMA-IR
homeostatic model assessment of insulin resistance
Footnotes
Author Contributions
Lisa B. VanWagner: Hypothesis, analytic plan, results interpretation, manuscript drafting, submission preparation and editing
Jane E. Wilcox: Hypothesis, analytic plan, results interpretation, manuscript editing Laura A. Colangelo: Performed analyses, manuscript editing (specific attention to statistical methods, results, tables, figures)
Donald M. Lloyd-Jones: Hypothesis, analytic plan, results interpretation, manuscript editing, CARDIA site principal investigator J. Jeffrey Carr: CT methods and design, manuscript editing
Joao A. Lima: Echocardiography methods and study design, manuscript editing
Cora E. Lewis: Analytic plan, results interpretation, manuscript editing and critical review
Mary E. Rinella: Results interpretation, manuscript editing and critical review
Sanjiv J. Shah: Hypothesis, analytic plan, results interpretation, manuscript editing and critical review
Disclosures
The authors have no conflicts of interest pertinent to this study.
REFERENCES
- 1.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 3.Ballestri S, Lonardo A, Bonapace S, et al. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(7):1724–45. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473–80. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 5.Akabame S, Hamaguchi M, Tomiyasu K, et al. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT). Circ J. 2008;72(4):618–25. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 6.Assy N, Djibre A, Farah R, et al. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254(2):393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 7.Volzke H, Robinson DM, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11(12):1848–53. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Musani SK, Bidulescu A, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224(2):521–5. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605–13. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235(2):599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Lautamaki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291(2):E282–90. doi: 10.1152/ajpendo.00604.2005. [DOI] [PubMed] [Google Scholar]
- 13.Perseghin G, Lattuada G, De Cobelli F, et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47(1):51–8. doi: 10.1002/hep.21983. [DOI] [PubMed] [Google Scholar]
- 14.Goland S, Shimoni S, Zornitzki T, et al. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40(10):949–55. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 15.Fotbolcu H, Yakar T, Duman D, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17(5):457–63. [PubMed] [Google Scholar]
- 16.Fallo F, Dalla Pozza A, Sonino N, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19(9):646–53. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Park BJ, Kim YJ, Kim DH, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23(6):900–7. doi: 10.1111/j.1440-1746.2007.05212.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 19.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 20.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307–12. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 21.Gidding SS, Liu K, Colangelo LA, et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation Cardiovascular imaging. 2013;6(5):769–75. doi: 10.1161/CIRCIMAGING.112.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi S, Armstrong A, Gidding SS, et al. Association of Obesity in Early Adulthood and Middle Age with Incipient Left Ventricular Dysfunction and Structural Remodeling: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JACC Heart Failure. 2014 doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Sert A, Aypar E, Pirgon O, et al. Left ventricular function by echocardiography, tissue Doppler imaging, and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am J Cardiol. 2013;112(3):436–43. doi: 10.1016/j.amjcard.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Karabay CY, Kocabay G, Kalayci A, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26(3):325–31. doi: 10.1097/MEG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 26.Pacifico L, Di Martino M, De Merulis A, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59(2):461–70. doi: 10.1002/hep.26610. [DOI] [PubMed] [Google Scholar]
- 27.Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–95. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim NH, Park J, Kim SH, et al. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100(12):938–43. doi: 10.1136/heartjnl-2013-305099. [DOI] [PubMed] [Google Scholar]
- 29.Ersboll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61(23):2365–73. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 30.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circulation Cardiovascular imaging. 2009;2(5):356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 31.Tsang TSM, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. American Journal of Cardiology. 2002;90(12):1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 32.Beinart R, Boyko V, Schwammenthal E, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44(2):327–34. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 33.Dahl JS, Videbæk L, Poulsen MK, et al. Noninvasive assessment of filling pressure and left atrial pressure overload in severe aortic valve stenosis: Relation to ventricular remodeling and clinical outcome after aortic valve HEP-14-2519 replacement. Journal of Thoracic and Cardiovascular Surgery. 2011;142(3):e77–e83. doi: 10.1016/j.jtcvs.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Meris A, Amigoni M, Uno H, et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: The VALIANT Echo Study. European heart journal. 2009;30(1):56–65. doi: 10.1093/eurheartj/ehn499. [DOI] [PubMed] [Google Scholar]
- 35.Poulsen MK, Dahl JS, Henriksen JE, et al. Left atrial volume index: relation to long-term clinical outcome in type 2 diabetes. J Am Coll Cardiol. 2013;62(25):2416–21. doi: 10.1016/j.jacc.2013.08.1622. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33(10):1190–200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 37.Bugianesi E. Nonalcoholic fatty liver disease (NAFLD) and cardiac lipotoxicity: Another piece of the puzzle. Hepatology. 2008;47(1):2–4. doi: 10.1002/hep.22105. [DOI] [PubMed] [Google Scholar]
- 38.Targher G. Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol. 2006;45(6):879–81. doi: 10.1016/j.jhep.2006.09.005. author reply 81-2. [DOI] [PubMed] [Google Scholar]
- 39.Chinali M, Devereux RB, Howard BV, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). Am J Cardiol. 2004;93(1):40–4. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Djousse L, Benkeser D, Arnold A, et al. Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ Heart Fail. 2013;6(5):964–9. doi: 10.1161/CIRCHEARTFAILURE.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rijzewijk LJ, Jonker JT, van der Meer RW, et al. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol. 2010;56(3):225–33. doi: 10.1016/j.jacc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 42.Kankaanpaa M, Lehto HR, Parkka JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91(11):4689–95. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 43.Iacobellis G, Lonn E, Lamy A, et al. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146(3):452–4. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 44.Dhingra R, Gona P, Wang TJ, et al. Serum gamma-glutamyl transferase and risk of heart failure in the community. Arterioscler Thromb Vasc Biol. 2010;30(9):1855–60. doi: 10.1161/ATVBAHA.110.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Tuomilehto J, Jousilahti P, et al. Serum gamma-glutamyltransferase and the risk of heart failure in men and women in Finland. Heart. 2013;99(3):163–7. doi: 10.1136/heartjnl-2012-302972. [DOI] [PubMed] [Google Scholar]
- 46.Wannamethee SG, Whincup PH, Shaper AG, et al. Gamma-glutamyltransferase, hepatic enzymes, and risk of incident heart failure in older men. Arterioscler Thromb Vasc Biol. 2012;32(3):830–5. doi: 10.1161/ATVBAHA.111.240457. [DOI] [PubMed] [Google Scholar]
- 47.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 48.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–92. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.