Abstract
Accumulating evidence suggests that working memory load is an important factor for the interplay between cognitive and facial-affective processing. However, it is unclear how distraction caused by perception of faces interacts with load-related performance. We developed a modified version of the delayed match-to-sample task wherein task-irrelevant facial distracters were presented early in the rehearsal of pseudoword memoranda that varied incrementally in load size (1-syllable, 2-syllables, or 3-syllables). Facial distracters displayed happy, sad, or neutral expressions in Experiment 1 (N=60) and happy, fearful, or neutral expressions in Experiment 2 (N=29). Facial distracters significantly disrupted task performance in the intermediate load condition (2-syllable) but not in the low or high load conditions (1- and 3-syllables, respectively), an interaction replicated and generalised in Experiment 2. All facial distracters disrupted working memory in the intermediate load condition irrespective of valence, suggesting a primary and general effect of distraction caused by faces. However, sad and fearful faces tended to be less disruptive than happy faces, suggesting a secondary and specific valence effect. Working memory appears to be most vulnerable to social-emotional information at intermediate loads. At low loads, spare capacity is capable of accommodating the combinatorial load (1-syllable plus facial distracter), whereas high loads maximised capacity and deprived facial stimuli from occupying working memory slots to cause disruption.
Keywords: Working memory, Facial distraction, Affective interference, Delayed match-to-sample, Social-emotional processing
A common approach for probing cognitive-emotional interactions is to examine cognitive vulnerability to attentional interference from task-irrelevant emotional stimuli. Accumulating evidence suggests that working memory load—the limited information currently in mind for further use—mediates this interaction (e.g., Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Van Dillen & Koole, 2007; Van Dillen, Heslenfeld, & Koole, 2009). For example, emotional faces are more likely to disrupt the cognitive process of gender judgement than neutral faces, even though emotional properties of faces are task irrelevant. However, recent data show that such disruptive effects are only observed when gender judgements are embedded within low working memory load trials and are abolished when embedded within high working memory load trials (Van Dillen & Koole, 2009). The prevailing explanation of such cognitive-emotional interactions is that low working memory load spares sufficient attentional resources for emotional stimuli to absorb whereas high working memory load consumes much of the attentional resources within capacity and spares little for distracter stimuli to absorb (Erber & Tesser, 1992; Erthal et al., 2005; Okon-Singer, Tzelgov, & Henik, 2007). The prevailing view predicts a linear relationship between working memory load and emotional modulation: high working memory load is correlated with low emotional interference. The purpose of the present study was to test this prediction utilising a new behavioural paradigm and confirm whether working memory load mediates the interplay between cognition and emotion.
Cognitive-emotional interactions have been described as a dynamic competition between task-driven (“top-down”) and stimulus-driven (“bottom-up”) control over attentional resources (e.g., Blair et al., 2007; MacNamara, Ferri, & Hajcak, 2011; Mitchell, Nakic, Fridberg, Kamel, Pine, & Blair, 2007; Okon-Singer et al., 2007; Van Dillen & Koole, 2009). Task-driven control of attentional resources has traditionally been seen as “cold”, indifferent to emotion, and achieved by effortfully keeping task-relevant information prioritised over task-irrelevant information (Knudsen, 2007; Lavie & De Fockert, 2005). Such control is critical for maintaining mental set and coping with interference (see Bledowski, Kaiser, & Rahm, 2010, for a review). In contrast, stimulus-driven control over attentional resources can be seen as “hot”, indifferent to task demands, and effortless insofar as stimulus processing is “automatically” carried out to completion without conscious monitoring (Tzelgov, 1997). Such control is vital for rapid and preconscious appraisal of stimuli that have social, biological, or survival importance (e.g., emotional expressions on faces; Bargh, 1989; Dijksterhuis & Aarts, 2003; Eastwood, Smilek, & Merikle, 2001; Okon-Singer et al., 2007; Yantis, 2000). Though emotional stimuli may be task-irrelevant, they have the intrinsic potential to disrupt task-driven cognition by automatically absorbing attentional resources and reducing working memory capacity. However, as noted above, so-called automatic processing of emotional stimuli may be contingent upon availability of sufficient attentional resources. As such, emotional processing modulates ongoing cognition only if the cognitive process at hand is easy and not attention demanding (low load) whereas task-driven processing of high loads seems resistant to emotional interference, thus the dynamic interplay.
The studies mentioned above examined the effect of emotional interference on cognitive load (e.g., mental arithmetic vs. no mental arithmetic; easy spatial judgements vs. difficult spatial judgements), working memory load (e.g., 1-digit rehearsal vs. 8-digit rehearsal), or attentional load (e.g., one-, two-, four-, or six-letter “set size” in a letter detection task). The general finding has been of a linear relationship between load and emotional modulation, with robust emotional interference in low load conditions but no emotional interference in high load conditions (e.g., Erthal et al., 2005; Okon-Singer et al., 2007; Van Dillen & Koole, 2009). However, a potentially significant limitation of these studies is the use of complex task instructions that confound the boundaries of load and permit emotional processing to be task driven rather than stimulus driven. For example, some studies instructed participants to “ignore” emotional stimuli while performing a cognitive task (e.g., Erthal et al., 2005; Okon-Singer et al., 2007) while other studies instructed participants to switch between emotional and cognitive trials (e.g., Van Dillen & Koole, 2009). With the instruction to ignore emotional stimuli, it is conceivable that participants experienced the paradoxical phenomenon of thought suppression that actually increases the incidence of unwanted thoughts (e.g., Wegner, Schneider, Carter, & White, 1987), which in this case add emotional content to the cognitive load rather than the intended “ignoring”. Similarly, switching between cognitive and emotional tasks adds the complexity of switching between instructional sets. In both instances, task instructions add to the overall processing load, reduce working memory capacity, and permit emotional processing to be task driven. One unintended consequence is that prior conceptualisations of “low load” may have been larger than intended given the additional load from task instructions. Because working memory is a limited-capacity storage system (Baddeley, 2003; Cowan, 2000) with cognitive and affective stimuli dynamically competing over attentional resources, it is important for behavioural paradigms not to confound load boundaries with complex instructions that absorb working memory resources and permit emotional processing to be task driven rather than stimulus driven.
We developed a modified version of the delayed match-to-sample task to address the issues mentioned above. Broadly, task-irrelevant emotional distracters were presented early in the rehearsal of pseudoword memoranda that varied incrementally in load size (i.e., 1-syllable pseudo-word, 2-syllable pseudoword, and 3-syllable pseudoword). Notably, pseudowords (pronounceable word-like letter strings) were selected as memoranda because load size (i.e., syllable number) has a far greater effect on pseudowords than words (Valdois et al., 2006). This is principally because pseudoword reading places more demand on the phonological-loop of working memory by inducing a serial mechanism for assembling novel articulatory programmes (e.g., Hagoort et al., 1999; Herbster, Mintun, Nebes, & Becker, 1997; Mechelli, Gorno-Tempini, & Price 2003; Price, Wise, & Frackowiak, 1996). Social-emotional distracters were memoranda-nonconfusable pictures of human faces displaying happy, sad, or neutral expressions in Experiment 1 and happy, fearful, and neutral expressions in Experiment 2. Facial stimuli were chosen as distracters because they are biologically anchored social signals of environmental conditions that are conducive to stimulus-driven processing (Langton, Law, Burton, & Schweinberger, 2008; Lewkowicz & Hansen-Tift, 2012).
Because our behavioural paradigm was designed to assess the interaction between task-driven working memory and stimulus-driven facial processing, it was necessary to present facial distracters under conditions that emphasised stimulus-driven processing while de-emphasising task-driven processing. For example, facial distracters were briefly presented (33 ms) and followed by a non-facial backward mask to disrupt sensory icon memory (Massaro, 1975). Reducing opportunity for apperception of faces meant that the completion of stimulus processing was likely to be independent of conscious monitoring. An important issue with respect to brief presentations of facial stimuli, in any experiment, is the possibility for certain facial affective expressions have lower (or higher) perceptual thresholds for apperception than other facial affective expressions. To address this issue in the present task, a post-task facial affective recognition test was administered to all participants to determine whether any observable effects (or interactions) were moderated by the differential apperception of facial affective expressions. To de-emphasise task-driven processing of facial stimuli, we did not inform participants of distracters prior to task administration. A common procedure is to inform participants that distracters will be present during the task and that they should “ignore” the distracter stimulus and instead attend to task-relevant memoranda. However, a problem with such a procedure is that processing of distracter stimuli unavoidably becomes task driven given that participants are instructed to process distracter stimuli (i.e., refuse to take notice, disregard intentionally). We reasoned that such task-driven processing would, in theory, confound our aim of emphasising automatic, stimulus-driven processing of social-emotional distracters. Given the biological unimportance of nonsense syllables and the evolutionary prepotency of facial stimuli for social processing, the present behavioural paradigm examined the role of working memory load in mediating the dynamic interplay between task-driven (top-down) cognition and stimulus-driven (bottom-up) social-emotional processing.
We hypothesised a linear relationship between working memory load and social-emotional modulation. For example, social-emotional distracters were predicted to be most disruptive to working memory performance (i.e., increased response time, decreased accuracy) in the low load condition (1-syllable pseudoword), moderately disruptive to working memory performance in the intermediate load condition (2-syllable pseudo-word), and undisruptive to working memory performance in the high load condition (3-syllable pseudoword). A priori trend analyses utilising orthogonal polynomials tested whether a linear versus nonlinear form best represented the cognition—emotion interaction.
EXPERIMENT 1
Methods
Participants
Participants were 60 healthy (34 females), undergraduate students, aged 18–52 (M=22 ± 0.6), with no history of neurological or psychiatric disease. All were right-handed (handedness quotient > 70) on the Edinburgh handedness inventory (Oldfield, 1971), spoke English as a first language, and reported normal or corrected-to-normal vision. Years of education ranged from 12–16 years (M=15 ± 0.2). Participants provided written informed consent, were given a post-task debriefing statement, and were given course credit in exchange for their voluntary participation. The University of California, San Diego (UCSD) Human Research Protection Program approved the protocol.
Design
The present experiment employed a 3×4 parametric factorial design, with the with-in-subjects factors being Load (three levels: 1-syllable vs. 2-syllables vs. 3-syllables) and Distraction (four levels: geometrical baseline control vs. happy face vs. sad face vs. neutral face). Dependent variables were response latencies and percentage correct. An a priori power analysis for interaction-level analysis of variance (ANOVA) with an alpha level of .05, a 3 × 4 factorial design, and an anticipated small-to-medium effect size (δ=.5) indicated a minimum sample size of 60 to achieve acceptable power (.81).
Stimuli
Stimuli included: (1) pronounceable non-words, i.e., pseudowords; (2) faces; and a (3) non-facial geometrical control. Below is a description of each in turn.
1. Pseudowords
A total of 216 consonant-vowel-consonant (CVC) pseudowords were utilised in Experiment 1 (see appendix). The 1-syllable condition consisted of 36 CVC pseudo-words, the 2-syllables condition consisted of 72 CVC pseudowords, and the 3-syllables condition consisted of 108 CVC pseudowords. The one-and two-syllable conditions were flanked on the right by strings of Xs, e.g., MAB-XXX-XXX, FUT-GIS-XXX, to control for perceptual processes associated with visual-field eccentricity during left to right reading. All CVC pseudo-words were presented only once to prevent proactive interference. Moreover, the current corpus of pseudowords was distilled of pseudohomophones to remove interference from lexical-level phonology and semantics. Pseudowords were generated using MCWord (Medler & Binder, 2005), an online tool for orthographic analysis and letter-string generation (http://www.neuro.mcw.edu/mcword/). The algorithm for letter-string generation used a Markov chain procedure based on position-specific English trigram statistics from the CELEX database. Chaining based on trigram statistics guarantees that pseudowords have word-like orthographic characteristics.
Table 1 displays the statistical properties of CVC pseudowords in all 12 experimental conditions. The CVC pseudowords utilised in all conditions were matched on multiple sublexical parameters, including orthographic neighbourhood size, orthographic form frequency, and position-constrained bigram frequencies. The following orthographic properties were matched across working memory load conditions (1-syllable, 2-syllables, 3-syllables): orthographic neighbourhood size, F(2, 213)=0.441,p=.644, ; orthographic form frequency, F(2, 213) =0.378, p=.686, ; and position-constrained bigram frequencies, F(2, 213) =0.946, p=390, ; thus, any effects from load are not likely to be attributed to differences in stimulus properties across load conditions. The following orthographic properties were matched across distracter conditions (geometrical baseline control, happy face, sad face, neutral face): orthographic neighbourhood size, F(3, 212) =1.576, p=.196, ; orthographic form frequency, F(3, 212) =0.650, p=.584, ; and position-constrained bigram frequencies, F(3, 212)=0.268, p=.849, ; thus, any effects from distracter types are not likely to be attributed to differences in stimulus properties across distracter conditions. The following orthographic properties were matched across each combination of these factors: orthographic neighbourhood size, F(11, 204) =0.860, p=.581, ; orthographic form frequency, F(11, 204) =0.735, p =. 704, ; and position-constrained bigram frequencies, F(11, 204) =0.636, p=.797, ; thus, any interaction effects are not likely to be attributed to differences in stimulus properties across factorial conditions. The goal of controlling for orthographic properties of CVC pseudowords across factors was to ensure that observable effects or interactions were due to experimental manipulations (i.e., load, distraction) and not stimulus properties.
Table 1.
Orthographic properties of CVC stimuli
| Condition | ONS M (SD) |
OF-F M (SD) |
PCB-F M (SD) |
|---|---|---|---|
| 1-Syllable | |||
| Baseline | 12 (4) | 339 (455) | 1517 (2243) |
| Happy | 14 (6) | 168 (222) | 1091 (1494) |
| Neutral | 10 (2) | 218 (363) | 1207 (2167) |
| Sad | 10 (4) | 300 (375) | 1363 (1431) |
| 2-Syllables | |||
| Baseline | 13 (4) | 303 (363) | 1652 (1699) |
| Happy | 12 (4) | 207 (252) | 1471 (2107) |
| Neutral | 12 (5) | 283 (356) | 1489 (1870) |
| Sad | 12 (4) | 231 (259) | 1207 (1287) |
| 3-Syllables | |||
| Baseline | 11 (4) | 107 (139) | 570 (769) |
| Happy | 13 (4) | 210 (370) | 1196 (1954) |
| Neutral | 12 (4) | 233 (331) | 1236 (1619) |
| Sad | 12 (5) | 314 (484) | 1443 (1504) |
Notes: CVC=consonant-vowel-consonant; ONS=Orthographic neighbourhood size; OF-F=Orthographic form frequency; PCB-F=Position-constrained bigram frequency. Analyses showed that orthographic properties among experimental conditions were not statistically different. Thus, whatever differences exist among manipulated factors cannot be attributed to orthographic stimulus properties.
2. Facial affective distracters
Affective distracters comprised faces of happy, sad and neutral expressions, which were taken from the Radboud Faces Database (Langner et al., 2010; http://facedb.blogspot.com/). There were a total of 27 faces per category of facial distracters, with each category comprised of nine Caucasian adult males, nine Caucasian adult females, and nine Moroccan adult males. All participants in the Radboud Faces database were trained in the Facial Action Coding System (FACS; Ekman, Friesen, &Hager, 2002). The database offers eight affectively valenced facial expressions (i.e., sad, neutral, angry, contemptuous, disgusted, surprised, fearful, and happy), three eye gaze directions (left, frontal, right), and five camera angles from left to right in increments of 45° (180°, 135°, 90°, 45°, 0°), all controlled on multiple technical factors (e.g., attractiveness, intensity, clarity, genuineness, valence). All facial stimuli in the present experiment displayed a frontal eye gaze and were pictured in a 908 camera angle. All face images were resized, cropped from the clavicle down, and converted to greyscale. All picture edits were accomplished using GIMP software (http://www.gimp.org).
3. Non-facial geometrical control stimulus
An oval-shaped figure containing three intersecting lines was created to control for distraction effects attributable to visual object processing (see Figure 1B). Moreover, the control stimulus was designed to possess a structural face-like configuration to further isolate the perceptual and emotional processes of interest: facial processing in general and facial affective processing in particular.
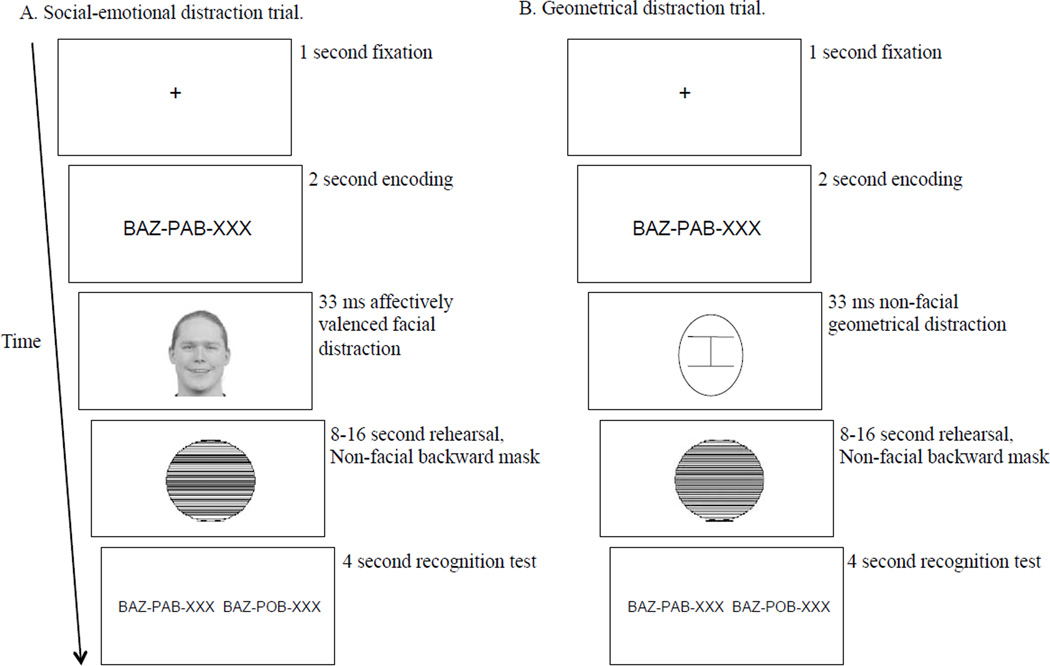
Figure 1.
Task diagram. Notably, images and stimuli are not displayed to scale. For example, alphabetic stimuli subtend a much smaller visual angle than is projected on the display. Picture of happy face taken from Radboud Faces Database (Langner et al., 2010). Permissions to display picture of happy face were obtained from the copyright holder of the Radboud Faces Database and publisher of the image.
Phonological-affective delayed match-to-sample (paDMTS) task
Participants were tested individually in a quiet room. Stimuli were presented on a 20-inch flat screen monitor with a resolution of 1,280 × 1,024. Alphabetic stimuli were presented in a 12-point Courier New font, in black against a white background. Notably, alphabetic stimuli were presented in upper-case to prevent participants from completing the task using visuoperceptual strategies based on unique sub-letter visual features, i.e., ascenders or descenders. Figure 1 depicts the task diagram; though, note that stimuli are not displayed to scale. At the outset, participants were given the general instruction to maintain fixation and not to look away during the entire task.
Each trial began with a cross (e.g., “ + ”) presented at central fixation for 1,000 ms and was comprised of three sequential phases. In the first phase (encoding), participants were given pseudowords comprised of one, two, or three syllables to subvocally read and memorise (2 s phase duration). Notably, participants were instructed “not to move their lips when reading”. During the second phase (rehearsal), participants were instructed to mentally rehearse the syllables presented in the first phase, with the interval duration varying between 8 and16 s in two-second increments (M=11.9 s, SD=2.8 s). The third phase (recognition) consisted of a recognition test in which two sets of pseudowords were presented and the participant was instructed to indicate (using the number pad on keyboard with dominant right hand) which set was from the first phase (4 s phase duration). Accuracy and speed were stressed. An equal number of correct responses were on the left and right side of the screen. Syllables within pseudowords in the recognition phase were also separated by hyphens to match the presentation format in the encoding phase. Within the recognition phase, the foil pseudoword differed from the target pseudoword by only one letter, with the location of the differing letter varying across trials to prevent participants from attending to particular locations to complete the task. A blank screen was presented during inter-trial intervals that randomly varied between 1,000, 1,250, 1,500, 1,750, and 2,000 ms (M=1,502 ms, SD =352 ms).
Unbeknownst to participants, a picture of a human face displaying happy, sad, or neutral expressions was briefly presented (33 ms) immediately after the pseudoword presentation phase. A non-facial neutral backward mask immediately replaced distracters and filled the duration of the rehearsal phase. As seen in Figure 1, the backward mask consisted of a circle with multiple horizontal lines varying in thickness. Participants were told the backward mask was a rehearsal indicator. Distracters were task-irrelevant and presented at fixation to ensure their location was within the focus of visual attention and therefore could not be unattended items. The location of facial distracters was presented so that the eyes and nose of each face aligned with the pseudoword targets. There were an equal number of happy, sad, and neutral distracter trials.
To summarise, each trial consisted of a fixation cross for 1,000 ms, a pseudoword encoding phase for 2,000 ms, a facial (or non-facial geometrical) distracter for 33 ms, a rehearsal phase varying between 8,000 and 16,000 ms, a recognition phase for 4,000 ms, and an inter-trial interval varying between 1,000 and 2,000 ms.
Response latencies were measured from the onset of the test phase until the participants’ response. Each participant was given a total of 108 experimental trials. Order of trial presentation was programmed by RSFgen (http://afni.nimh.nih.gov/) to pseudorandomly vary among participants so that the same condition was never presented thrice in a row. Participants were given practice trials prior to the experiment proper. None of the stimuli utilised in the practice phase were utilised in the experiment proper. Feedback was given during this practice phase to ensure comprehension of task demands, but was not given during the experiment proper. Two breaks were built into the experiment proper, each given after 36 trials. The total duration of the computerised task lasted approximately 40 minutes. Stimulus presentation and behavioural recordings were controlled using E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA).
All stimuli were presented in the central visual field. Participants sat at a viewing distance of approximately 89 cm. All alphabetic stimuli presented at encoding, including flanking XXXs, had a physical width of 72 mm and subtended a viewing angle of 4° wide. Facial distracters had a physical width of 65 mm and subtended a viewing angle of 3° wide. The backward mask had a physical width of 95 mm and subtended a viewing angle of 5° wide.
Post-task facial affect recognition test
Following administration of the paDMTS task, participants were asked in an open-ended manner whether they “noticed anything in the task”. Our aim with this open-ended question was to determine whether participants were able to detect a face at any point during the task. If a participant did not freely report detecting a face in the task, then the task administrator informed the participant of facial distracters and proceeded with the debriefing statement. If, however, participants reported detecting a face during the task, then they were administered the facial affect recognition test prior to presentation of the debriefing statement. This test consisted of document showing example facial expressions of each of the eight emotional expressions (angry, contemptuous, disgusted, fearful, happy, neutral, sad, and surprised) in the Radboud Faces Database (Langner et al., 2010). Participants were instructed to circle three emotional expressions that they might have seen in the task. Our aim was not to determine whether participants could recognise facial identities, but whether they were aware of emotional expressions during the task. A secondary aim of this test was to determine whether apperception of faces during completion of the task moderated main effects and/or interactions. The percentage of total participants that reported seeing a particular facial expression was calculated at the end of the experiment. Notably, there were no example images of the non-facial geometrical distracter in this post-task test.
A priori data analysis
Repeated-measures ANOVAs were performed separately for response latency and accuracy data to test for main effects (i.e., load, facial distracter type) and factorial interaction. Effect sizes for ANOVAs were estimated using partial eta-square (), of which small, medium, and large effect sizes are defined respectively as .01, .06, and .14 (Cohen, 1988). To test for general effects of distraction caused by perception of faces (i.e., facial distraction), ANOVAs were followed by paired sample t-tests comparing task performance in the context of each facial distracter with task performance in the context of non-facial geometrical distraction for each load condition (e.g., 1-syllable/happy face distraction vs. 1-syllable/non-facial geometrical distraction; 2-syllable/sad face distraction vs. 2-syllable/non-facial geometrical distraction). Effect sizes from these comparisons were calculated from means and standard deviations using Cohen’s d, of which small, medium, and large effect sizes are defined respectively as 0.2, 0.5, and 0.8 (Cohen, 1988). To test linearity in facial affective modulation of incremented load performance, contrast variables were created by computing the difference in performance between facial distraction and non-facial distraction for each load condition and were submitted to post hoc orthogonal polynomial comparisons, linear (1 0 − 1); quadratic (−1 2 − 1). This analysis tested the linearity hypothesis of greatest facial affective disruption in the 1-syllable condition, intermediate disruption in the 2-syllable condition, and negligible disruption in the 3-syllable condition. Here, one-sample t-tests indicated whether polynomial coefficients were significantly different from zero. Post hoc within-subjects (simple) contrasts tested for effects of facial-affective valence in those load conditions wherein facial distracters were significantly disruptive to performance relative to geometrical control.
In separate analyses of post-task facial affective recognition data, Kruskal–Wallis H and Mann–Whitney U tests were performed to determine whether apperception of facial affect (happy, neutral, and sad) moderated main effects and/ or interactions. Kruskal–Wallis H and Mann–Whitney U tests were chosen because of unequal sample sizes and non-normal distributions.
Results
Overall accuracy ranged from 76% to 100% (M=94±1). Outliers were identified and removed at the individual level such that response latencies below or above 2.5 standard deviations away from each participant’s mean were removed from statistical analyses. With respect to response latencies, only correct response latencies were treated to statistical analyses. Condition-specific latencies and percentage correct for Experiment 1 are displayed in Table 2.
Table 2.
Experiment 1 behavioural performance
| Distracter type | ||||
|---|---|---|---|---|
| WM load | Geometrical M±SEM |
Happy M±SEM |
Neutral M±SEM |
Sad M±SEM |
| 1-Syllable | ||||
| RT | 1,399±31 | 1,382±32 | 1,382±34 | 1,361±35 |
| ACC | 97±1 | 95±1 | 96±1 | 98±1 |
| 2-Syllables | ||||
| RT | 1,703±34 | 1,879±39 | 1,834±40 | 1,801±39 |
| ACC | 98±1 | 94±1 | 94±1 | 94±1 |
| 3-Syllables | ||||
| RT | 2,288±39 | 2,285±41 | 2,316±42 | 2,251±42 |
| ACC | 90±1 | 93±1 | 89±2 | 92±1 |
Notes: SEM=standard error of mean; WM=working memory; RT=response time; ACC=accuracy.
Response latencies
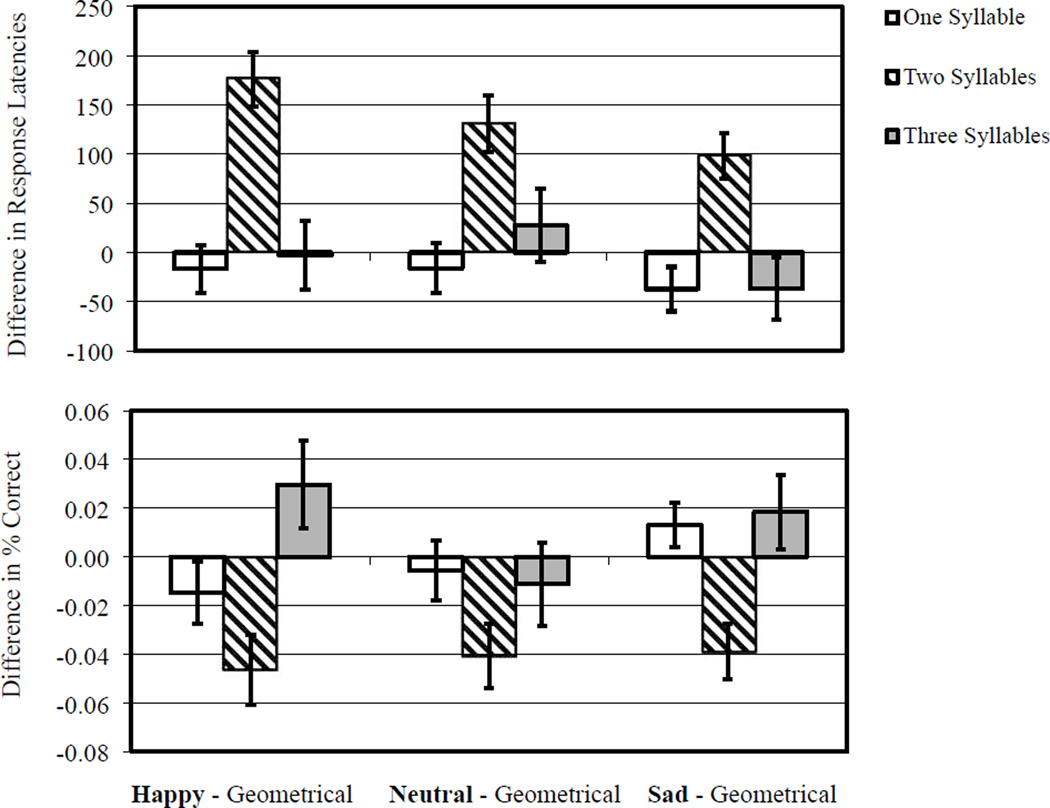
Load had a significant effect on response latencies, F(2, 58)=657.31,p <.001, MSE =52,176.68, , indicating that latencies were quickest for the 1-syllable pseudo-word condition (1,381 ±29 ms), intermediately quick for the 2-syllable pseudoword condition (1,804 ± 34 ms), and slowest for the 3-syllable pseudoword condition (2,285 ±34 ms). Facial distraction had a significant effect on response latencies, F(3, 57) =4.57, p=.006, MSE =25,008.47, , indicating that happy and neutral facial distracters were more disruptive (1,849 ±32 ms, 1,844 ±33 ms, respectively) than sad faces and non-facial geometric distracters (1,805 ±34 ms, 1,797± 29 ms, respectively). The interaction between load and facial distraction was significant, F(6, 54)=6.20, p<.001, MSE =28,444.10, , with Figure 2 displaying this interaction by showing each facial distracter contrasted against the geometrical baseline. Pairwise comparisons localised the source of this interaction to the 2-syllable pseudoword condition wherein all facial distracters (happy, neutral, sad) significantly increased latencies compared to the geometrical baseline, t(59)=− 6.40,p<.001, t(59)=− 4.50,p<.001, t(59)=−4.21, p<.001, respectively). Conversely, the disruptive effects of happy, neutral, and sad facial distracters in the 1-syllable and 3-syllable load conditions were not significantly different from non-facial geometric distracters (ps>.05). The joint effect of facial disruption (irrespective of facial distracter type) and incremented load on performance was not consistent with a linear trend (ps > .05; Cohen’s d ranged from −0.03 to 0.35). Rather, the joint effect for all facial distracters and incremented load was best captured by a quadratic trend (happy facial distracters and load performance: t(59) =4.99, p<.001, Cohen’s d=1.30; neutral facial distracters and load performance: t(59) =3.49, p <.001, Cohen’s d=0.91; sad facial distracters and load performance: t(59) =4.25, p<.001, Cohen’s d=1.10). Given the significant quadratic trend, analyses of the effects of valence were constrained to the intermediate load condition (2-syllables). Post hoc within-subjects (simple) contrasts of happy versus neutral facial distracters and sad versus neutral facial distracters were nonsignificant (p=.168 and p=.189; respectively) whereas the contrast of happy versus sad facial distracters was significant, F(1, 59)=7.77, MSE=47,062.19,p =.007, .
Figure 2.
Differences between task performance in the context of facial distraction and task performance in the context of non-facial geometrical distraction for Experiment 1. Zero axis value indicates performance in the context of non-facial geometrical distraction. Positive values in the response latency indicates relative behavioural disruption, whereas negative values in percent correct indicates relative behavioural disruption.
Accuracy
Load had a significant effect on accuracy, F(2, 58)=19.39, p<.001, MSE=0.007; , indicating that responses were most accurate for the 1-syllable pseudoword condition (97±1%), intermediately accurate for the 2-syllables condition (95 ±1%), and least accurate for the 3-syllable pseudoword condition (91 ± 1%). Facial distraction did not have a significant main effect on accuracy, F(3, 57) =1.64, p=.189, MSE =0.006, . The interaction between load and facial distraction was significant, F(6, 54) =4.102, p=.002, MSE=0.006, , with Figure 2 displaying this interaction by showing each facial distracter contrasted against a non-facial geometrical baseline. Pairwise comparisons localised the source of this interaction to the 2-syllable pseudo-word condition wherein happy, neutral, and sad facial distracters significantly reduced accuracy compared to the geometrical baseline, t(59)=−3.39, p<.001, t(59)=−3.23, p=.002, t(59)=−3.08, p=.003, respectively. The potentially disruptive effects of the individual types of facial distracters were not significantly different from non-facial geometrical baseline in the 1- and 3-syllable pseudoword conditions (ps>.05). The joint effect of facial disruption (irrespective of facial distracter type) and incremented load on performance was not consistent with a linear trend (ps > .05, Cohen’s d ranged from −0.07 to 0.56). Rather, the combined effect of facial distraction for all face types and incremented load was best captured by a quadratic trend, happy facial distracters and load performance: t (59) =2.99, p=.004, Cohen’s d=0.78, neutral facial distracters and load performance: t(59)=1.96, p=.05, Cohen’s d=0.51, sad facial distracters and load performance: t(59) =3.91, p<.001, Cohen’s d=1.02. Given the significant quadratic trend, analyses of the effects of valence were constrained to the intermediate load condition (2-syllables). Post hoc within-subjects (simple) contrasts of happy versus neutral, happy versus sad, and sad versus neutral were all non-significant (p=.713, p=.576, and p=.909, respectively).
Post-task facial affect recognition
All participants reported detecting a face during the task. On the post-task facial affect recognition test, twenty-one (35.0%) participants correctly identified at least one facial expression, 32 (53.3%) correctly identified two facial expressions, and seven (11.6%) correctly identified all three facial expressions. The percentage of participants that recognised particular affective expressions varied across emotion type, as reported below.
Forty-five (75%) participants reported that they were able to recognise happy faces. Participants who recognised happy faces demonstrated shorter response latencies than those participants who did not recognise happy faces in the following conditions: 1-syllable/geometric distracter (z=−2.313,p=.021); 1-syllable/neutral distracter (z=−1.938, p=.053); 2-syllable/happy distracter (z= − 2.842, p=.004); and 3-syllable/ neutral distracter (z=− 2.142, p=.032). Recognising happy faces did not significantly moderate performance on any another task condition (ps > .05).
Sixteen (27%) participants reported that they were able to recognise sad faces; however, this did not significantly moderate performance on any task condition (ps >.05). Forty-five (75%) participants reported that they were able to recognise neutral faces; however, this did not significantly moderate performance on any task condition (ps > .05).
Experiment 1: Summary
Participants did well on the paDMTS task, as indicated by mean total accuracy of 94%. As expected, there was a significant main effect of load on response latency and task accuracy, with incrementally poorer performance as load increased. The interaction between working memory load and facial distraction was significant and consistent with a large effect size (). Compared to non-facial geometric distracters, facial distracters significantly disrupted working memory performance (i.e., increased RT, decreased accuracy) in the intermediate load condition (2-syllable pseudoword) but not the low or high load conditions (1- and 3-syllable pseudo-words, respectively). While all three facial dis-tracters disrupted working memory performance in the intermediate load condition relative to the geometrical baseline, there was a stepwise pattern wherein happy faces were the most disruptive, then neutral faces, then sad faces. The effect of valence (i.e., positive vs. negative) in the intermediate load condition was significant, with happy facial distracters causing more disruption than sad facial distracters, but only for response latencies.
EXPERIMENT 2
Experiment 2 was designed to replicate and generalise the findings of Experiment 1. While happy faces tended to be more disruptive than sad faces in Experiment 1, we questioned whether other types of negative facial expressions would more deleteriously affect working memory performance. Fearful faces seemed the most appropriate candidate given that they are known for being stronger provocateurs of amygdala activation relative to both happy and sad faces (see Fusar-Poli et al., 2009, for a meta-analytic review). Thus, in Experiment 2, fearful facial distracters replaced sad facial distracters. Moreover, we sought to determine whether varying the length of the rehearsal interval altered the interaction between load and facial distracters. This inquiry was predicated on evidence showing that load-dependent effects on working-memory-related brain regions (e.g., superior, middle and inferior frontal gyri, intraparietal sulcus, fusiform gyrus) differs as a function of the length of the delay interval, with load effects modulating activation of these brain regions 6–9 s following stimulus onset but not over longer time intervals (Jha & McCarthy, 2000). Thus, we also shortened the rehearsal interval in Experiment 2. In sum, Experiment 2 differed from Experiment 1 in that fearful faces replaced sad faces and the length of the rehearsal interval was shortened.
Methods
Participants
In Experiment 1, the effect sizes for the interaction between load and facial distracters were large for both response latencies () and accuracy (). However, changes in task parameters (i.e., fearful faces, shorter rehearsal interval) called into question whether such effects would remain high in Experiment 2; thus, we estimated a medium effect size when calculating power. For Experiment 2, an a priori power analysis for an interaction-level ANOVA with an alpha level of .05, a 3 × 4 factorial design, and an anticipated medium effect size (δ=.75) indicated a minimum sample size of 27 to achieve acceptable power (.80).
Participants were 29 healthy, undergraduate college students (22 females), aged 18–23 (M=19 ± 1), with no history of neurological or psychiatric disease. All were right-handed (hand-edness quotient > 70) on the Edinburgh handedness inventory (Oldfield, 1971), spoke English as a first language, and reported normal or corrected-to-normal vision. Years of education ranged from 13–15 years (M=13 ±1). Participants provided written informed consent, were given a post-task debriefing statement, and were given course credit in exchange for their voluntary participation. The San Diego State University (SDSU) Independent Review Board approved the protocol.
Design
We employed the same experimental design utilised in Experiment 1 with the exception that fearful faces replaced sad faces. The within-subjects factors in Experiment 2 were Load (1-syllable vs. 2-syllables vs. 3-syllables) and Facial Distraction (geometrical baseline vs. happy face vs. fearful face vs. neutral face). Each participant was given a total of 108 experimental trials. Dependent variables were response latencies and percentage correct.
Stimuli
Same as Experiment 1.
Phonological-affective delayed match-to-sample (paDMTS) task
Same as Experiment 1 with the exception that the duration of the rehearsal interval randomly varied between 2 s, 3 s, and 4 s (M=3s, SD=821 ms). All other task parameters, procedures, and instructions remained unchanged from Experiment 1.
Post-task facial affect recognition test
Same as Experiment 1.
A priori data analysis
Same as Experiment 1.
Results
Overall accuracy ranged from 68% to 99% (M=94 ± 1). Outliers were identified and removed at the individual level such that response latencies below or above 2.5 standard deviations away from each participant’s mean were removed from statistical analyses. Notably, only correct response latencies were treated to statistical analyses concerning response latencies. Condition-specific response latencies and percentage correct for Experiment 2 are displayed in Table 3.
Table 3.
Experiment 2 behavioural performance
| Distracter type | ||||
|---|---|---|---|---|
| WM load | Geometrical M±SEM |
Happy M±SEM |
Neutral M±SEM |
Fearful M±SEM |
| 1-Syllable | ||||
| RT | 1,187±52 | 1,162±54 | 1,166±52 | 1,128±46 |
| ACC | 98±1 | 98±1 | 96±2 | 98±2 |
| 2-Syllables | ||||
| RT | 1,472±51 | 1,642±59 | 1,570±64 | 1,521±55 |
| ACC | 98±1 | 96±1 | 95±2 | 94±2 |
| 3-Syllables | ||||
| RT | 1,981±72 | 2,001±71 | 1,938±57 | 1,974±80 |
| ACC | 90±2 | 91±2 | 89±3 | 92±2 |
Notes: SEM=standard error of mean; WM=working memory; RT=response time; ACC=accuracy.
Response latencies
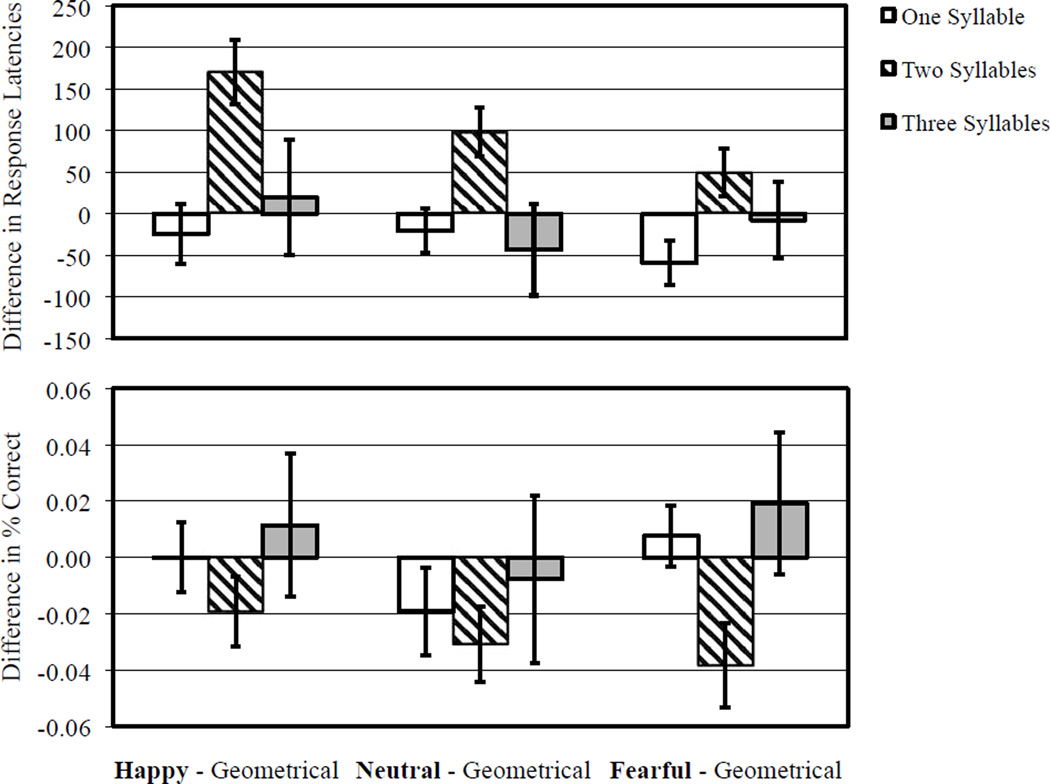
Load had a significant effect on response latencies, F(2, 27)=129.98, p < .001, MSE=90,854.15, , indicating that latencies were quickest for the 1-syllable pseudo-word condition (1,161 ± 48 ms), intermediately quick for the 2-syllable pseudoword condition (1,551 ±53 ms), and slowest for the 3-syllable condition (1,973 ± 60 ms). Facial distraction did not have a significant main effect on task response latencies, F(3, 26) =1.33, p=.284, MSE=29,550.30, . The interaction between load and facial distraction was significant, F(6, 23) =2.50, p=.052, MSE =27,502.40, , with Figure 3 displaying this interaction by showing each facial distracter contrasted against the geometrical baseline. Pairwise comparisons localised the source of this interaction to the 2-syllable pseudoword condition in which happy and neutral facial distracters significantly increased latencies compared to the geometrical baseline, t(28)=−4.41, p<.001, t(28) =−3.389, p=.002, while the disruptive effect of fearful faces relative to geometrical baseline was marginally significant, t(28)=− 1.71, p=.098. The potentially disruptive effects of happy, neutral, and fearful facial distracters in the 1-syllable and 3-syllable load conditions were not significantly different from non-facial geometric distracters (ps>.05). The joint effect of facial disruption (irrespective of facial distracter type) and incremented load on performance was not consistent with a linear trend (ps > .05; Cohen’s d ranged from −0.35 to − 0.14). Rather, the joint effect of all facial distracters and incremented load on performance was best captured by a quadratic trend (happy facial distracters and load performance: t(59) =3.26, p=.003, Cohen’s d=1.23, neutral facial distracters and load performance: t(59) =2.78, p=.009, Cohen’s d=1.05, fearful facial distracters and load performance: t(59) =2.12, p=.04, Cohen’s d=0.80. Given the significant quadratic trend, analyses of the effects of valence were constrained to the intermediate load condition (2-syllables). Post hoc within-subjects (simple) contrasts of happy versus neutral facial distracters and sad versus neutral facial distracters were non-significant (p=.159, p=.176) whereas the contrast of happy versus fearful facial distracters was significant, F(1, 28) =9.93, MSE=42,593.63, p=.004, .
Figure 3.
Differences between task performance in the context of facial distraction and task performance in the context of non-facial geometrical distraction for Experiment 2. Zero axis value indicates performance in the context of non-facial geometrical distraction. Positive values in the response latency indicates relative behavioural disruption, whereas negative values in percent correct indicates relative behavioural disruption.
Accuracy
Load had a significant effect on accuracy, F(2, 27)=18.581, p <.001, MSE =0.006, , indicating that responses were most accurate for the 1- and 2-syllable pseudoword conditions (97±1% and 96 ± 1%, respectively), and least accurate for the 3-syllable pseudoword condition (90 9 1%). Facial distraction did not have a significant main effect on task accuracy, F(3, 27) =1.017, p=.401, MSE =0.005, . Although the interaction between load and facial distraction was not significant, the observed effect size was quite large, F (6, 23) =1.411, p=.253, MSE =0.006, .
Post-task facial affect recognition
All participants reported detecting a face during the task. On the post-task facial affect recognition test, twelve (41%) participants correctly identified at least one facial affect, 14 (48%) correctly identified two facial affect, and one (3%) correctly identified all three facial affect. The percentage of participants that recognised particular affective expressions varied across emotion type, as reported below.
Sixteen (55%) participants reported that they were able to recognise happy faces during the task. Participants who recognised happy faces demonstrated higher accuracy latencies than those participants who did not recognise happy faces in the 3-syllable/neutral distracter condition (z= −2.283, p=.022). Being able to recognise happy faces during the task did not significantly moderate performance on any other task condition (ps >.05).
Six (20%) participants reported that they were able to recognise fearful faces during the task. Participants who recognised fearful faces demonstrated higher accuracy than those participants who did not recognise fearful faces in the following conditions: 1-syllable/geometric distracter, 1-syllable/fearful distracter, and 2-syllable/ geometric distracter (z=−2.419, p=.016, z=− 1.958, p=.050, and z=− 2.088, p=.037, respectively). Recognising fearful faces during the task did not significantly moderate performance on any other task condition (ps > .05).
Twenty (68%) participants reported that they were able to recognise neutral faces during the task; however, this did not significantly moderate performance on any task condition (ps > .05).
Experiment 2: Summary
Response latencies from Experiment 2 replicated the pattern of results in Experiment 1, despite replacing sad with fearful faces and shortening the length of the rehearsal interval. Participants in Experiment 2 obtained a mean total accuracy of 94%, showing remarkable consistency with Experiment 1. As expected, load had a significant effect on response latency and task accuracy, with performance worsening with increasing load. As with Experiment 1, the interaction between load and facial distraction in Experiment 2 was significant with respect to response latency data, showing a similarly large effect size (; response latency). The source of this interaction stemmed from facial distracters being disruptive to working memory performance in the intermediate load condition (2-syllable pseudoword) but not in the low or high load conditions (1- and 3-syllable pseudowords, respectively). Within the intermediate load condition, happy facial distracters were the most disruptive, then neutral facial distracters, then fearful facial distracters. Notably, the disruption of fearful facial distracters relative to geometrical baseline fell short of the significance threshold (p=.09). The effect of valence (i.e., positive vs. negative) in the intermediate load condition was significant, with happy facial distracters causing more disruption than fearful facial distracters, but only for response latencies. The fact that both sad (Experiment 1) and fearful (Experiment 2) faces were least disruptive compared to happy faces strengthens the interpretation that negative emotion is generally less disruptive to working memory than positive emotion. Unlike Experiment 1, the interaction between load and facial distraction in Experiment 2 was not significant with respect to accuracy, though the direction of the effects were similar.
GENERAL DISCUSSION
Our behavioural paradigm revealed a curvilinear relationship between working memory load and social-emotional modulation wherein task-irrelevant facial distracters significantly increased response latency and decreased accuracy in the intermediate load condition (2-syllable pseudo-word) but not in the low or high load conditions (1-and 3-syllable pseudowords, respectively). This curvilinear pattern in response latencies was replicated and generalised in a second experiment wherein fearful faces replaced sad faces and the length of the rehearsal interval was shortened. All facial distracters disrupted working memory performance in the intermediate load condition irrespective of valence, suggesting a primary and general facial processing effect. However, negatively valenced faces tended to be less disruptive to working memory performance than positively valenced faces, suggesting a secondary and specific valence effect. The present curvilinear relationship between working memory load and facial affective modulation did not support our hypothesis nor did it support the prevailing linear view (i.e., greatest disruption in low load, mild disruption in intermediate load, least disruption in high load), suggesting a more nuanced view wherein working memory spare capacity plays a determinative role.
Curvilinear relationship between working memory load and social-emotional modulation
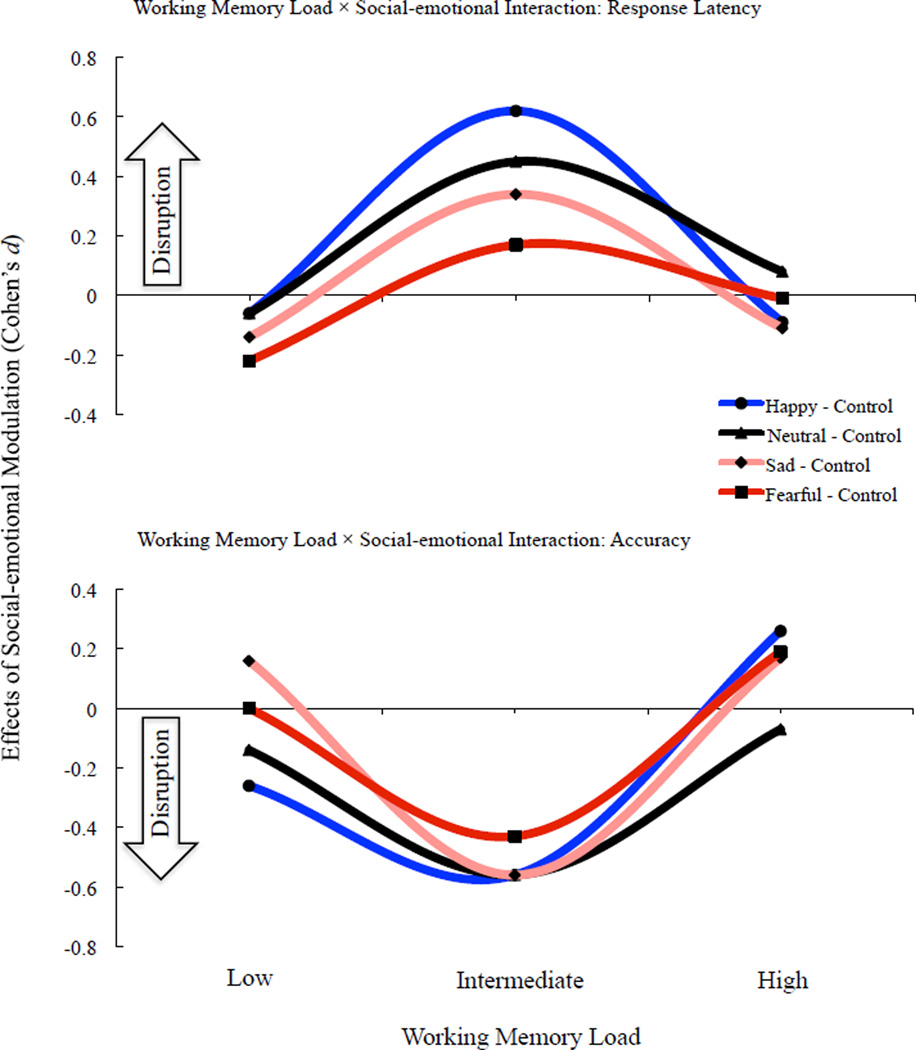
Figure 4 depicts the curvilinear relationship between working memory load (x-axis) and social-emotional modulation (y-axis) obtained from Experiments 1 and 2, which presents the effects of facial distracters relative to non-facial geometrical distracters (zero axis) in Cohen’s d effect sizes. Before presenting our account of this relationship, however, it is important to first discuss the assumptions underlying our explanation. First, a critical component of our account of the curvilinear findings is the concept of working memory capacity, which is believed by many to vary among three to five units or slots of information chunks (i.e., 4±1; Cowan, 2000). One syllable, for example, occupies one slot whereas two syllables occupy two slots. When load size approaches the limits of working memory capacity by occupying many of the available slots, performance decrements will ensue. Notably, orthographically controlled pseudo-words with clear syllabic boundaries allowed us to control load size given that pseudowords lack semantic associations yet can easily be chunked on the basis of frequently occurring orthographic-phonological representations. The prevailing view, described in the introduction, seems to have favoured an attentional resource model to explain past results; however, we favour an item-limit (or fixed slot) model because it explains present data better and is more theoretically interpretable (see Cowan & Rouder, 2009, for a discussion). Second, our account of present curvilinear results assumes that a single facial distracter occupies a working memory slot, but only when slots are available within working memory spare capacity (e.g., Erthal et al., 2005; Okon-Singer et al., 2007; Pessoa et al., 2002; Van Dillen et al., 2009). In contrast, a non-facial geometrical distracter is not assumed to occupy a working memory slot because it lacks intrinsic value. Finally, we assume that the act of facial distracters occupying working memory slots adds to the pseudoword memoranda load to produce a combinatorial load (i.e., syllables plus facial distraction), even though facial distracters were task-irrelevant and unbeknownst to participants prior to task administration.
Figure 4.
Modulation effects of facial valence are displayed relative to non-facial geometric distracters. The zero axis value represents working memory performance in the context of non-facial geometrical distracters. In the top graph depicting response latencies relative to non-facial geometrical distracters, positive effect sizes connote behavioural disruption while negative effect sizes connote behavioural facilitation. In the bottom graph depicting accuracy relative to non-facial geometrical distracters, positive effect sizes connote behavioural facilitation while negative effect sizes connote behavioural disruption. Notably, effects sizes for happy, neutral, and sad facial distracters are reported from Experiment 1 while effect sizes for fearful facial distracters are reported from Experiment 2.
When memorandum load size is low (1-syllable pseudoword) and well below the limits of working memory capacity (4±1; Cowan, 2000), there is no performance difference in the context of either facial or non-facial geometric distracters (Figure 4) because the combined memoranda are below capacity limits in both instances. Available working memory slots easily accommodated the additional load from facial distraction given that the combined load size remains below working memory capacity. Thus, the lack of facial disruption during low load processing stemmed from working memory spare capacity capable of accommodating the combinatorial load of one syllable plus facial-distraction. This position differs from the prevailing view insofar as it does not presume that automatic absorption of working memory slots by emotional stimuli obligatorily disrupts working memory performance. For example, the prevailing linear view predicts that facial distracters would be most disruptive to working memory performance in the low load condition (1-syllable pseudoword) given that spare resources are highest in this condition. Instead, it is conceivable that emotional stimuli can both “automatically” occupy working memory slots yet not cause behavioural disruption, so long as the combinatorial processing load remains well below capacity limits. The critical moderating variable here, therefore, is working memory spare capacity.
When memoranda load is intermediately sized (2-syllable pseudoword) and approaching the limits of working memory capacity (4±1; Cowan, 2000), there is a significant performance decrement in the context of facial distraction relative to non-facial geometrical distraction (Figure 4) because the combinatorial load of the former occupy more working memory slots than the latter. In other words, working memory capacity had greater difficulty accommodating the combinatorial load of “two syllables plus facial distraction” relative to the combinatorial load of “two syllables plus non-facial geometrical distraction”. Consistent with the prevailing view of cognitive-emotional interactions, our account assumes that facial distracters occupied available working memory slots. However, the presence of facial disruption during intermediate load processing stemmed from the combinatorial load of pseudoword memoranda plus facial distraction approaching the limits of working memory capacity, not singularly from facial distracters automatically absorbing available attentional resources within working memory capacity.
When memoranda load is high (i.e., 3-syllable pseudoword) and reaching the limits of working memory capacity (4±1; Cowan, 2000), there is no performance difference in the context of either facial or non-facial geometrical distracters because high load processing occupied the majority of working memory slots and spared few (if any) for facial stimuli to occupy. This interpretation, notably, is consistent with the absorption hypothesis (Erber & Tesser, 1992) and the prevailing view of cognitive-emotional interactions cited above. Working memory capacity was nearly maximised by 3-syllable pseudoword memoranda, depriving facial stimuli of spare slots to occupy and cause disruption. Thus, there was no performance difference between the combinatorial load of “3-syllables plus facial-distraction” and the combinatorial load of “3-syllables plus non-facial geometrical control”. Interestingly, neuroimaging research suggests a neural mechanism for such effects (Van Dillen et al., 2009), whereby high load processing “tunes down” or down-regulates activity in affective neural circuitry when emotional stimuli are present. The lack of facial disruption relative to non-facial geometrical distraction during high load processing implies that “automaticity” of social-emotional processing is contingent upon the availability of working memory resources left over by task demands.
Brief presentation of facial distraction and importance of task instructions
Though facial distracters were presented very briefly (33 ms) and followed immediately by a non-facial backward mask, all participants reported detecting a face during the task when given an open-ended question following task administration. This observation is not inconsistent with prior reports of low perceptual thresholds for facial stimuli (Kirouac & Doré, 1984; Maxwell & Davidson, 2004; Pessoa, Japee, & Ungerleider, 2005), with some authors suggesting the threshold for detecting faces to be well below 30 ms (Milders, Sahraie, & Logan, 2008). It is conceivable that all participants reported detecting a face because of the dissimilarity between the control stimulus (non-facial circle with lines) and faces, given the large number of trials and the weak masking effect from our non-facial backward mask. Research has demonstrated that the masking effect is greater when masks closely resemble their target (Loffler, Gordon, Wilkinson, Goren, & Wilson, 2005); notably, faces were not targets in our behavioural paradigm.
In prior working memory × emotion studies (e.g., Okon-Singer et al., 2007; Pessoa et al., 2002; Van Dillen et al., 2009), stimulus duration for distracters was longer (≥ 100 ms) and in most cases the distracters were incorporated into task instructions (e.g., “ignore” task-irrelevant stimuli). Such task parameters are likely to confound load boundaries and the interaction between task-driven working memory and stimulus-driven processing of social-emotional distraction. For example, distracters on screen for relatively long durations are likely to “hold” attention, while mentioning distracters in task instructions adds the complexity of dual-task processing and permits processing of emotional distracters to be task driven, both of which have the net effect of increasing load size by consuming additional working memory resources. Taken together, it is conceivable that prior conceptualisations of “low” load processing were in fact larger than intended by design. More specifically, prior conceptualisations of “low load” may have actually been “intermediate load”.
Ongoing research in our laboratory is testing whether the presently observed curvilinear pattern is replicated if participants are informed of the facial distracters prior to task administration and the stimulus duration of distracters is increased (e.g., 500 ms). If adopting these parameters has the net effect of increasing working memory load, then “low” load will become “intermediate” load and we should therefore observe significant facial disruption in the 1-syllable condition relative to non-facial geometrical distraction. It is therefore conceivable that a more linear (less curvilinear) “load × emotion interaction” would emerge by increasing the stimulus duration of distracters and incorporating distraction into task instructions, which would support the prevailing linear view. In the present study, the behavioural paradigm was designed to assess the interaction between task-driven working memory and stimulus-driven processing of social-emotional distraction. As such, we designed the behavioural paradigm so that facial distracters were briefly presented (33 ms), backward masked by a non-facial stimulus, and omitted from task instructions. These procedures maximised the probability that completion of facial processing was independent of conscious monitoring and ensured that social-emotional processing was uninstructed. Moreover, omission of facial distracters from task instructions permitted us to assess the incidental, and perhaps intrinsic, ability of working memory to cope with facial distraction. A final point to emphasise regarding the presently observed curvilinear pattern is the importance of broadening the load parameter beyond the typical dichotomous “low versus high” or “simple versus complex”.
Source of social-emotional modulation: Primary effect of general facial processing?
Relative to non-facial geometrical distracters, all facial distracters disrupted working memory performance in the intermediate load condition irrespective of valence. That all facial distracters disrupted intermediate working memory load processing—including emotionally neutral faces—suggests a primary and general modulation effect of facial processing. Interpreting present findings as cognitive vulnerability to general facial processing effects stands to reason given the strong biological and social importance intrinsic to faces. However, a significant problem with this interpretation stems from our use of a non-facial geometrical distraction that did not fully control for differences in perceptual and semantic complexity. Indeed, facial distracters were more perceptually and semantically complex than our non-facial geometrical control. As a result, it is conceivable that the presently observed interaction effects stemmed from greater perceptual and/or semantic complexity among faces relative to non-facial geometrical distracters. Future research is needed with the present paradigm utilising non-social yet semantically rich distracters (i.e., pictures of tools, furniture, plants) to test whether the presently observed interaction is specific to social information processing or broader semantics.
Source of social-emotional modulation: Secondary effect of affective valence?
Though all facial distracters disrupted intermediate working memory load processing relative to non-facial geometrical baseline, the disruptive effects of affectively valenced faces compared to emotionally neutral faces (i.e., happy vs. neutral; sad vs. neutral; fearful vs. neutral) was not significantly different. While there is longstanding precedence and justification for such comparisons, it may be imprecise to assume that emotionally neutral faces are completely devoid of valence or social meaning. For example, emotionally neutral faces are systematically seen as more negative than positive in a forced-choice task (e.g., Arce et al., 2009). It is conceivable that disruptive effects of valence were too subtle to be detected by comparisons with “emotionally neutral” faces. Indeed, results were different when comparing the disruptive effects of positively valenced faces directly with the disruptive effects of negatively valenced faces (sad and angry faces). Here, we found that happy facial distracters were significantly more disruptive to intermediate working memory load processing than negatively valenced faces (sad and angry faces), but only for response latencies. Collectively, these results suggest that valence had a disruptive effect, though secondary to the primary facial processing effect and seemingly specific to positive valence.
Notwithstanding the potential limitations of using neutral faces for controlling valence, happy faces tended to be most disruptive to intermediate load working memory performance than sad and fearful faces, suggesting a specific yet secondary effect of valence. However, our finding of secondary valence effects needs to be interpreted within the context of inconsistent findings in the literature, which show that working memory might be enhanced or hindered by affectively charged memoranda. For example, some report that memoranda valence has no effect on working memory performance (LoPresti et al., 2008; Perlstein, Elbert, & Stenger, 2002) while others report that positive and negative valence memoranda enhance working memory (Leven & Phelps, 2008). And yet others report that negatively valenced memoranda hinder working memory (Kensinger & Corkin, 2003). Two recent studies of the effect of valence on working memory reported an “angry face benefit” whereby maintenance of facial memoranda is better for angry faces than for happy faces (Jackson, Wolf, Johnston, Raymond, & Linden, 2008; Jackson, Wu, Linden, & Raymond, 2009), which was interpreted to reflect a phenomenon whereby negatively valenced stimuli enhance visuospatial-sketchpad systems of working memory (Baddeley, 2003) and therefore improve performance. It is conceivable that negatively valenced facial distracters were relatively less disruptive to working memory than positively valenced faces because working memory can more easily accommodate negative information than positive. Such an interpretation is not inconsistent with the “angry face benefit” (Jackson et al., 2008, 2009). However, such an interpretation may seem counterintuitive given that negative information about immediate social environment ought to, in theory, reprioritise working memory contents to favour emotionally provocative stimuli.
Conversely, it is conceivable that positively valenced facial distracters were relatively more disruptive to intermediate working memory load than negatively valenced facial distracters because of an attentional bias—in healthy participants— towards more positive stimuli and away from more negative stimuli (e.g., Frewen, Dozois, Joanisse, & Neufeld, 2008). For example, recent work has shown the “face in the crowd effect” to be stronger for happy (not angry faces) whereby positively valenced faces are more efficiently detected than negatively valenced faces, which tend to be avoided (e.g., Becker, Anderson, Mortensen, Neufeld, & Neel, 2011; Becker et al., 2012). In fact, it is conceivable that preference for happy faces and avoidance of negatively valenced faces (e.g., sad, fearful) explained results from the post-task facial affect recognition test, which showed that participants were more likely to notice happy than sad and fearful faces. Notably, there were several methodological limitations with our post-task facial affect recognition test, such as the forced-choice format and not including the geometrical distracter. Thus, participants may have selected happy faces more often because of the “happiness superiority effect”, confounding our attempt to test whether participants were aware of facial valence during the task. Despite the limitations with the design of our post-task affect recognition test, behavioural results from our paradigm suggest a unique interaction between the “happiness superiority effect” and working memory load, which warrants further inquiry.
Conclusions
We found a curvilinear relationship between working memory load and social-emotional modulation wherein facial distracters disrupted working memory performance in the intermediate load condition (2-syllable pseudoword) but not in the low or high load condition (1- and 3-syllable pseudowords, respectively). These results disagree with the linear relationship predicted by the prevailing view, yet support the notion that working memory load and spare capacity play pivotal roles in determining cognitive vulnerability and resistance to facial distraction. All facial distracters disrupted working memory in the intermediate load condition irrespective of valence, suggesting a general yet primary facial processing effect; however, sad and fearful faces tended to be less disruptive than happy faces, suggesting a specific yet secondary valence effect. The issue of why negatively valenced faces tended to be less disruptive to working memory warrants further inquiry.
Acknowledgments
This research was supported by the Department of Veteran Affairs Office of Academic Affiliations Advanced Fellowship Program affiliated with the Desert-Pacific Mental Illness Research, Education and Clinical Centre (MIRECC) (QRM, GGB, and RA). Funding sources had no further role in writing of this manuscript or decision to submit for publication. RA is now affiliated with the Department of Psychology at the University of Missouri – Kansas City.
The authors would like to thank Natalie Varnay, Cassandra Elrod, Lijun Yang, and Patrick Chen for their assistance in data collection. The authors would also like to thank the anonymous reviewers for their thoughtful comments and suggestions to improve the quality of the manuscript.
APPENDIX
| 1-Syllable condition | 2-Syllable condition | 3-Syllable condition |
|---|---|---|
| LAL-XXX-XXX | MIG-VOP-XXX | CUG-SUG-DAC |
| FEV-XXX-XXX | FUT-GIS-XXX | HEG-HOF-KAD |
| LUT-XXX-XXX | NAD-NOP-XXX | PUV-SEF-SIF |
| FIM-XXX-XXX | GAN-GOC-XXX | HEJ-HOV-KED |
| RUC-XXX-XXX | RUP-TIZ-XXX | ZUN-TEF-BIP |
| TEV-XXX-XXX | VEN-GAK-XXX | RAZ-RAL-DIF |
| TID-XXX-XXX | NAR-NOZ-XXX | BAF-PEB-SIG |
| HUS-XXX-XXX | VOR-ZAD-XXX | PUZ-SEG-BEP |
| WAJ-XXX-XXX | VOD-PAJ-XXX | TAJ-SOF-GAZ |
| BUV-XXX-XXX | RIN-BOZ-XXX | HEV-GEC-GOM |
| ZAT-XXX-XXX | LEB-MAJ-XXX | JUB-VOB-TAF |
| MAB-XXX-XXX | DUT-FOD-XXX | NEN-PEJ-SEK |
| LEF-XXX-XXX | TOF-VUT-XXX | PES-SAB-SEP |
| JAT-XXX-XXX | MAV-NEP-XXX | WOB-DUS-CAS |
| SUT-XXX-XXX | TIV-KIG-XXX | ZID-TEP-FAP |
| TEM-XXX-XXX | REJ-ZOP-XXX | ZOT-LOM-LIG |
| FEF-XXX-XXX | FOT-GES-XXX | POB-GUP-CED |
| JOS-XXX-XXX | TOZ-RUV-XXX | WAZ-HIN-JID |
| SIZ-XXX-XXX | GUD-SUS-XXX | ZET-BAV-BOV |
| FAF-XXX-XXX | VUN-TOB-XXX | GOS-HEZ-JAD |
| BOF-XXX-XXX | FOF-GEF-XXX | PAF-TAV-CAJ |
| LEM-XXX-XXX | FOM-GEG-XXX | SES-PEV-SAF |
| FAJ-XXX-XXX | TOJ-WAB-XXX | HAB-HIF-JAS |
| TAS-XXX-XXX | MAZ-NES-XXX | ZIM-JIT-PEM |
| TER-XXX-XXX | MEP-NIS-XXX | JOM-PEF-RIT |
| LEP-XXX-XXX | REZ-WOD-XXX | PIM-SAZ-SEZ |
| CUV-XXX-XXX | VAD-JUM-XXX | TAM-FUP-CET |
| BUP-XXX-XXX | KET-MAF-XXX | GEB-GOG-HET |
| SEJ-XXX-XXX | DIJ-FIS-XXX | VOT-ZAN-BAZ |
| FOZ-XXX-XXX | SIJ-TIS-XXX | RIS-NEB-NUR |
| LEN-XXX-XXX | FOS-GEP-XXX | TAZ-SOV-CAZ |
| FAZ-XXX-XXX | ZOD-ZOB-XXX | PID-SAJ-SEV |
| ZON-XXX-XXX | MEC-NID-XXX | HAR-HIG-JED |
| FEP-XXX-XXX | NOF-MUN-XXX | TEB-JOP-TEZ |
| TES-XXX-XXX | GIM-PAG-XXX | HEF-WIS-JOR |
| LER-XXX-XXX | VED-WID-XXX | PUM-SEB-SID |
REFERENCES
- Arce E, Simmons AN, Stein MB, Winkielman P, Hitchcock C, Paulus MP. Association between individual differences in self-reported emotional resilience and the affective perception of neutral faces. Journal of Affective Disorders. 2009;114:286–293. doi: 10.1016/j.jad.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews: Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bargh JA. Conditional automaticity: Varieties of automatic influence in social perception and cognition. In: Uleman JS, Bargh JA, editors. Unintended thought. New York, NY: Guilford Press; 1989. pp. 3–51. [Google Scholar]
- Becker DV, Anderson US, Mortensen CR, Neufeld S, Neel R. The face in the crowd effect unconfounded: Happy faces, not angry faces, are more efficiently detected in the visual search task. Journal of Experimental Psychology: General. 2011;140:637–659. doi: 10.1037/a0024060. [DOI] [PubMed] [Google Scholar]
- Becker DV, Neel R, Srinivasan N, Neufeld S, Kumar D, Fouse S. The vividness of happiness in dynamic facial displays of emotion. PLoS ONE. 2012;7(1):e26551. doi: 10.1371/journal.pone.0026551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DGV, Morton J, Vythilingam M, Pessoa L, et al. Modulation of emotion by cognition and cognition by emotion. NeuroImage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: Contributions from functional imaging studies. Behavioural Brain Research. 2010;214(2):172–179. doi: 10.1016/j.bbr.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2000;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Rouder JN. Comment on “dynamic shifts of limited working memory resources in human vision”. Science. 2009;323(5916):877. doi: 10.1126/science.1166478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis A, Aarts H. On wildebeests and humans: The preferential detection of negative stimuli. Psychological Science. 2003;14:14–18. doi: 10.1111/1467-9280.t01-1-01412. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance by unattended faces expressing positive and negative emotion. Perception & Psychophysics. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Hager JC. Facial Action Coding System: The manual. Salt Lake City, UT: Research Nexus; 2002. [Google Scholar]
- Erber R, Tesser A. Task effort and the regulation of mood: The absorption hypothesis. Journal of Experimental Social Psychology. 1992;28:339–359. [Google Scholar]
- Erthal FS, De Oliviera L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E. Load-dependent modulation of affective picture processing. Cognitive, Affective, Behavioral Neuroscience. 2005;5:388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Joanisse MF, Neufeld RWJ. Selective attention to threat versus reward: Meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Brown C, Indefrey P, Herzog H, Steinmetz H, Seitz R. The neural circuitry involves in the reading of German words and pseudowords: A PET study. Journal of Cognitive Neuroscience. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jackson MC, Wolf C, Johnston SJ, Raymond JE, Linden DEJ. Neural correlates of enhanced visual short-term memory for angry faces: An fMRI study. PLoS ONE. 2008;3(10):e3536. doi: 10.1371/journal.pone.0003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MC, Wu C-Y, Linden DEJ, Raymond JE. Enhanced visual short-term memory for angry faces. Journal of Experimental Psychology: Human Perception andPeformance. 2009;35(2):363–374. doi: 10.1037/a0013895. [DOI] [PubMed] [Google Scholar]
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: An event-related functional MRI study. Journal of Cognitive Neuroscience. 2000;12(2):90–105. doi: 10.1162/089892900564091. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3(4):378–393. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Kirouac G, Doré FY. Judgment of facial expressions: An approach to the relations between phenomenal experience and perceptual processes. Cognitive Psychology. 1984;15:238–300. doi: 10.1016/0010-0285(83)90010-5. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annual Review of Neuroscience. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition and Emotion. 2010;24(8):1377–1388. [Google Scholar]
- Langton SRH, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107:330–342. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Lavie N, De Fockert J. The role of working memory in attentional capture. Psychonomic Bulletin & Review. 2005;12:669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Emotion processing effects on interference resolution in working memory. Emotion. 2008;8(2):267–280. doi: 10.1037/1528-3542.8.2.267. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Hansen-Tift AM. Infants deploy selective attention to the mouth of a talking face when learning speech. PNAS USA. 2012;109(5):1431–1436. doi: 10.1073/pnas.1114783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler G, Gordon G, Wilkinson F, Goren D, Wilson H. Configural masking of faces: Evidence for high-level interactions in face perception. Vision Research. 2005;45:2287–2297. doi: 10.1016/j.visres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celene KA, Stern CE. Working memory for social cues recruits orbito-frontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. The Journal of Neuroscience. 2008;28(14):3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Ferri J, Hajcak G. Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective, and Behavioral Neuroscience. 2011;11:321–331. doi: 10.3758/s13415-011-0036-z. [DOI] [PubMed] [Google Scholar]
- Massaro DW. Experimental psychology and information processing. Chicago, IL: Rand McNally; 1975. [Google Scholar]
- Maxwell J, Davidson R. Unequally masked: Indexing differences in the perceptual saliency of “unseen” facial expressions. Cognition and Emotion. 2004;18:1009–1026. [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudo-word reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–270. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An on-line orthographic database of the English Language. 2005 Available at: http://www.neuro.mcw.edu/mcword/
- Milders M, Sahraie A, Logan S. Minimum presentation time for masked facial expression discrimination. Cognition and Emotion. 2008;22(1):63–82. [Google Scholar]
- Mitchell DGV, Nakic M, Kamel N, Pine DS, Blair RJR. The impact of processing load on emotion. NeuroImage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Tzelgov J, Henik A. Distinguishing between automaticity and attention in the processing of emotionally significant stimuli. Emotion. 2007;7:147–157. doi: 10.1037/1528-3542.7.1.147. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsycho-logia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Ungerleider L. Visual awareness and the detection of fearful faces. Emotion. 2005;5:243–247. doi: 10.1037/1528-3542.5.2.243. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Science, USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Science, USA. 2002;99(3):1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Tzelgov J. Specifying the relations between automaticity and consciousness: A theoretical note. Consciousness and Cognition. 1997;6:441–451. [PubMed] [Google Scholar]
- Valdois S, Carbonnel S, Juphard A, Baciu M, Ans B, Peyrin C, et al. Polysyllabic pseudo-word processing in reading and lexical decision: Converging evidence from behavioral data, connectionist simulations and functional MRI. Brain Research. 2006;1085:149–162. doi: 10.1016/j.brainres.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45:1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: A working memory model of distraction from negative feelings. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. How automatic is “automatic vigilance”? The role of working memory in attentional interference of negative information. Cognition and Emotion. 2009;23(6):1106–1117. [Google Scholar]
- Wegner DM, Schneider DJ, Carter SR, III, White TL. Paradoxical effects of thought suppression. Journal of Personality and Social Psychology. 1987;53:5–13. doi: 10.1037//0022-3514.53.1.5. [DOI] [PubMed] [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. In: Monsell S, Driver J, editors. Attention andpeformance. Vol. 18. Cambridge, MA: MIT Press; 2000. pp. 73–103. [Google Scholar]