Abstract
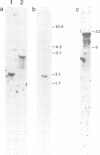
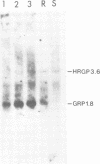
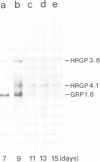
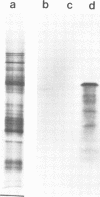
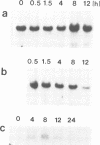
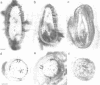
A single genomic clone (14 kb) isolated from bean (Phaseolus vulgaris L.) contains two genes that encode glycine-rich proteins. These genes are present as single copies in the genome, are separated by 2.85 kb and encode transcripts of 1.8 kb and 1.0 kb respectively. The encoded proteins contain 60% glycine and have amino-terminal signal peptides. The 1.8 kb transcript is present in young hypocotyls and in ovary tissue. Excision-wounding transiently induced this transcript in old, but not in young hypocotyl tissue. Antibodies raised against regions of the glycine-rich protein 1.8, expressed as a lacZ fusion protein in bacteria, react with a protein of 53 kd in a protein fraction extracted from cell walls of bean ovaries. Tissue imprints of bean ovaries treated with anti-glycine-rich protein antibodies showed that the glycine-rich protein was distributed in a regular pattern of small, highly localized discrete sites. The immunoreactive regions correspond to the pattern of vascular tissue in the pod. In young hypocotyls, glycine-rich protein is present at four pairs of discrete sites symmetrically arranged on the inner side of the vascular ring. These results suggest a close relationship between glycine-rich proteins and development of the vascular system.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averyhart-Fullard V., Datta K., Marcus A. A hydroxyproline-rich protein in the soybean cell wall. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1082–1085. doi: 10.1073/pnas.85.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab G. I., Nieto-Sotelo J., Cooper J. B., van Holst G. J., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol. 1985 Mar;77(3):532–535. doi: 10.1104/pp.77.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab G. I., Varner J. E. Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol. 1987 Dec;105(6 Pt 1):2581–2588. doi: 10.1083/jcb.105.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. M., Meagher R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol Cell Biol. 1987 Dec;7(12):4273–4279. doi: 10.1128/mcb.7.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Cross-linking of soluble extensin in isolated cell walls. Plant Physiol. 1984 Oct;76(2):414–417. doi: 10.1104/pp.76.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Insolubilization of hydroxyproline-rich cell wall glycoprotein in aerated carrot root slices. Biochem Biophys Res Commun. 1983 Apr 15;112(1):161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Keller B., Sengstag C., Kellenberger E., Bickle T. A. Gene 68, a new bacteriophage T4 gene which codes for the 17K prohead core protein is involved in head size determination. J Mol Biol. 1984 Nov 5;179(3):415–430. doi: 10.1016/0022-2836(84)90073-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L., Corey R. B. Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets. Proc Natl Acad Sci U S A. 1951 Nov;37(11):729–740. doi: 10.1073/pnas.37.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. S., Poovaiah B. W. Accumulation of a glycine rich protein in auxin-deprived strawberry fruits. Biochem Biophys Res Commun. 1987 Sep 30;147(3):885–891. doi: 10.1016/s0006-291x(87)80153-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. D. Bone marrow grafts and tolerance. Nature. 1986 Sep 11;323(6084):110–111. doi: 10.1038/323110b0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]