Abstract
As the rapid development of nanotechnology in the past three decades, titanium dioxide nanoparticles (TiO2 NPs), for their peculiar physicochemical properties, are widely applied in consumer products, food additives, cosmetics, drug carriers, and so on. However, little is known about their potential exposure and neurotoxic effects. Once NPs are unintentionally exposed to human beings, they could be absorbed, and then accumulated in the brain regions by passing through the blood–brain barrier (BBB) or through the nose-to-brain pathway, potentially leading to dysfunctions of central nerve system (CNS). Besides, NPs may affect the brain development of embryo by crossing the placental barrier. A few in vivo and in vitro researches have demonstrated that the morphology and function of neuronal or glial cells could be impaired by TiO2 NPs which might induce cell necrosis. Cellular components, such as mitochondrial, lysosome, and cytoskeleton, could also be influenced as well. The recognition ability, spatial memory, and learning ability of TiO2 NPs-treated rodents were significantly impaired, which meant that accumulation of TiO2 NPs in the brain could lead to neurodegeneration. However, conclusions obtained from those studies were not consistent with each other as researchers may choose different experimental parameters, including administration ways, dosage, size, and crystal structure of TiO2 NPs. Therefore, in order to fully understand the potential risks of TiO2 NPs to brain health, figure out research areas where further studies are required, and improve its bio-safety for applications in the near future, how TiO2 NPs interact with the brain is investigated in this review by summarizing the current researches on neurotoxicity induced by TiO2 NPs.
Keywords: Titanium dioxide, TiO2, Anatase, Rutile, Nanoparticles, Brain, CNS, Neurotoxicity
Review
Introduction
Nanomaterial, with one dimension in the range of 1 to 100 nm at least, possesses unique physicochemical [1], optical [2], and electrical properties [3]. Because of its peculiar features, nanomaterial is widely applied in cosmetics [4], food and personal care products [5], medical devices [6], and so on. As nanotechnology is advancing rapidly, more concerns on health risks about exposure to nanoparticles have been arising. The TiO2 particles are believed to possess low toxicity and thus are widely used in biomedical applications for their excellent biocompatibility [7–9]. However, when the size of TiO2 is diminished to nanoscale, the bioactivity and physiochemical properties of nano-sized TiO2 are significantly different from the properties of their bulk analogue. As a consequence, the toxic effects of TiO2 NPs on human beings could not be simply determined by traditional methods. What’s more, the understanding about the risk assessments of NPs is insufficient and often lags behind their rapid advancement and widespread applications [10–13]. TiO2 NPs-containing products are widely used as well, which could unintentionally lead to human exposure and environmental pollution. The TiO2 NPs might be potentially absorbed mainly through inhalation, indigestion, and skin penetrations into the circulation of human beings. And then they may be redistributed into other tissues (such as the liver, heart, lung, etc.), which could induce impairments on organs after unintentional exposure. As the concerns about unintentional exposure of NPs on human beings arise, an increasing number of researches have been performed to study the potential toxic effects of TiO2 NPs in recent years. Several in vivo researches adopted rats or mice for the experimental models, and they were exposed to TiO2 NPs for bio-safety assessment. The main administration routes in in vivo studies included inhalation [14], intratracheal [15] or nasal instillation [16], oral gavage and dermal exposure [17], intragastric feeding [18], intraperitoneal [19], and intravenous injection [15]. Numerous reports have revealed that when the TiO2 NPs were administrated and transported into second targets, they could induce renal fibrosis, change cell cycle of lung epithelial cell, disturb the metabolism of hepatocytes, and impair the spleen [20–23].
The central nervous system (CNS), including the brain and spinal cord, is an extremely important system for human beings. Its functions are mainly composed of the following: (1) to transfer, store, and process information; (2) to generate a variety of psychological activities; and (3) to command and control all the behaviors of human beings. Several in vivo studies have investigated the bio-distribution of TiO2 NPs. After rats or mice were exposed to TiO2 NPs, the NPs were capable of reaching most parts of the brain zones, and the Ti contents in the brain were increased. The main pathways for TiO2 NPs to be transported into the brain included (1) the blood–brain barrier (BBB) pathway [24], (2) the olfactory nerve translocation pathway [25], and (3) the placental barrier pathway [26]. However, the processes of translocation of TiO2 NPs into the brain would be regulated by several parameters, such as administration routes, size, and surface modification. Once the TiO2 NPs were transported into the brain regions, major cells in the CNS, including the neurons and the glial cells, would be affected by NPs. Reactive oxygen species (ROS), apoptosis, and inflammation would be induced by TiO2 NPs, which may lead to cell death and disturb the CNS functions or even induce neurodegenerative disease. Some in vitro studies also indicated that when neurons or glial cells were incubated with TiO2 NPs, the viability, cell cycle, cell morphology, antioxidant capability, and cellular components would be affected [27–30].
However, current knowledge about neurotoxicity induced by TiO2 NPs is insufficient and more detailed and standardized researches are needed. Therefore, in order to fully understand the potential risks of TiO2 NPs to brain health, figure out research areas where further studies are required, and improve its bio-safety for applications in the near future, how TiO2 NPs can be translocated into the brain and how they influenced the CNS function are investigated in this review via summarizing relevant in vivo and in vitro researches.
The main routes of TiO2 NPs into the brain
Due to peculiar physicochemical properties of NPs [31], TiO2 NPs are widely used in many fields, such as photovoltaic appliances [32], sensors [33], renewable energy devices [34], functional building blocks [35], textiles [10], sunscreens [11], food [13], and medical applications [36]. These widespread applications, however, put humans at a high risk of getting exposed to TiO2 NPs, probably through inhalation, ingestion, skin penetration, medical applications, and so on. Therefore, it is urgent to evaluate the bio-safety of TiO2 NPs at length. The CNS is an extremely important system for human beings. Numerous studies have already demonstrated that once NPs were absorbed, they were able to be transported to the second targets, including the brain, liver, lung, spleen, and so on. In in vivo studies, after the experimental rodents were exposed to TiO2 NPs, these NPs can be transported into the brain regions mainly through the following routes.
Translocation of TiO2 NPs from the blood to the brain
The BBB is an effectively protective structure, which is mainly composed of endothelial cells, astrocytes, and pericytes [37]. The endothelial cells are connected with each other through complicated tight junctions, while the connections are supported by the astrocytes and pericytes. On account of this sophisticated structure, only specific substances with small size or low-molecular weight (MW) could be allowed to pass through the BBB by means of three main transport patterns (passive diffusion, active transport, and endocytosis). In another word, BBB is capable of protecting the healthy and functional CNS from being affected by harmful chemicals, toxins, and drugs in the circulatory system. Whereas, NPs possess unique chemical–physical characteristics and tiny size which make them be similar to biomolecule. Therefore, they are able to pass through the BBB and enter into the CNS [38–40] (Fig. 1). On the other hand, the permeability of BBB can be altered by NPs, which could assist in influx of exogenous substances into the brain. As a result, NPs induced inflammation, edema, and cell injury or even cell death in brain regions.
Fig. 1.
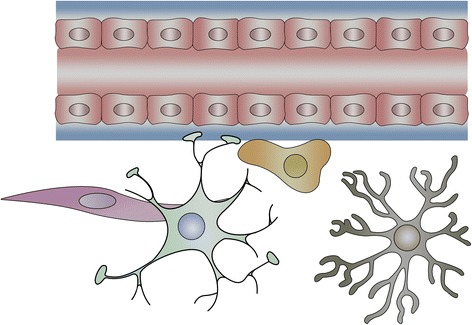
A diagram of the blood–brain barrier structure
In this in vivo study [41], TiO2 NPs with 3 nm diameter were repeatedly administrated on mice through intratracheal instillations in a chronic way. After 4 weeks, compared with the control group, the percentage of brain-to-body weight was downregulated. Through histopathological examination, inflammatory cell aggregation and cell necrosis were presented in the brain zones. The amount of Ti in the brain was upregulated measured by inductively coupled plasma mass spectrometry (ICP-MS). These results indicated that (1) after mice were intratracheally instilled with TiO2 NPs, those NPs were transported into the blood, then they passed through the BBB, and finally accumulated in the brain. (2) TiO2 NPs which were transported into the CNS could induce its injury. In another research [42], rats were treated with TiO2 NPs suspension of different diameters (10, 20, and 200 nm) through aerosol inhalation. Seventy two hours later, TiO2 NPs with diameters of 10 and 20 nm were both transported into the brain, inducing cerebral injury in a dosage-dependent way. However, TiO2 NPs with diameter of 200 nm did not cause any significant changes in the brain. From these results, we could infer that the ability of passing through the BBB for TiO2 NPs was associated with the nanoparticle diameter.
TiO2 NPs could not only pass through the BBB but also disrupt the integrity of the BBB. The toxic effects of early, acute, and long-term exposures of TiO2 NPs on the BBB were investigated on the basis of an in vitro BBB model. The model, mimicking the specific characteristics of an in vivo one [43], was composed of rat primary endothelial cells (BECs) and astrocytes. After the BBB model was treated with TiO2 NPs for acute or long-term exposure, the expression levels of P-glycoprotein (P-gp), claudin 5, caveolin-1, and caveolin-2, which regulated the integrity of BBB, were reduced. These results indicated that direct harmful impacts of TiO2 NPs on BBB integrity were presented during the acute and long-term exposure. While in this study, authors still discovered that after exposure for 4 h the mRNA expression levels of CXC chemokines, CC chemokines, ADAM17, Ccl2, Tgfβ1, ICAM, and VCAM were increased, paralleled by the decreased mRNA expression levels of ABC transporters. The upregulated expression levels of those target genes were reported to be related with the decreased permeability of BBB [44–50], which could facilitate the transportation of other exogenous substances into brain. These findings meant that besides direct impairments, the indirect harmful impacts (inflammatory effects) of TiO2 NPs on BBB integrity also occurred. On the other hand, if the NPs were eliminated from the brain slowly, they might induce long-term adverse effects after the exposure.
Axonal translocation of TiO2 NPs from the nose to the brain
Axonal transport is defined as the process that proteins and other substances synthesized in neurosome which are transported to the nerve endings through the cytoskeleton [51]. However, some low-molecular weight or small-size substances such as NPs could be taken up by the nerve ending, and then transported to the neurosome. This process is called retrograde axonal transport [52]. The olfactory and trigeminal nerve endings are abundant in the nasal areas. Once NPs were instilled through the nose, they can enter the circulation system and pass through the BBB, or they can bypass the BBB to be transported into the brain regions along the axons. The second direct pathway is reported to be the major route for NPs of being transported to the brain zones after intranasal instillation (Fig. 2).
Fig. 2.
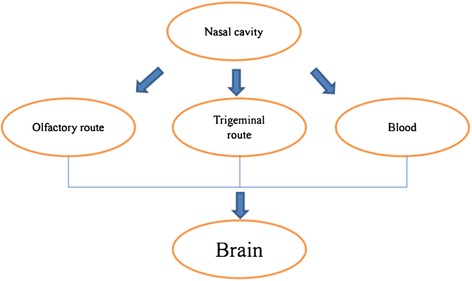
A simple diagram of nose–brain pathway after intranasal administration
Wang et al. [16] confirmed that after the female mice were exposed to TiO2 NPs of different sizes with two crystal types (80 nm for rutile and 155 nm for anatase) through intranasal instillation, the Ti concentration was significantly increased in the brain as compared with the control. Wang et al. [25] discovered that when female mice were treated with TiO2 NPs (80 nm rutile, 155 nm anatase) through intranasal instillation every other day, the titanium content was detected in the brains after 30 days. The Ti contents determined by synchrotron radiation X-ray fluorescence (SRXRF) analysis and ICP–MS were significantly upregulated in the hippocampus, followed by the olfactory bulb, cerebellum, and cerebral cortex. These findings suggested that the TiO2 NPs could be transported to the mice brain through the olfactory bulb after intranasal instillation. The same research group conducted another study [53], which also detected Ti contents in the hippocampus, olfactory bulb, cerebellum, and cerebral cortex by ICP-MS in a time-dependent way. Both the two studies indicated that there was a high Ti content in the hippocampus, so this brain area would be easily affected by TiO2 NPs. It is generally accepted that the hippocampus is mainly in charge of memory and learning [54, 55]. Therefore, impairments on it might probably induce neurodegenerative diseases, such as Alzheimer’s diseases [56–58].
In another in vivo study [59], the female mice were treated with TiO2 NPs by intranasal instillation. The experimental mice were divided into four groups according to different sizes, coatings, and shapes of the TiO2 particles. Groups A and B shared the same insoluble property, but with various size (micro- and nano-sized TiO2 particles) and no surface coatings. Groups C and D were hydrophilic NPs and silica-coated with different shapes. After treatment every other day for 30 days, the titanium contents in the brain were determined by ICP-MS. These data demonstrated that groups C and D showed higher Ti concentration in the cerebral cortex and striatum than groups A and B, while group A was detected with no Ti content in the sub-brain zones. These results indicated that the size, surface modification, and shape of TiO2 NPs played an important role on their transport to the brain from the nose. In this study [60], CD-1 (ICR) female mice were exposed to TiO2 NPs with different dosages (2.5, 5, and 10 mg/kg body weight). After nasal administration for 90 consecutive days, the TiO2 NPs were detected in the brain, and this accumulation could induce CNS dysfunctions.
Translocation into the brain of offspring through the placental barrier
Placental barrier, composed of both maternal and fetal tissues, is another internal barrier that can protect the development of embryo [61]. It could protect the fetus from being affected by harmful substances in maternal blood circulation, while the fetus could get nutrients and oxygen from the mother via the placenta (Fig. 3). However, a great number of studies [62, 63] have already revealed that after pregnant mice/rats were exposed to exogenous substances, such as nanoparticles, those substances could be detected in the brain of fetus, and then they can disturb the homeostasis of CNS or even induce neuronal death. Those harmful impacts on fetus brain have been demonstrated to be related with psychiatric disorders such as autism, schizophrenia, depression, and so on in their later life [64, 65]. As a consequence, those findings suggest that placental barrier plays an important part on fetal growth and development.
Fig. 3.
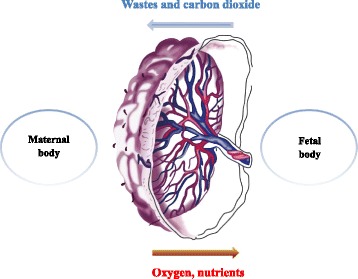
Substance exchange between the mother and fetus through placenta barrier
In this study [66], pregnant Wistar rats were administrated with TiO2 NPs intragastrically daily from gestational day 2 to 21. Then, the 1-day-old neonates were sacrificed, and the TiO2 NPs concentration in the hippocampus, determined by ICP-MS, was significantly upregulated as compared with the control group. Yamashita et al. [67] also detected silica (70 nm) and TiO2 (35 nm) NPs in the placenta and fetal brain after the pregnant mice were injected intravenously with these NPs, which led to the pregnancy complication.
In another study [68], the authors discovered that when pregnant ICR mice were treated with TiO2 NPs, the levels of dopamine and its metabolites were increased in some regions of the fetus brain on postnatal day 21 as compared with the control group. Another study [69] adopted microarray to assess gene expression changes in the brains of male fetus and pups after the pregnant ICR mice were exposed to TiO2 NPs. Data showed that the expression levels of genes related with dopamine neuron system were altered. Microarray was also applied in this study [70], and pregnant ICR mice were administrated with anatase TiO2 NPs. By analyzing the gene expression alternations in the brains of male fetus and pups, data obtained revealed that the expression levels of genes related with oxidative stress, neurotransmitters, and psychiatric diseases were dysregulated. The neurobehavioral performance of the offspring might be moderately altered due to maternal exposure to TiO2 NPs [71]. Similarly, Cui et al. [72] discovered that when Sprague–Dawley rats were injected subcutaneously with TiO2 NPs, the antioxidant ability of pups’ brain was impaired. Although these researches did not measure the contents of TiO2 NPs in the brain directly, those data collected indirectly demonstrated that the TiO2 NPs in maternal circulation system would affect the development of embryo. Then, they could impair the brain development and finally lead to CNS dysfunctions in their later life.
Bio-distribution and elimination of TiO2 NPs from the brain
When TiO2 NPs were absorbed into circulation, they were capable of being redistributed to second organs (Fig. 4). At present, several researches have been performed to study the bio-distribution of TiO2 NPs after administrations (Table 1). In this study [73], when rats were treated with TiO2 NPs (5 mg/kg body weight) by intravenous injection, TiO2 NPs can be detected in the liver, spleen, lung, and kidney except blood cells, plasma, brain, and lymph nodes. The BALB/c female mice were exposed to TiO2 NPs at a dose of 560 mg/kg by intravenous injection (i.v.) or 5600 mg/kg by subcutaneous injection (s.c.). The TiO2 NPs were detected by energy dispersive X-ray spectroscopy (EDS) in the lung, liver, lymph node, spleen, and kidney from i.v.-administrated mice but only in the liver, lymph node, and spleen of s.c.-administrated mice, while the content of NPs was not detected in the brain [74]. Another study [75] also did not detect TiO2 NPs in the brain of male mice except blood and liver after i.v. injection. However, after hairless mice were treated with TiO2 NPs (21 nm) by dermal exposure for 60 days, significant pathological alterations were presented in the skin and liver and the NPs were also detected in the brain without pathological changes [76]. Wang et al. [16] studied the bio-distribution of TiO2 NPs (50 mg/kg) after female mice were treated with NPs by intranasal instillation every other day for 30 days. The biochemical parameters of the liver, spleen, heart, and serum were not affected by NPs as compared with the control group; while the concentration of NPs was apparently enhanced in the lung and brain regions. Another study investigated the bio-distribution of TiO2 NPs after rats were repeatedly orally administrated for 13 weeks. Even in the highest dosage group (1041.5 mg/kg BW), the Ti content in the brain was minimal with no statistical significance. Geraets et al. [77] compared the different distributions of TiO2 NPs in rats after oral and intravenous administration. The data obtained demonstrated that the Ti concentrations were not detectable in tissues, including the brain after oral administration. However, the Ti contents were detected in the liver, spleen, kidney, lung, heart, brain, thymus, and reproductive organs after intravenous injection. It could be inferred from those studies that (1) intranasal instillation might be the most effective routes for TiO2 NPs transported to the brain and (2) Ti content could be undetectable in the brain regions after intravenous injection. Undoubtedly, those conclusions drawn from abovementioned in vivo researches might not be convincing, because the translocations of TiO2 NPs into the brain would be influenced by several parameters, such as administration routes, size, dosage, and so on, which would be discussed in later chapters.
Fig. 4.
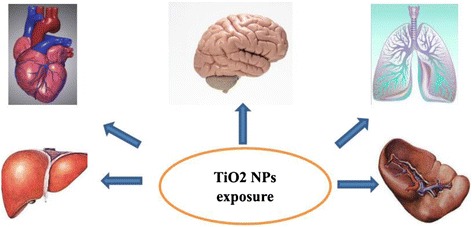
A simple diagram of bio-distribution of Ti after TiO2 NPs exposure
Table 1.
Bio-distribution of TiO2 NPs after rat/mice were administrated by different routes
| Crystal type | Animal | Administration | Parameters/dose | Bio-distribution | Reference |
|---|---|---|---|---|---|
| Both anatase and rutile forms (70/30) | Male Wistar rats | Intravenous injection | 20–30 nm; no surface coating; 5 mg/kg body weight (BW); single injection | Liver, spleen, lung, and kidney detected; blood cells, plasma, lymph nodes, and brain not detected | [73] |
| Both anatase and rutile forms (80/20) | BALB/c female mice | Subcutaneous (s.c.) injection | Hydrodynamic diameters ranging from 114 to 122 nm; 5600 mg/kg BW; 2 consecutive days | Liver, lymph node and spleen detected; brain not detected | [74] |
| Intravenous (i.v.) injection | Hydrodynamic diameters ranging from 114 to 122 nm; 560 mg/kg BW; 2 consecutive days | Lung, liver, lymph node, spleen and kidney detected; brain not detected | |||
| Rutile | Male mice | Intravenous injection | Primary particle diameter 15 nm, secondary particle size 120 nm; 1813 μg/animal | Liver, kidney, blood detected; brain not detected | [75] |
| Rutile | CD-1 (ICR) female mice | Intranasal instillation | 80 nm; 50 mg/kg BW; every other day for 30 days | Lung and brain detected; liver, heart, and spleen not detected | [16] |
| Anatase | 155 nm; 50 mg/kg BW; every other day for 30 days | ||||
| Degussa P25 | Rats | Intravenous administration | 21 nm; spherical; 0.95 mg/kg BW; single injection | Liver (highest), spleen, lung, kidney, heart and blood detected; brain not detected | [113] |
| Anatase 80: 20 rutile | Sprague–Dawley rats | Oral administration | 21 nm; spherical; 260.4, 520.8, and 1041.5 mg/kg/day BW; every day for 13 weeks (7 days/week) | Low absorption in other organs and brain not detected | [78] |
| Anatase | Male Kunming mice | Inhalation exposure | 20 nm; steady concentration (6.34 ± 0.22 mg m−3); 8 h per day for 3 weeks | Lungs, liver, blood, and urine detected; kidney and brain not detected | [114] |
Although TiO2 NPs are capable of entering into the brain regions through specific routes by a variety of delivery ways, the capability of excretion would keep the brain from being affected by NPs. But few researches about the elimination of TiO2 NPs from the brain were published. In Cho et al.’s study [78], when the Sprague–Dawley rats were administrated with TiO2 NPs by intravenous injection (a single or repeated dosage of 1 ml for 5 consecutive days), the contents of NPs were determined on days 2/6, 14, 30, and 90 after administration. The Ti contents were detected in the liver (the highest), spleen, kidney, lung, heart, brain, thymus, and reproductive organs on day 2/6. During the observation period, Ti concentrations in the feces and urine demonstrated no increase as compared with control group. Also, Ti contents in the brain demonstrated no detectable alterations.
It can be inferred from the results that (1) the excretion of TiO2 NPs from the brain is limited and (2) even negligible dosage of TiO2 NPs could be accumulated in the brain with minimal elimination, so chronic or long-term exposure might be potentially risky for the brain health. TiO2 NPs are thought to be low toxic. However, the long-term, repeated exposure, and durative existence of TiO2 NPs in the brain regions made it necessary to re-evaluate their bio-safety. Therefore, more in vivo researches are needed to further investigate the parameters of TiO2 NPs which might influence their elimination rates from the brain. Then, the health risks of TiO2 NPs to the brain are expected to be lowered.
Neurotoxicity of titanium dioxide nanoparticle
Once the TiO2 NPs are translocated into the CNS through the abovementioned pathways, they may accumulate in the brain regions. For their slow elimination rates, those NPs could remain in the brain zones for a long period, and the Ti contents would gradually increase with repeated exposure. This would induce pathologic changes, such as inflammation, immunological response, edema, cell injury, cell necrosis, and so on, which would ultimately lead to CNS dysfunctions, including neurodegenerative diseases and psychiatric disorders. Generally, the neurons and glial cells are the main cell types in the CNS. Therefore, the toxic effects of TiO2 NPs on them would lead to impairments on the brain as a consequence.
Toxic effects on CNS in in vivo studies
Several in vivo studies have demonstrated that the TiO2 NPs could be transported into the brain regions, and then accumulated in the CNS, eventually leading to CNS dysfunctions (Table 2).
Table 2.
Toxic effects of TiO2 NPs on CNS in in vivo studies
| Crystal type | Animals | Cell type | Parameters/dose | Main findings | Reference |
|---|---|---|---|---|---|
| Rutile 80 nm anatase 155 nm | CD-1 (ICR) female mice | Nasal instillation | 500 μg; every other day for 30 days | Ti contents detected in the brain; GFAP-positive cell, CAT, SOD, MDA, protein carbonyls, AChE activities, glutamic acid, and NO increased | [25] |
| Anatase bulk | CD-1 (ICR) female mice | Delivered to the abdominal cavity | 5 nm; 5, 10, 50, 100, 150 mg/kg BW; every day for 14 days | Ti contents detected in brain; O2, H2O2, MDA, NOS, NO increased; Glu contents, antioxidative enzymes, non-enzymatic antioxidant contents, and AChE activity decreased | [79] |
| Anatase | CD-1 male mice | Intranasal administration | 5–6 nm; 2. 5, 5, 10 mg/kg BW. every day for 90 days | Ti contents detected in brain; no daily behavioral changes; O2, H2O2, MDA, protein carbonyl, 8-OHdg, p38, JNK, NF-κB, Nrf-2, and HO-1 increased | [80] |
| Anatase | Sprague–Dawley rats (male and female) | Subcutaneous injection | 5 nm; 500 μl (1 μg/μl) on GD 6, 9, 12, 15, and 18 | CAT, GSH-PX, and T-AOC decreased; MDA and 8-hydroxydeoxyguanosine (8-OHdG) increased | [72] |
| Rutile 80 nm anatase 155 nm | CD-1 (ICR) female mice | Intranasal instillation | 500 μg; every other day for 30 days; evaluated at 2, 10, 20, and 30 days of post-instillation time points | Ti contents detected in brain; GSH-Px, GST, SOD and GSH not changed; MDA, TNF-α and IL-1β increased | [53] |
| Anatase | CD-1 (ICR) female mice | Intranasal administration | 5–6 nm; 2.5, 5, 10 mg/kg BW; every day for 90 days | TLR2, TLR4, TNF-α, IKK1, IKK2, NF-κB, NF-κBP52, NF-κBP65, NIK, and IL-1β increased; spatial recognition memory and locomotor activity affected | [81] |
| Rutile | Male C57BL/6 mice | Intraperitoneal injection | Fine (<1 μm), ultrafine (21 nm); 40 mg/kg BW; one injection 30 min after LPS or vehicle injection | IL-1β, TNF-α, iNOS, ROS production, and OX-42 enhanced by ultrafine TiO2 in the LPS-stimulated group | [82] |
| Anatase | CD-1 (ICR) female mice | Intragastric administration | 6.5 nm; 5, 10, 50 mg/kg BW; every day for 60 days | Ti contents in the hippocampus increased; caspase-9, caspase-3, Bax, cytochrome c, O2 and H2O2 upregulated; Bcl-2, SOD, CAT, APx, and GSH-Px reduced | [83] |
| Anatase | CD-1 female mice | Nasal administration | 5–6 nm; 2.5, 5, 10 mg/kg BW; every day for 90 days | NR2A, NR2B, CREB-1, CREB-2, FosB/DFosB, CaMKIV, and pCREB decreased | [85] |
| Anatase | CD-1 female mice | Intragastric administration | 5 nm; 5, 10, 50 mg/kg BW; every day for 60 days | Ti contents in brain upregulated; reduction in the activities of Na+/K+-ATPase, Ca2+-ATPase, Ca2+/Mg2+-ATPase; Ache, Glu, and NO elevated; NE, DA, DOPAC, 5-HT, and 5-HIAA reduced | [86] |
| Anatase | Pregnant ICR mice | Subcutaneous injection | 2570 nm; 100 μg, injection on GD 6, 9, 12, 15 | Genes related with cell death, apoptosis, oxidative stress, inflammation and neurotransmitters changed | [70] |
| Rutile | Pregnant BALB/c mice | Intravenous injection | 35 nm; 0.8 mg, injections on GD 16 and 17 | Lower uterine weights and smaller fetuses; fetal resorption and retarded fetal growth | [67] |
| Anatase | Pregnant Wistar rats | Intragastric administration | 10 nm; 100 mg/kg BW, every day from GD 2 to 21 | Ti contents elevated and Ki-67-positive cells reduced; learning and memory in offspring disrupted | [66] |
Wang et al. [25] revealed that after the female mice were treated with TiO2 NPs (80 nm, rutile and 155 nm, anatase; 500 μg) through intranasal instillation every other day for 30 days, the Ti contents were detected in the brain regions, including the olfactory bulb, hippocampus, cerebellum, and cerebral cortex. This accumulation induced increased glial fibrillary acidic protein (GFAP)-positive cell, CAT activity, MDA content, protein carbonyls content, AChE activities, glutamic acid, and NO content, accompanied by cell lost in both experimental groups. While, SOD level was increased only in the 155 nm group. In another study [79], the ICR female mice were injected with TiO2 NPs (anatase; 5 nm; 5, 10, 50, 100, 150 mg/kg BW) into the abdominal cavity every day for 14 days. The coefficients of the brain-to-body weight, antioxidative enzymes (SOD, CAT, APx, GSH-Px), non-enzymatic antioxidant contents (ASA/DASA,GSH/GSSG), Glu contents, and AChE activity were decreased, but the Ti contents in the brain, the levels of O2, H2O2, MDA, NOS, and NO were increased in a dose-dependent way. Ze et al. [80] discovered that when CD-1 male mice were intranasally treated with TiO2 NPs (5–6 nm; 2.5, 5, 10 mg/kg BW) every day for 90 days, the mRNA expressions of genes regulating oxidative stress, including p38, JNK, NF-κB, Nrf-2, and HO-1, were increased besides upregulated Ti concentrations and levels of O2, H2O2, MDA, protein carbonyl, and 8-OHdg,.
However, in another study [53], after the CD-1 (ICR) female mice were exposed to TiO2 NPs (rutile 80 nm and anatase 155 nm) every other day for 30 days by intranasal instillation, the activities of GSH-Px, GST, SOD, and GSH level in the brain were not changed at 30 days. Yet, the level of MDA was enhanced all the same. The Ti contents were highest in the hippocampus, followed by olfactory bulb, cerebellum, and cerebral cortex. However, the levels of TNF-α and IL-1β in brain were upregulated in the 155 nm group, which indicated that intranasal instillation with anatase TiO2 NPs would induce inflammation in the brain of mice. Ze et al. [81] also demonstrated that TiO2 NPs could induce inflammation in mice brain. In their study, the mRNA and protein levels of Toll-like receptor (TLR)2, TLR4, TNF-α, IKK1, IKK2, NF-κB, NF-κBP52, NF-κBP65, NIK, and IL-1β in the brain were enhanced after the female mice were treated with TiO2 NPs (5–6 nm; 2.5, 5, 10 mg/kg BW) every day for 90 days. However, the mRNA expression and protein level of IκB were downregulated significantly. Moreover, the spatial recognition memory and locomotor activity were impaired mostly due to the inflammation response to TiO2 NPs. In this study [82], ultrafine TiO2 (21 nm, 40 mg/kg BW, one injection 30 min after vehicle administration) could not induce detectable impairments on the brain when the male C57BL/6 mice were exposed by intraperitoneal injection. However, when mice were pretreated with lipopolysaccharide (LPS), the mRNA expression levels of IL-1β, TNF-α, and iNOS in the cortex and hippocampus were markedly upregulated at 2 h after LPS injection, accompanied by significantly increased protein level of IL-1β at 6 h after the usage of LPS pretreatment in the LPS-stimulated group. The ROS production and the expression of OX-42 were significantly increased in the cortex and hippocampus by ultrafine TiO2 at 24 h after LPS injection in the LPS-treated group. It was inferred from these findings that ultrafine TiO2 could augment the damage in the pre-inflamed but not the healthy brain.
Hu et al. [83] revealed that after CD-1 (ICR) female mice were exposed to TiO2 NPs (6.5 nm; 5, 10, 50 mg/kg BW) by intragastric administration every day for 60 days, the expressions of apoptosis-related genes were affected. The mRNA and protein expression levels of caspase-9, caspase-3, Bax, and cytochrome c were upregulated with downregulated level of Bcl-2. However, the caspase-8 level was not affected, which indicated that this apoptosis of the hippocampus induced by TiO2 NPs might result from intrinsic pathway. Moreover, levels of O2 and H2O2 were increased and the activities of SOD, CAT, APx, and GSH-Px were downregulated. The ratios of AsA to DAsA and GSH to GSSGG were decreased as well. These results indicated that the antioxidant capabilities of the brain were impaired by TiO2 NPs. The time spending exploring the novel arm was significantly reduced in the experimental groups as compared with the control one, which inferred that the spatial recognition memory of mice was impaired for the apoptosis of neurons in hippocampus. In this study [84], when Wistar rats were injected intravenously with nano-TiO2 (21 nm; 5, 25, and 50 mg/kg BW) once a week for 4 weeks, the Ti contents were detected in the brain regions. Moreover, oxidative stress was induced, which led to inflammation and changed levels of neurotransmitters. Ultimately, mitochondria-mediated apoptosis were found.
Although low-dose exposure could not induce any acute neurotoxicity, chronic exposure to low dose TiO2 NPs might lead to dramatic damage to the brain. In Ze et al.’s study [85], the CD-1 female mice were treated with TiO2 NPs (5–6 nm; 2.5, 5, 10 mg/kg BW) every day for 90 days. The histopathological changes, including rarefaction of glial cells, dispersive replication of pyramidal cells, and reduced size of cell volume, were presented in the hippocampus. The neuronal ultrastructure was found to be affected as well, such as mitochondrial swelling and nuclear membrane collapse. TiO2 NPs-treated mice learned the training task more slowly than the control group and showed apparently downregulation of LTP amplitudes of fEPSP, which were consistent with the reduction in mRNA and protein levels of NR2A, NR2B, CREB-1, CREB-2, FosB/DFosB, and CaMKIV. These results suggested that the spatial recognition memory in mice should be impaired, due to long-term exposure of low-dose TiO2 NPs. Spatial recognition memory could be influenced by disturbance of the trace elements in mice’ s CNS due to chronic exposure to TiO2 NPs as well. In this study [86], CD-1 female mice were exposed to TiO2 NPs (5 nm; 5, 10, 50 mg/kg BW) every day for 60 days by intragastric administration. The Ti concentrations in the brain were upregulated in a dose-dependent way. The concentrations of Ca2+ and Na+ were markedly elevated, accompanied by decreased levels of Mg2+, K+, Zn2+, and Fe3+, all of which were consistent with reduction in the activities of Na+/K+-ATPase, Ca2+-ATPase, and C3a2+/Mg2+-ATPase of the brain. The levels of Ach, Glu, and NO were elevated, while the contents of NE, DA, DOPAC, 5-HT, and 5-HIAA were reduced. The disturbance in such elements, neurotransmitters, and enzymes may potentially impair the spatial recognition memory of experimental mice. In another research [87], male Wistar rats were intraperitoneally administrated with TiO2 NPs (20 mg/kg body weight) every 2 days for 20 days. This subacute exposure changed neurobehavioral performance of rats.
As mentioned above, TiO2 NPs are able to be translocated through the placenta barrier, influencing the fetal development. The fetal brain is another target of TiO2 NPs. Shimizu et al. [70] adopted DNA microarrays to determine altered genes expressions in the brain of male mice fetuses (ED 2) and pups (PD 2, 7, 14, 21) after their mothers were injected subcutaneously with TiO2 NPs (2570 nm; 100 μg, injection on gestational day (GD) 6, 9, 12, 15). They used GO category and MeSH term to analyze the microarray dataset and discovered that those changed genes were related with cell death, apoptosis, oxidative stress, inflammation, and neurotransmitters in the brain. This suggested that maternal exposure to TiO2 NPs would affect the brain development of fetuses and pups of mice. Cui et al. [72] found out that when pregnant Sprague–Dawley rats were exposed to TiO2 NPs (5 nm; 500 μl (1 μg/μl)) on gestational day (GD 6, 9, 12, 15, 18), the antioxidant capabilities of the brain in their pups would be affected. The levels of CAT, GSH-PX, and T-AOC in newborn pups were downregulated, while the level of MDA was enhanced in the experimental groups. The 8-hydroxydeoxyguanosine (8-OHdG) content was also upregulated, which might indicate that the maternal exposure to TiO2 NPs could induce oxidative stress to the nucleic acids in the fetal brain. Moreover, behavioral tests were finished during postnatal day (PD) 40 to 44. The experimental groups spent less time exploring the novel object and consumed less sucrose water. The time of immobility in the force swimming test was increased. These results inferred that the impairments in the brain of the offspring due to prenatal exposure to TiO2 NPs could affect the antioxidant capabilities of the newborn pups’ brain, leading to depressive-like behaviors during adulthood. In this study [66], when pregnant Wistar rats were treated with TiO2 NPs (10 nm; 100 mg/kg BW) every day from GD 2 to 21, the Ti contents were significantly elevated and the number of Ki-67-positive cells were reduced significantly in the hippocampus of PD 1 newborn as compared with the control group. Moreover, the learning and memory in offspring (PD21, PD60) were markedly disrupted. Yamashita et al. [67] had also demonstrated that after pregnant BALB/c mice were exposed to TiO2 NPs (35 nm, 0.8 mg) by intravenous injection on GD 16 and 17, Ti contents were detected in the placenta and fetal brain. Their uterine weights and fetuses sizes were reduced as well, leading to retarded development of the fetal brain.
Toxic effects on CNS in in vitro studies
In addition to in vivo studies, several in vitro researches (Table 3) investigated the neurotoxicity of TiO2 NPs as well. Xue et al. [88] treated primary microglia derived from Sprague–Dawley rats with 0.25 or 0.5 mg/ml TiO2 NPs (20 nm) for 24 or 48 h. The NO production was remarkably elevated, accompanied by increased mRNA and protein levels of iNOS. And the mRNA expressions of MCP-1 and MIP-1α were significantly enhanced in the experimental group as well. NF-κB binding activity was also increased markedly. However, the mRNA expression level of Th was significantly inhibited by TiO2 NPs. When PC12 cells were co-incubated with supernatant of TiO2 NPs-treated microglia for 48 h, the viability of PC12 cells, measured by MTT assay, was markedly reduced. In another study [89], PC12 cells were co-incubated with different concentrations of TiO2 NPs (range from 20 to 50 nm; 1, 10, 50, 100 μg/ml) for different times (6, 12, 24, 48 h). After 6 h incubation, only the 100 μg/ml TiO2 NPs-treated group showed significant reduction in cell viability. However, the viability of PC12 cells was decreased in all experimental groups except the 1 μg/ml after 12-, 24-, and 48-h incubation. Moreover, as the concentrations of TiO2 NPs increased, the percentage of dihydrodichlorofluorescein (DCF)-positive cells was elevated. Both the 10 and 50 μg/ml TiO2 NPs-treated groups demonstrated that there was an increased ratio of cell apoptosis after 24 h incubation. But pre-treated with N-MPG (ROS scavenger) could ameliorate the harmful effects on PC12 cells induced by TiO2 NPs. Wu et al. [90] also adopted PC12 cells as an model of dopaminergic neurons to study the neurotoxicity caused by TiO2 NPs on CNS. The PC12 cells were co-incubated with different concentrations of anatase or rutile TiO2 NPs (20 nm; 25, 50, 100, 200 μg/ml) for 24 h. The two crystal types induced apparent cytotoxicity. However, anatase TiO2 NPs led to significant lactate dehydrogenase (LDH) leakage at all concentrations. It induced significant elevation in DCF fluorescence intensity in 200 μg/ml anatase group, which was higher than rutile group with the same concentration. The levels of GSH and SOD were decreased by anatase (50, 100, 200 μg/ml) and rutile (200 μg/ml) TiO2 NPs, while the level of MDA was elevated by anatase (100, 200 μg/ml) and rutile (200 μg/ml) TiO2 NPs. Furthermore, the reduction in mitochondrial membrane potential in anatase (200 μg/ml) type was higher than that in rutile (200 μg/ml) one. Cell apoptosis and necrosis were also presented in TiO2 NPs-treated groups at the concentration of 200 μg/ml. The elevated percentage of G2/M phase cells was caused by anatase (100, 200 μg/ml) and rutile (200 μg/ml) TiO2 NPs as well. The protein levels of p-JNK, JNK, p-c-Jun, Jun, p-P53, p53, p21 GADD45, Bcl-2, and Bax were disrupted, too. The activity of caspase-3 was increased by anatase (50, 100, 200 μg/ml) and rutile (200 μg/ml) TiO2 NPs. Those changes might contribute to apoptosis or necrosis and cell cycle arrest in PC12 cells. In this study [91], primary hippocampal neurons from 1-day-old Sprague–Dawley rat were incubated with TiO2 NPs (approximate 5 nm; 5, 15, 30, 40, 50 μg/ml) for 6, 12, 24, or 48 h. The cytotoxicity, determined by MTT assay, demonstrated that the cell viabilities were reduced in a time-dependent and dose-dependent way. The LDH activity was significantly enhanced as well. Observed by TEM, it is revealed that changes of the ultrastructure of cells related with apoptosis were presented in cytoplasm and the apoptotic rate was elevated assessed by TUNEL method. Mitochondrial membrane potential (MMP) was markedly reduced, suggesting mitochondrial impairments. The Ca2+ concentration in cytoplasm was also apparently increased. Apoptotic cytokine levels, including cytochrome c, caspase-3, Bax, caspase-12, GRP78, and CHOP, were markedly enhanced with significant reduction in Bcl-2 level.
Table 3.
Toxic effects of TiO2 NPs on CNS in in vitro studies
| Crystal type | Cell type | Parameters/dose | Main findings | Reference |
|---|---|---|---|---|
| Unknown | Primary microglia from Sprague–Dawley rats and PC12 | 20 nm; 0.25 or 0.5 mg/ml; 24 or 48 h | NO, iNOS, MCP-1, MIP-1α, and NF-κB binding activity increased and Th inhibited in microglia; marked cytotoxicity in PC12 after incubation with supernatant of NPs-treated microglia | [88] |
| Anatase | PC12 | Average 21 nm (range from 20 to 50 nm); 1, 10, 50, 100 μg/ml; incubated for 6, 12, 24, 48 h | Viability of cells decreased except 1 μg/ml group; DCF-positive cells and ratio of PC12 apoptosis elevated | [89] |
| Anatase and rutile | PC12 | 20 nm; 25, 50, 100, 200 μg/ml for 24 h | Apparent cytotoxicity; GSH, SOD, and mitochondrial membrane potential decreased; MDA and G2/M phase cells elevated; p-JNK, JNK, p-c-Jun, Jun, p-P53, p53, p21 GADD45, Bcl-2, and Bax disrupted | [90] |
| Anatase (96 %) | C6 U373 | 40–200 nm; 2.5, 5, 10, 20, 40 μg/ml; 24, 48 or 96 h | Apoptosis; cellular proliferation depressed; morphology and cytoskeleton changed; reduction in immune-location of F-actin fibers | [96] |
| Unknown | C6 U373 | 50 nm; 20 μg/cm2 for 2, 4, 6, 24, 48, 72 h | Imbalance in GPx, SOD and catalase; fluorescence of cis-parinaric acid and Rh123 downregulated; H2DCFDA and MitoTracker Green FM staining elevated | [97] |
| Rutile coated by SiO2 | Mouse NSCs line C17.2 | 80–100 nm; 50, 100, 150, 200, 250 μg/ml exposed for 12, 24, 36, 48, 60, 72 h, or 7 days | Inhibition on cellular proliferation; β-tubulin positive cells detected; Cx43 elevated; PKCε reduced | [99] |
| Unknown | HCECs (human cerebral endothelial cells) | 21 nm; 2 mg/ml; 0.12, 0.6, 3, 15, 75 μg/cm2 for 4, 24, 48, or 72 h | Significant cytotoxicity, ROS production, and marked DNA damage detected; cathepsin D and LC3-II upregulated | [98] |
| Anatase | Primary hippocampal neurons | 5 nm; 5, 15, 30, 40, 50 μg/ml for 6, 12, 24, or 48 h | Cell viabilities and MMP reduced; LDH activities, apoptotic rate, and cytoplasmic Ca2+ elevated; ultrastructure of cells altered; apoptotic cytokine disturbed | [91] |
| Anatase (S) Anatase (80 %) + rutile (D) | Human SHSY5Y neuronal cells | 25 nm; 20, 40, 60, 80, 100, 120, 140, 160 μg/ml for 3, 6, 24 h | No cytotoxicity; cell cycle changed; apoptotic cells elevated; genotoxicity detected; no oxidative damage | [92] |
| Anatase | Human neural stem cell line | 80 nm; 0.01, 0.1, 1 mg/ml for 7 days | Morphology changed; mitochondrial activity not changed; Nestin, neurofilament heavy polypeptide, and high mobility group AT-hook 1 elevated | [100] |
The function of human neuronal cells could be affected by TiO2 NPs. Valdiglesias et al. [92] treated human SH-SY5Y neuronal cells with anatase TiO2 NPs (TiO2-S) and TiO2 NPs (anatase (80 %) + rutile) (TiO2 NPs-D) at different concentrations (20, 40, 60, 80, 100, 120, 140, 160 μg/ml) for 3, 6, and 24 h. Cytotoxicity, assessed by MTT and NRU assays, was not induced by both types of TiO2 NPs, but NPs were apparently internalized by cells, observed by flow cytometry. Cell cycle, determined by analyzing the relative DNA content, was changed in the TiO2-S group. The elevated percentage of apoptotic cells, measured by flow cytometry, and genotoxicity, determined by Comet assay, were presented in both experimental groups. Both TiO2-S and TiO2-D induced no oxidative stress in human SH-SY5Y neuronal cells. Mao et al. [93] also employed the SH-SY5Y to investigate the neurotoxicity of TiO2 NPs. After the SH-SY5Y were exposed to TiO2 NPs (0.1, 1, 10, and 100 μg/ml), the cell viability was not affected in all groups. However, the microtubules of cells were disrupted, which contributed to the neurotoxic effects of TiO2 NPs. But a conflicting conclusion was obtained in a recent study [94]. After SH-SY5Y cell lines were treated with TiO2 NPs, the mitochondrial function was affected and cell membrane was damaged after both acute and chronic exposures. Hong et al. [95] reported that after the primary cultured hippocampal neurons were exposed to 5, 15, and 30 mg/ml nano-TiO2 for 24 h, the protein expressions of NMDAR were reduced. At the same time, the nitric oxide, nitrice synthase, and ADP/ATP ratios were upregulated. Those changes disrupted neurite outgrowth of hippocampal neurons.
As mentioned above, the glial cell could be another target of TiO2 NPs. In this study [96], C6 (rat’s glial cell) and U373 (human glial cell) were incubated with TiO2 NPs (40–200 nm; 2.5, 5, 10, 20, 40 μg/cm2) for 24, 48, or 96 h. The proliferation of C6 and U373 was depressed in a dose-dependent way after both cell lines were incubated with TiO2 NPs for 48 h. Morphological alterations of both cell lines were induced by TiO2 NPs after 96 h exposure at the concentration of 20 μg/cm2. TiO2 NPs were internalized by both cell lines after 24 h incubation which was observed by TEM. The immune-locations of F-actin fibers in C6 and U373 were observed after 24 and 96 h exposure. Those data demonstrated that the fluorescence of F-actin in C6 and U373 was decreased and the degrees of this reduction were closely related with concentration, exposure time, and cell type. Moreover, apoptosis was induced by TiO2 NPs in both cell lines, which was determined by DAPI nuclear staining. In another study [97], C6 and U373 were incubated with TiO2 NPs (50 nm; 20 μg/cm2) for 2, 4, 6, 24, 48, or 72 h. Data obtained from the research demonstrated that the H2DCFDA oxidation in both cell lines was significantly enhanced by treatment with TiO2 NPs for 2 h, reaching its maximum at 6 h, and was reduced at 24 h. The mRNA levels of antioxidant enzyme, including GPx, catalase, and SOD, were determined by RT-PCR, which demonstrated that their expressions were elevated at an early exposure time and then decreased at a later period in both C6 and U373. Reduction in the fluorescence of cis-parinaric acid and Rh123 indicated oxidation of lipids and disturbance of mitochondrial function, respectively. The mitochondrial depolarization was assessed by MitoTracker Green FM, and the staining was markedly increased in both cell lines after treatment with TiO2 NPs for 24 and 48 h. These results suggested that TiO2 NPs were able to cause oxidative stress and the mitochondrion could be impaired in both C6 and U373 glial cell lines in vitro study.
Once NPs were absorbed into the circulation system after exposure, they have to cross the BBB to enter into the brain regions. Therefore, the integrity of the BBB could be affected by NPs as well. In this in vitro study [98], human cerebral endothelial cells (HCECs) were exposed to 21-nm TiO2 NPs at different concentrations for different times. After 24 h incubation, TiO2 NPs were internalized by HCECs which were observed by TEM. Significant cytotoxicity reduction was determined by MTT assay after 72 h exposure. Carboxy-H2DCFDA demonstrated that TiO2 NPs induced apparent elevation in ROS production after 4 h treatment. Marked DNA damage was also observed in cells by Comet assay after 24 and 48 h incubation. Moreover, the levels of activated cathepsin D and LC3-II were upregulated, which indicated that TiO2 NPs induced autophagy.
The TiO2 NPs still have some positive effects on the brain in addition to neurotoxicity. In this research [99], mouse neural stem cells (NSCs) line C17.2 were incubated with TiO2 NPs (coated with SiO2; 80–100 nm; 50, 100, 150, 200, 250 μg/ml) for 12, 24, 36, 48, 60, 72 h, or 7 days to determine the effects of TiO2 NPs on the differentiation trend of neural stem cells. It was discovered that after C17.2 cells were incubated with TiO2 NPs for 7 days, the β-tubulin positive cells were obviously increased as compared with that in the control group. This finding indicated that the TiO2 NPs could induce the C17.2 differentiating into neurons. But once the HB1.F3 human neural stem cells (hNSCs) were incubated with TiO2 NPs (80 nm; 0.01, 0.1, 1 mg/ml) for 7 days, the cells were aggregated and the morphology of cells changed with no change in mitochondrial activity. The levels of proteins, linked to hNSC differentiation including Nestin (stem cell marker) and neurofilament heavy polypeptide (N-FH; neuron marker), were elevated with the increase in mRNA level of high mobility group AT-hook 1 (HMGA1) after 24 h exposure [100].
We can draw a conclusion from the abovementioned in vivo and in vitro researches that for the typical properties of TiO2 NPs, exposure to them might pose a high risk on the brain health. Molecular mechanisms underlying the neurotoxicity of TiO2 NPs might mainly include oxidative stress, apoptosis, inflammation, and disturbance of ATPases or neurotransmitters. Similarly, these sorts of mechanisms could be present in other types of nanomaterials besides TiO2 NPs [101–104]. But what factors mainly influence the neurotoxicity of TiO2 NPs are still unclear.
Major factors influence the neurotoxicity of TiO2 NPs
The neurotoxic effects of TiO2 NPs could be modulated by its peculiar physicochemical characteristics, administration routes, dosage, and so on. Therefore, although the abovementioned studies were all focused on the harmful impacts of TiO2 NPs on the brain, different conclusions had been obtained. As a consequence, for the purpose of assessing their neurotoxicity in a standard way, it is vital to discuss the major factors that might influence the neurotoxicity of TiO2 NPs.
Crystal type
TiO2 NPs, unlike other NPs, have two crystal types, i.e., the anatase and rutile [31]. Both of them possess subtle different physicochemical characteristics, which lead to different toxicities. It was reported that the toxicity of anatase form was higher than that of rutile [105–107]. However, some studies did not draw the same conclusions [108, 109]. Concerning the TiO2 NPs, how different crystal forms of TiO2 NPs affect neurotoxicity is still unclear. In this study [25], CD-1 (ICR) female mice were treated with anatase (155 nm) or rutile (80 nm) TiO2 NPs. The levels of GFAP protein, MDA, AChE activity, and glutamic acid in anatase group were higher than that in rutile group. However, the numbers of cell lost in both groups were similar, with no statistical difference. In another report of the same research group [53], the elevated levels of GSH-Px, GST, GSH, and SOD in rutile group were significantly higher than that in control and anatase groups at the time point of 10 days after CD-1 (ICR) female mice were treated with TiO2 NPs. But the levels of IL-1β and TNF-α in anatase group were apparently higher than that in the control and rutile groups after 30 days exposure. The rutile TiO2 NPs did not induced elevation in the levels of IL-1β and TNF-α.
PC12 cells were exposed to anatase or rutile TiO2 NPs (20 nm), the reduction of cell viability, mitochondria membrane potential (MMP), and levels of GSH and SOD in the anatase group were remarkably higher than that in the rutile one at the concentration of 200 μg/ml. The levels of LDH and MDA, the caspase 3 activity, and the percentage of necrosis in the anatase group were increased, which were higher than that in the rutile group at the concentration of 200 μg/ml [90]. However, an inconsistent conclusion was drawn from this study that the effects of both TiO2-S (100 % anatase NPs) and TiO2-D (80 % anatase + 20 % rutile) on the CNS were similar with no statistical difference [92].
Size of NPs
Several researches have revealed that dimension of nanomaterial is another vital factor which can influence the nanotoxicity [110]. When it comes to the effects of TiO2 NPs on the CNS, little is known about how different sizes affect the neurotoxicity of TiO2 NPs. Available data collected from current researches only compared the toxicity of the micro-TiO2 with that of nanosized-TiO2. Micro-TiO2 was found to be not detected in the brain regions. Thus, it was demonstrated to have no toxic effects [59, 79, 82, 90, 100]. Therefore, for fully understanding the neurotoxicity of TiO2 NPs, more studies are needed to investigate the effects of TiO2 NPs on the CNS with different sizes at the nanoscale level.
Administration route
As for in vivo studies, the administration routes play an important part on neurotoxicity of NPs [111, 112]. The Ti contents in the brain regions of mice/rats could not be detected when the TiO2 NPs were administrated via intravenous injection [73–75, 113]. But when the pregnant mice were treated with TiO2 NPs, their uterine weights were lowered with smaller fetuses, which indirectly suggested that the TiO2 NPs could induce fetal resorption and retard fetal growth via intravenous injection [67]. Ti could not be detected in the brain when mice were exposed to TiO2 NPs by inhalation [114]. Based on current studies, the intranasal instillation or nasal administration was the most effective pathway for TiO2 NPs to be translocated into the brain [16]. This might be due to the retrograde axonal translocation of NPs directly from the nose to the brain. Therefore, more researches are needed to investigate how and why the different administration routes lead to different NP bio-distributions.
Shape and Surface modification
The morphology of NPs is crucial to their toxicity. The effects of NPs on organisms might be regulated by morphological structure [115–119]. However, how different shapes (mainly including nanobelts, nanorods, nanotubes, and nanospheres) of TiO2 NPs modulate their transportation to the brain, how they get excretion from the CNS, and how they have toxic effects on neurons or glia cells are still largely unknown. Moreover, the surface coating can regulate the physicochemical properties of TiO2 NPs, which might influence their toxicity [120, 121]. However, whether TiO2 NPs coated with inorganic or organic materials could alleviate or exacerbate the harmful impacts on the CNS is unclear as well. In this study [99], mouse NSCs line C17.2 were treated with SiO2-coated rutile TiO2 NPs. This exposure induced elevation of the β-tubulin positive cells, which indicated that the TiO2 NPs can induce the C17.2 differentiating into neurons. In another study [100], the HB1.F3 human neural stem cells (hNSCs) were incubated with uncoated TiO2 NPs for 7 days, the cells were aggregated and the morphology of cells was changed. Results from the two researches might suggest that the coating on TiO2 NPs could make NPs possess beneficial properties. Zhang et al. [59] investigated how different sizes, morphology, and surface modification of TiO2 NPs in rutile form regulated their toxic effects on the brain after CD-1 (ICR) female mice were exposed by intranasal instillation every other day for 30 days. The experimental groups included A (micro-sized, hydrophobic, rod-like, and no coating), B (nano-sized, hydrophobic, needle-like, and no coating), C (nano-sized, hydrophilic, needle-like, and coated with silica), and D (nano-sized, hydrophilic, rod-like, and coated with silica). Results collected from the study demonstrated that (1) micro-sized TiO2 could not be detected in the brain regions. The Ti contents in the cerebellum region demonstrated no significant difference in the five groups (four experimental groups and one control group). Significantly increased Ti contents in the cerebral cortex were detected in C and D groups. In the striatum region, the Ti contents were markedly elevated in the B, C, and D groups; (2) the neuron loss in cerebral cortex and hippocampus CA1 region was significant in B, C, and D groups; (3) the levels of norepinephrine in the hippocampus, cerebral cortex, cerebellum, and striatum were significantly reduced in groups C and D; and (4) the levels of DA, DOPAC, HVA, 5-HT, and 5-HIAA in the four sub-brain regions were significantly affected in groups C and D. These findings suggested that the size, shape, and surface modification could modulate the toxic effects of TiO2 NPs in the brain. However, studies about effects of shape and surface modification on neurotoxicity of TiO2 NPs were limited, which needed further investigations.
Conclusions
As the rapid development of nanotechnology, numerous nanomaterial-based products are widely used at present, such as consumer products, food additives, cosmetics, drug carriers, and so on. Meanwhile, concerns on health risks about unexpected exposure to TiO2 NPs are arising. In in vivo studies, once animals were exposed to TiO2 NPs, the NPs could be translocated into the brain mainly through the blood–brain barrier (BBB) and nose–brain pathway. Besides, TiO2 NPs may affect the brain development of embryo by crossing the placental barrier. The Ti contents accumulated in the brain regions are tiny at one exposure, but its elimination from the brain was limited. Therefore, long-term or chronic exposure to TiO2 NPs could potentially lead to the gradually increased Ti contents in the brain, which may eventually induce impairments on the neurons and glial cells and lead to CNS dysfunction as a consequence.
Several in vivo and in vitro studies have demonstrated that TiO2 NPs, for their nanoscale, possessed toxic properties on the brain. However, as the experimental parameters used in all of the current studies were not standardized (such as different administration routes, experimental animals, crystal forms, different shapes and sizes), the conclusions from those studies are not comparable and even some of them might be conflicting. Therefore, it is urgent to standardize experiments on assessing the neurotoxicity of TiO2 NPs. In addition, all the research objects in those experiments were only consisted of animals (such as mice and rats) or cells (such as PC12, U373, C6). In consequence, neurotoxic data of TiO2 NPs collected from those studies might be inappropriate to determine their neurotoxic effects on humans. Therefore, in order to fully understand the neurotoxicity of TiO2 NPs, using human exposures or cells derived from humans to do experiment are needed. On the other hand, the toxic effects of different physicochemical characteristics of TiO2 NPs on the brain are unclear and should be investigated intensively, which includes the crystal forms, shape, size, surface modifications, and so on. In order to reduce translocation rate of TiO2 NPs into the brain and neurotoxicity, it is urgent to seek out the optimum parameters of physicochemical properties of TiO2 NPs to improve the bio-safety of TiO2 NPs-based products. Moreover, because TiO2 NPs could induce neurons or glial cells death and disturb the homeostasis in the brain, the possible relationship between the TiO2 NPs exposure and neurodegenerative diseases or psychiatric disorders needs further investigation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31070857, 50973045, 51172283, 81400557) and the Project on the Integration of Industry, Education and Research of Guangdong Province, China (2012B091000147).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LS presented the idea. BS collected and reviewed the data and drafted the manuscript. JL, XF, and LW helped in modifying the draft in the first version and after revision. All authors approved the final manuscript.
Contributor Information
Bin Song, Email: 17055224@qq.com.
Jia Liu, Email: liujia1988dr@163.com.
Xiaoli Feng, Email: 867770038@qq.com.
Limin Wei, Email: dentwlm@163.com.
Longquan Shao, Phone: +86 15989283921, Email: shaolongquan@smu.edu.cn.
References
- 1.Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev. 2009;61(6):457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy CJ, San TK, Gole AM, Orendorff CJ, Gao JX, Gou L, et al. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B. 2005;109(29):13857–13870. doi: 10.1021/jp0516846. [DOI] [PubMed] [Google Scholar]
- 3.Wessels JM, Nothofer HG, Ford WE, von Wrochem F, Scholz F, Vossmeyer T, et al. Optical and electrical properties of three-dimensional interlinked gold nanoparticle assemblies. J Am Chem Soc. 2004;126(10):3349–3356. doi: 10.1021/ja0377605. [DOI] [PubMed] [Google Scholar]
- 4.Auffan M, Pedeutour M, Rose J, Masion A, Ziarelli F, Borschneck D, et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ Sci Technol. 2010;44(7):2689–2694. doi: 10.1021/es903757q. [DOI] [PubMed] [Google Scholar]
- 5.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerman K, Saito M, Yamamura S, Takamura Y, Tamiya E. Nanomaterial-based electrochemical biosensors for medical applications. Trac-Trends in Analytical Chemistry. 2008;27(7):585–592. doi: 10.1016/j.trac.2008.05.004. [DOI] [Google Scholar]
- 7.Logan N, Sherif A, Cross AJ, Collins SN, Traynor A, Bozec L, et al. TiO2-coated CoCrMo: improving the osteogenic differentiation and adhesion of mesenchymal stem cells in vitro. J Biomed Mater Res Part A. 2015;103(3):1208–1217. doi: 10.1002/jbm.a.35264. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Li J, Zhang W, Qian H, She W, Pan H, et al. Antibacterial property, angiogenic and osteogenic activity of Cu-incorporated TiO2 coating. J Mat Chem B. 2014;2(39):6738–6748. doi: 10.1039/C4TB00923A. [DOI] [PubMed] [Google Scholar]
- 9.Catauro M, Bollino F, Papale F, Marciano S, Pacifico S. TiO2/PCL hybrid materials synthesized via sol–gel technique for biomedical applications. Mater Sci Eng C. 2015;47:135–141. doi: 10.1016/j.msec.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Montazer M, Pakdel E. Functionality of nano titanium dioxide on textiles with future aspects: focus on wool. J Photochem Photobiol C-Photochem Rev. 2011;12(4):293–303. doi: 10.1016/j.jphotochemrev.2011.08.005. [DOI] [Google Scholar]
- 11.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61(4):685–692. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Wang X, Jia Y, Chen X, Han H, Li C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem Rev. 2014;114(19):9987–10043. doi: 10.1021/cr500008u. [DOI] [PubMed] [Google Scholar]
- 13.Martirosyan A, Schneider Y-J. Engineered nanomaterials in food: implications for food safety and consumer health. Int J Environ Res Public Health. 2014;11(6):5720–5750. doi: 10.3390/ijerph110605720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafsson A, Jonasson S, Sandstrom T, Lorentzen JC, Bucht A. Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles. Toxicology. 2014;326:74–85. doi: 10.1016/j.tox.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara N, Oshima Y, Kobayashi T, Imatanaka N, Nakai M, Ichinose T, et al. Dose-dependent clearance kinetics of intratracheally administered titanium dioxide nanoparticles in rat lung. Toxicology. 2014;325:1–11. doi: 10.1016/j.tox.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Li Y, Li W, Chen C, Li B, Zhao Y. Biological effect of intranasally instilled titanium dioxide nanoparticles on female mice. Nano. 2008;3(4):279–285. doi: 10.1142/S1793292008001325. [DOI] [Google Scholar]
- 17.Auttachoat W, McLoughlin CE, White KL, Jr, Smith MJ. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticles. J Immunotoxicol. 2014;11(3):273–282. doi: 10.3109/1547691X.2013.844750. [DOI] [PubMed] [Google Scholar]
- 18.Hong F, Hong J, Wang L, Zhou Y, Liu D, Xu B, et al. Chronic exposure to nanoparticulate TiO2 causes renal fibrosis involving activation of the Wnt pathway in mouse kidney. J Agric Food Chem. 2015;63(5):1639–1647. doi: 10.1021/jf5034834. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29(4):330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 20.Huang K-T, Wu C-T, Huang K-H, Lin W-C, Chen C-M, Guan S-S, et al. Titanium nanoparticle inhalation induces renal fibrosis in mice via an oxidative stress upregulated transforming growth factor-beta pathway. Chem Res Toxicol. 2015;28(3):354–364. doi: 10.1021/tx500287f. [DOI] [PubMed] [Google Scholar]
- 21.Medina-Reyes EI, Bucio-Lopez L, Freyre-Fonseca V, Sanchez-Perez Y, Garcia-Cuellar CM, Morales-Barcenas R, et al. Cell cycle synchronization reveals greater G2/M-phase accumulation of lung epithelial cells exposed to titanium dioxide nanoparticles. Environ Sci Pollut Res. 2015;22(5):3976–3982. doi: 10.1007/s11356-014-3871-y. [DOI] [PubMed] [Google Scholar]
- 22.Sheng L, Wang L, Sang X, Zhao X, Hong J, Cheng S, et al. Nano-sized titanium dioxide-induced splenic toxicity: a biological pathway explored using microarray technology. J Hazard Mater. 2014;278:180–188. doi: 10.1016/j.jhazmat.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Filippi C, Pryde A, Cowan P, Lee T, Hayes P, Donaldson K, et al. Toxicology of ZnO and TiO2 nanoparticles on hepatocytes: impact on metabolism and bioenergetics. Nanotoxicology. 2015;9(1):126–134. doi: 10.3109/17435390.2014.895437. [DOI] [PubMed] [Google Scholar]
- 24.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J Drug Target. 2004;12(9–10):635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183(1–3):72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Tsyganova NA, Khairullin RM, Terentyuk GS, Khlebtsov BN, Bogatyrev VA, Dykman LA, et al. Penetration of pegylated gold nanoparticles through rat placental barrier. Bull Exp Biol Med. 2014;157(3):383–385. doi: 10.1007/s10517-014-2572-3. [DOI] [PubMed] [Google Scholar]
- 27.Gheshlaghi ZN, Riazi GH, Ahmadian S, Ghafari M, Mahinpour R. Toxicity and interaction of titanium dioxide nanoparticles with microtubule protein. Acta Biochim Biophys Sin. 2008;40(9):777–782. doi: 10.1093/abbs/40.9.777. [DOI] [PubMed] [Google Scholar]
- 28.Wu W-h, Sun X, Yu Y-p, Hu J, Zhao L, Liu Q, et al. TiO2 nanoparticles promote beta-amyloid fibrillation in vitro. Biochem Biophys Res Commun. 2008;373(2):315–318. doi: 10.1016/j.bbrc.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Xu S, Zhang Z, Schluesener HJ. Apoptosis induced by titanium dioxide nanoparticles in cultured murine microglia N9 cells. Chin Sci Bull. 2009;54(20):3830–3836. doi: 10.1007/s11434-009-0548-x. [DOI] [Google Scholar]
- 30.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Mao SS. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107(7):2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, Mora-Sero I, De Angelis F, Bisquert J, Wang P. Titanium dioxide nanomaterials for photovoltaic applications. Chem Rev. 2014;114(19):10095–10130. doi: 10.1021/cr400606n. [DOI] [PubMed] [Google Scholar]
- 33.Bai J, Zhou B. Titanium dioxide nanomaterials for sensor applications. Chem Rev. 2014;114(19):10131–10176. doi: 10.1021/cr400625j. [DOI] [PubMed] [Google Scholar]
- 34.Kapilashrami M, Zhang Y, Liu Y-S, Hagfeldt A, Guo J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem Rev. 2014;114(19):9662–9707. doi: 10.1021/cr5000893. [DOI] [PubMed] [Google Scholar]
- 35.Sang L, Zhao Y, Burda C. TiO2 nanoparticles as functional building blocks. Chem Rev. 2014;114(19):9283–9318. doi: 10.1021/cr400629p. [DOI] [PubMed] [Google Scholar]
- 36.Shrivas K, Hayasaka T, Sugiura Y, Setou M. Method for simultaneous imaging of endogenous Low molecular weight metabolites in mouse brain using TiO2 nanoparticles in nanoparticle-assisted laser desorption/ionization-imaging mass spectrometry. Anal Chem. 2011;83(19):7283–7289. doi: 10.1021/ac201602s. [DOI] [PubMed] [Google Scholar]
- 37.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview—structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Barbu E, Molnar E, Tsibouklis J, Gorecki DC. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood–brain barrier. Expert Opin Drug Deliv. 2009;6(6):553–565. doi: 10.1517/17425240902939143. [DOI] [PubMed] [Google Scholar]
- 39.Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu AM, et al. Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer's disease. J Control Release. 2005;108(2–3):193–214. doi: 10.1016/j.jconrel.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez A, Suarez-Merino B, Goni-de-Cerio F. Nanoparticles and blood–brain barrier: the key to central nervous system diseases. J Nanosci Nanotechnol. 2014;14(1):766–779. doi: 10.1166/jnn.2014.9119. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Li J, Yin J, Li W, Kang C, Huang Q, et al. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J Nanosci Nanotechnol. 2010;10(12):8544–8549. doi: 10.1166/jnn.2010.2690. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Xu Z, Li X. Cytotoxicity of titanium dioxide nanoparticles in rat neuroglia cells. Brain Inj. 2013;27(7–8):934–939. doi: 10.3109/02699052.2013.793401. [DOI] [PubMed] [Google Scholar]
- 43.Brun E, Carriere M, Mabondzo A. In vitro evidence of dysregulation of blood–brain barrier function after acute and repeated/long-term exposure to TiO2 nanoparticles. Biomaterials. 2012;33(3):886–896. doi: 10.1016/j.biomaterials.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Baello S, Iqbal M, Bloise E, Javam M, Gibb W, Matthews SG. TGF-beta 1 regulation of multidrug resistance P-glycoprotein in the developing male blood–brain barrier. Endocrinology. 2014;155(2):475–484. doi: 10.1210/en.2013-1472. [DOI] [PubMed] [Google Scholar]
- 45.Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR plus chemokines during acute viral encephalomyelitis. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louboutin J-P, Strayer DS. Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy. CNS Neurol Disord-Drug Targets. 2013;12(6):815–829. doi: 10.2174/18715273113126660173. [DOI] [PubMed] [Google Scholar]
- 47.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158(3):983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Aversa TG, Eugenin EA, Lopez L, Berman JW. Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood–brain barrier disruption: implications for the pathogenesis of multiple sclerosis. Neuropathol Appl Neurobiol. 2013;39(3):270–283. doi: 10.1111/j.1365-2990.2012.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang W, Lv P, Geng X, Shang E, Yang Q, Sha L, et al. Penetration of verapamil across blood brain barrier following cerebral ischemia depending on both paracellular pathway and P-glycoprotein transportation. Neurochem Int. 2013;62(1):23–30. doi: 10.1016/j.neuint.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Mestre L, Inigo PM, Mecha M, Correa FG, Hernangomez-Herrero M, Loria F, et al. Anandamide inhibits Theiler's virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB1 receptors. J Neuroinflamm. 2011 doi: 10.1186/1742-2094-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Vos KJ, Grierson AJ, Ackerley S, Miller CCJ. Role of axonal transport in neurodegenerative diseases. Annual Review of Neuroscience. 2008;31:151–73. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 52.Perlson E, Maday S, Fu M-m, Moughamian AJ, Holzbaur ELF. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33(7):335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Liu Y, Jiao F, Lao F, Li W, Gu Y, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology. 2008;254(1–2):82–90. doi: 10.1016/j.tox.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9(3):182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 55.Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- 56.Ashbrook DG, Williams RW, Lu L, Stein JL, Hibar DP, Nichols TE, et al. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. Bmc Genomics. 2014 doi: 10.1186/1471-2164-15-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceccariglia S, D'Altocolle A, Del Fa A, Silvestrini A, Barba M, Pizzolante F, et al. Increased expression of aquaporin 4 in the Rat hippocampus and cortex during trimethyltin-induced neurodegeneration. Neuroscience. 2014;274:273–288. doi: 10.1016/j.neuroscience.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 58.Lin T-W, Shih Y-H, Chen S-J, Lien C-H, Chang C-Y, Huang T-Y, et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol Learn Mem. 2015;118:189–197. doi: 10.1016/j.nlm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Bai R, Li B, Ge C, Du J, Liu Y, et al. Rutile TiO2 particles exert size and surface coating dependent retention and lesions on the murine brain. Toxicol Lett. 2011;207(1):73–81. doi: 10.1016/j.toxlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Ze Y, Hu R, Wang X, Sang X, Ze X, Li B, et al. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res Part A. 2014;102(2):470–478. doi: 10.1002/jbm.a.34705. [DOI] [PubMed] [Google Scholar]
- 61.Chu M, Wu Q, Yang H, Yuan R, Hou S, Yang Y, et al. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small. 2010;6(5):670–678. doi: 10.1002/smll.200902049. [DOI] [PubMed] [Google Scholar]
- 62.Semmler-Behnke M, Lipka J, Wenk A, Hirn S, Schaeffler M, Tian F, et al. Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Particle and Fibre Toxicology. 2014 doi: 10.1186/s12989-014-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Bona KR, Xu Y, Ramirez PA, DeLaine J, Parker C, Bao Y, et al. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod Toxicol. 2014;50:36–42. doi: 10.1016/j.reprotox.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry. 2010;67(8):841–849. doi: 10.1001/archgenpsychiatry.2010.92. [DOI] [PubMed] [Google Scholar]
- 65.O'Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- 66.Mohammadipour A, Fazel A, Haghir H, Motejaded F, Rafatpanah H, Zabihi H, et al. Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol. 2014;37(2):617–625. doi: 10.1016/j.etap.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6(5):321–328. doi: 10.1038/nnano.2011.41. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi Y, Mizuo K, Shinkai Y, Oshio S, Takeda K. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cotex and neostriatum of mice. J Toxicol Sci. 2010;35(5):749–756. doi: 10.2131/jts.35.749. [DOI] [PubMed] [Google Scholar]
- 69.Umezawa M, Tainaka H, Kawashima N, Shimizu M, Takeda K. Effect of fetal exposure to titanium dioxide nanoparticle on brain development—brain region information. J Toxicol Sci. 2012;37(6):1247–1252. doi: 10.2131/jts.37.1247. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu M, Tainaka H, Oba T, Mizuo K, Umezawa M, Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Particle and Fibre Toxicology. 2009 doi: 10.1186/1743-8977-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hougaard KS, Jackson P, Jensen KA, Sloth JJ, Loeschner K, Larsen EH, et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Particle and Fibre Toxicology. 2010 doi: 10.1186/1743-8977-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui Y, Chen X, Zhou Z, Lei Y, Ma M, Cao R, et al. Prenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult rats. Chemosphere. 2014;96:99–104. doi: 10.1016/j.chemosphere.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 73.Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, van Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol. 2008;82(3):151–157. doi: 10.1007/s00204-007-0253-y. [DOI] [PubMed] [Google Scholar]
- 74.Patri A, Umbreit T, Zheng J, Nagashima K, Goering P, Francke-Carroll S, et al. Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice. J Appl Toxicol. 2009;29(8):662–672. doi: 10.1002/jat.1454. [DOI] [PubMed] [Google Scholar]
- 75.Sugibayashi K, Todo H, Kimura E. Safety evaluation of titanium dioxide nanoparticles by their absorption and elimination profiles. J Toxicol Sci. 2008;33(3):293–298. doi: 10.2131/jts.33.293. [DOI] [PubMed] [Google Scholar]
- 76.Wu J, Liu W, Xue C, Zhou S, Lan F, Bi L, et al. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett. 2009;191(1):1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 77.Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, et al. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Particle and Fibre Toxicology. 2014 doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho W-S, Kang B-C, Lee JK, Jeong J, Che J-H, Seok SH. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Particle and Fibre Toxicology. 2013 doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma L, Liu J, Li N, Wang J, Duan Y, Yan J, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105. doi: 10.1016/j.biomaterials.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 80.Ze Y, Zheng L, Zhao X, Gui S, Sang X, Su J, et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere. 2013;92(9):1183–1189. doi: 10.1016/j.chemosphere.2013.01.094. [DOI] [PubMed] [Google Scholar]
- 81.Ze Y, Sheng L, Zhao X, Hong J, Ze X, Yu X, et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PLoS One. 2014 doi: 10.1371/journal.pone.0092230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Shin JA, Lee EJ, Seo SM, Kim HS, Kang JL, Park EM. Nanosized titanium dioxide enhanced inflammatory responses in the septic brain of mouse. Neuroscience. 2010;165(2):445–454. doi: 10.1016/j.neuroscience.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 83.Hu R, Zheng L, Zhang T, Gao G, Cui Y, Cheng Z, et al. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;191(1–3):32–40. doi: 10.1016/j.jhazmat.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 84.Meena R, Kumar S, Paulraj R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. Journal of Nanoparticle Research. 2015 [Google Scholar]
- 85.Ze Y, Sheng L, Zhao X, Ze X, Wang X, Zhou Q, et al. Neurotoxic characteristics of spatial recognition damage of the hippocampus in mice following subchronic peroral exposure to TiO2 nanoparticles. J Hazard Mater. 2014;264:219–229. doi: 10.1016/j.jhazmat.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 86.Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, et al. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31(31):8043–8050. doi: 10.1016/j.biomaterials.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 87.Ben Younes NR, Amara S, Mrad I, Ben-Slama I, Jeljeli M, Omri K, et al. Subacute toxicity of titanium dioxide (TiO2) nanoparticles in male rats: emotional behavior and pathophysiological examination. Environ Sci Pollut Res. 2015;22(11):8728–8737. doi: 10.1007/s11356-014-4002-5. [DOI] [PubMed] [Google Scholar]
- 88.Xue Y, Wu J, Sun J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett. 2012;214(2):91–98. doi: 10.1016/j.toxlet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Liu S, Xu L, Zhang T, Ren G, Yang Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267(1–3):172–177. doi: 10.1016/j.tox.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Wu J, Sun J, Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett. 2010;199(3):269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Sheng L, Ze Y, Wang L, Yu X, Hong J, Zhao X, et al. Mechanisms of TiO2 nanoparticle-induced neuronal apoptosis in rat primary cultured hippocampal neurons. J Biomed Mater Res Part A. 2015;103(3):1141–1149. doi: 10.1002/jbm.a.35263. [DOI] [PubMed] [Google Scholar]
- 92.Valdiglesias V, Costa C, Sharma V, Kilic G, Pasaro E, Teixeira JP, et al. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem Toxicol. 2013;57:352–361. doi: 10.1016/j.fct.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Mao ZL, Xu B, Ji XL, Zhou K, Zhang XM, Chen MJ, et al. Titanium dioxide nanoparticles alter cellular morphology via disturbing the microtubule dynamics. Nanoscale. 2015;7(18):8466–8475. doi: 10.1039/C5NR01448D. [DOI] [PubMed] [Google Scholar]
- 94.Coccini T, Grandi S, Lonati D, Locatelli C, De Simone U. Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology. 2015;48:77–89. doi: 10.1016/j.neuro.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 95.Hong FS, Sheng L, Ze YG, Hong J, Zhou YJ, Wang L, et al. Suppression of neurite outgrowth of primary cultured hippocampal neurons is involved in impairment of glutamate metabolism and NMDA receptor function caused by nanoparticulate TiO2. Biomaterials. 2015;53:76–85. doi: 10.1016/j.biomaterials.2015.02.067. [DOI] [PubMed] [Google Scholar]
- 96.Gissela Marquez-Ramirez S, Laura Delgado-Buenrostro N, Irasema Chirino Y, Gutierrez Iglesias G, Lopez-Marure R. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology. 2012;302(2–3):146–156. doi: 10.1016/j.tox.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Huerta-Garcia E, Antonio Perez-Arizti J, Gissela Marquez-Ramirez S, Laura Delgado-Buenrostro N, Irasema Chirino Y, Gutierrez Iglesias G, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med. 2014;73:84–94. doi: 10.1016/j.freeradbiomed.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 98.Kenzaoui BH, Bernasconi CC, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441:813–821. doi: 10.1042/BJ20111252. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Ren X, Deng X, Huo Y, Xie J, Huang H, et al. A protein interaction network for the analysis of the neuronal differentiation of neural stem cells in response to titanium dioxide nanoparticles. Biomaterials. 2010;31(11):3063–3070. doi: 10.1016/j.biomaterials.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 100.Fujioka K, Hanada S, Inoue Y, Sato K, Hirakuri K, Shiraishi K, et al. Effects of silica and titanium oxide particles on a human neural stem cell line: morphology, mitochondrial activity, and gene expression of differentiation markers. Int J Mol Sci. 2014;15(7):11742–11759. doi: 10.3390/ijms150711742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niska K, Santos-Martinez MJ, Radomski MW, Inkielewicz-Stepniak I. CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: protective effect of crocetin. Toxicol Vitro. 2015;29(4):663–671. doi: 10.1016/j.tiv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Xie YL, Wang YY, Zhang T, Ren GG, Yang Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J Biomed Sci. 2012;19:11. doi: 10.1186/1423-0127-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJS. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol. 2014;37(3):336–347. doi: 10.3109/01480545.2013.866134. [DOI] [PubMed] [Google Scholar]
- 104.Huang CL, Hsiao IL, Lin HC, Wang CF, Huang YJ, Chuang CY. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ Res. 2015;136:253–263. doi: 10.1016/j.envres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Gitrowski C, Al-Jubory AR, Handy RD. Uptake of different crystal structures of TiO2 nanoparticles by Caco-2 intestinal cells. Toxicol Lett. 2014;226(3):264–276. doi: 10.1016/j.toxlet.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Lin X, Li J, Ma S, Liu G, Yang K, Tong M, et al. Toxicity of TiO2 nanoparticles to Escherichia coli: effects of particle size, crystal phase and water chemistry. PLoS One. 2014 doi: 10.1371/journal.pone.0110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clemente Z, Castro VL, Feitosa LO, Lima R, Jonsson CM, Maia AHN, et al. Biomarker evaluation in fish after prolonged exposure to nano-TiO2: influence of illumination conditions and crystal phase. J Nanosci Nanotechnol. 2015;15(7):5424–5433. doi: 10.1166/jnn.2015.10021. [DOI] [PubMed] [Google Scholar]
- 108.Numano T, Xu J, Futakuchi M, Fukamachi K, Alexander DB, Furukawa F, et al. Comparative study of toxic effects of anatase and rutile type nanosized titanium dioxide particles in vivo and in vitro. Asian Pac J Cancer Prev. 2014;15(2):929–935. doi: 10.7314/APJCP.2014.15.2.929. [DOI] [PubMed] [Google Scholar]
- 109.Sekar D, Falcioni ML, Barucca G, Falcioni G. DNA damage and repair following in vitro exposure to Two different forms of titanium dioxide nanoparticles on trout erythrocyte. Environ Toxicol. 2014;29(1):117–127. doi: 10.1002/tox.20778. [DOI] [PubMed] [Google Scholar]
- 110.Chen LQ, Fang L, Ling J, Ding CZ, Kang B, Huang CZ. Nanotoxicity of silver nanoparticles to red blood cells: size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 2015;28(3):501–509. doi: 10.1021/tx500479m. [DOI] [PubMed] [Google Scholar]
- 111.Espinosa-Cristobal LF, Martinez-Castanon GA, Loyola-Rodriguez JP, Patino-Marin N, Reyes-Macias JF, Vargas-Morales JM, et al. Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. Journal of Nanoparticle Research. 2013 [Google Scholar]
- 112.Choi J, Kim H, Kim P, Jo E, Kim H-M, Lee M-Y, et al. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: single intravenous injection and single oral administration. J Toxicol Env Health Part A. 2015;78(4):226–243. doi: 10.1080/15287394.2014.949949. [DOI] [PubMed] [Google Scholar]
- 113.Shinohara N, Danno N, Ichinose T, Sasaki T, Fukui H, Honda K, et al. Tissue distribution and clearance of intravenously administered titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2014;8(2):132–141. doi: 10.3109/17435390.2012.763001. [DOI] [PubMed] [Google Scholar]
- 114.Yin J, Kang C, Li Y, Li Q, Zhang X, Li W. Aerosol inhalation exposure study of respiratory toxicity induced by 20 nm anatase titanium dioxide nanoparticles. Toxicol Res. 2014;3(5):367–374. doi: 10.1039/C4TX00040D. [DOI] [Google Scholar]
- 115.Medina-Reyes EI, Deciga-Alcaraz A, Freyre-Fonseca V, Delgado-Buenrostro NL, Flores-Flores JO, Gutierrez-Lopez GF, et al. Titanium dioxide nanoparticles induce an adaptive inflammatory response and invasion and proliferation of lung epithelial cells in chorioallantoic membrane. Environ Res. 2015;136:424–434. doi: 10.1016/j.envres.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 116.Tong T, Shereef A, Wu J, Chu Thi Thanh B, Kelly JJ, Gaillard J-F, et al. Effects of material morphology on the phototoxicity of nano-TiO2 to bacteria. Environ Sci Technol. 2013;47(21):12486–12495. doi: 10.1021/es403079h. [DOI] [PubMed] [Google Scholar]
- 117.Silva RM, TeeSy C, Franzi L, Weir A, Westerhoff P, Evans JE, et al. Biological response to nano-scale titanium dioxide (Tio2): role of particle dose, shape, and retention. J Toxicol Env Health Part A. 2013;76(16):953–972. doi: 10.1080/15287394.2013.826567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oosthuizen MA, Oberholzer HM, Scriba MR, van der Spuy WJ, Pretorius E. Evaluation of the morphological changes in the lungs of BALB/c mice after inhalation of spherical and rod-shaped titanium nanoparticles. Micron. 2012;43(8):863–869. doi: 10.1016/j.micron.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Hamilton RF, Jr, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Particle and Fibre Toxicology. 2009 doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rossi EM, Pylkkanen L, Koivisto AJ, Vippola M, Jensen KA, Miettinen M, et al. Airway exposure to silica-coated TiO2 nanoparticles induces pulmonary neutrophilia in mice. Toxicol Sci. 2010;113(2):422–433. doi: 10.1093/toxsci/kfp254. [DOI] [PubMed] [Google Scholar]
- 121.Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt R, Schilde C, et al. Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One. 2011 doi: 10.1371/journal.pone.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]


