Abstract
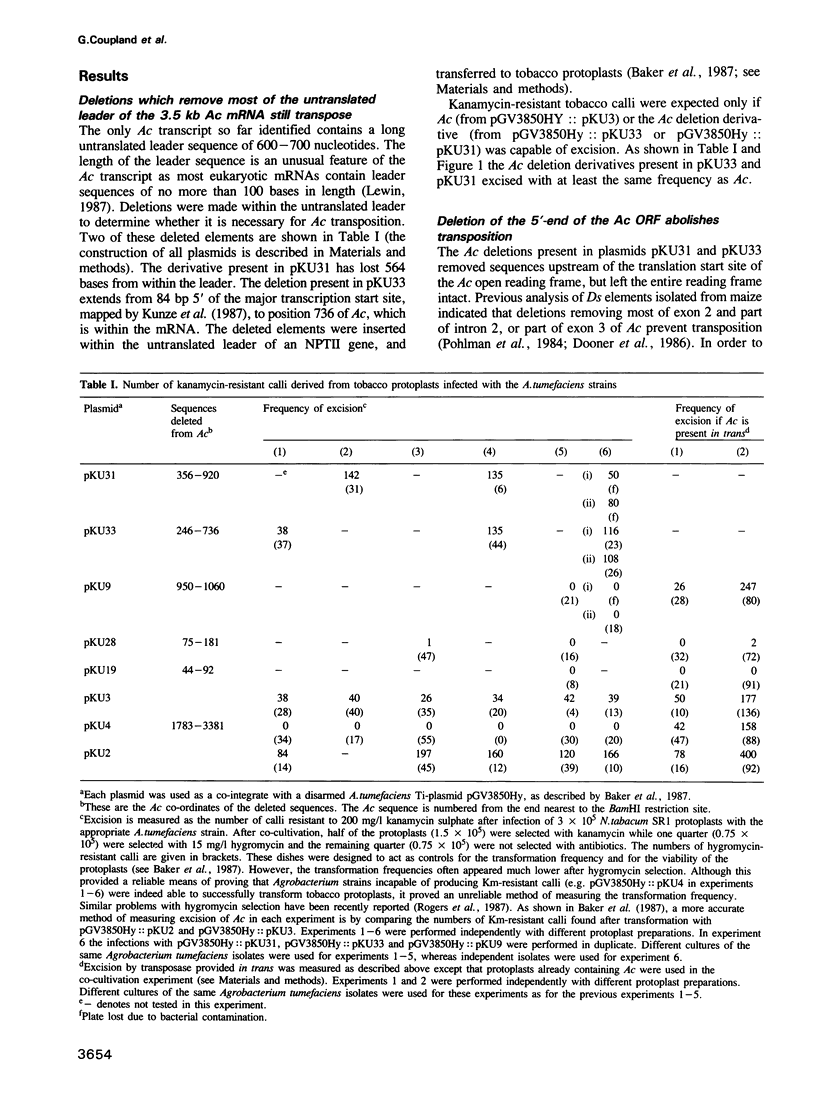
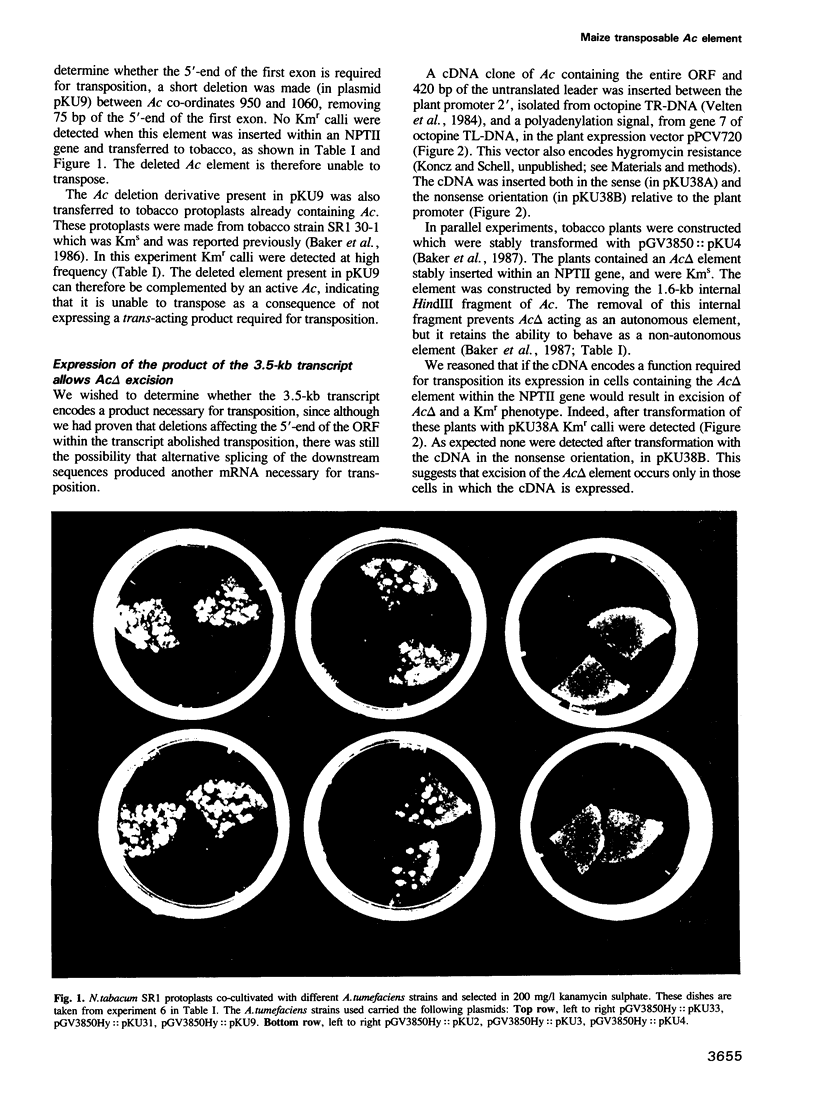
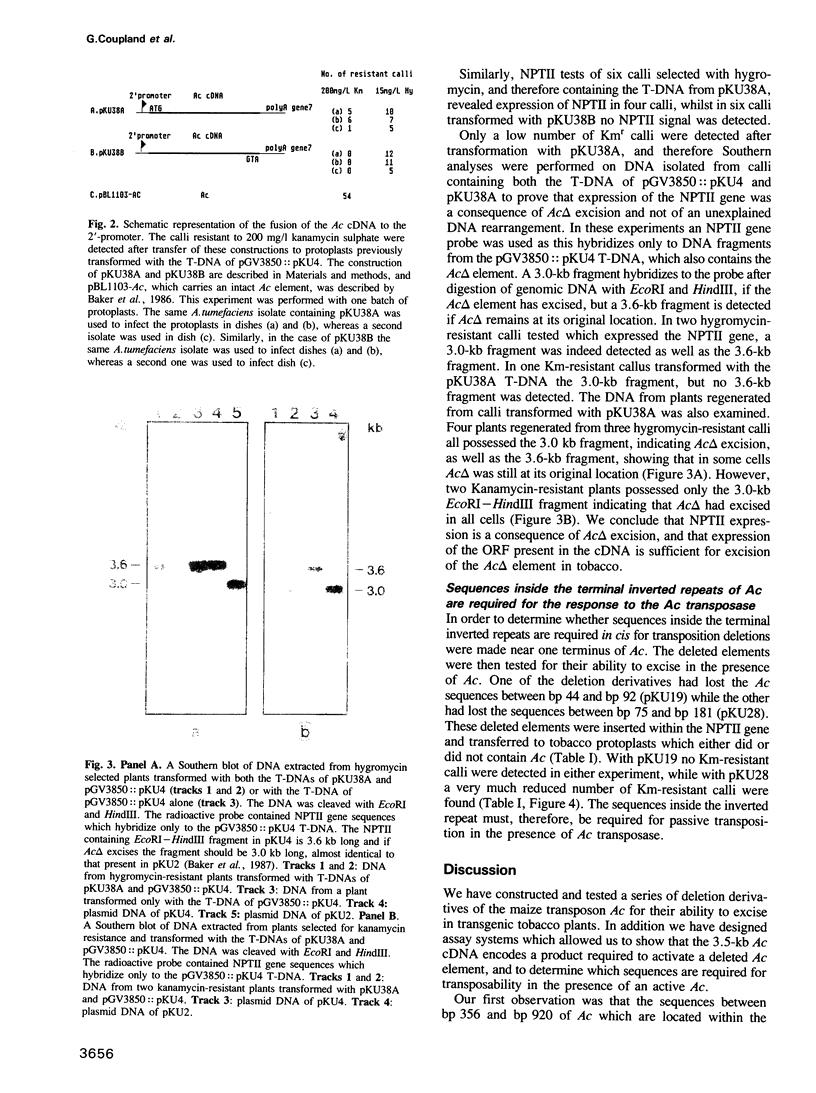
We have used the ability of Ac to transpose in tobacco to determine which Ac sequences are required for transposition, using a phenotypic assay for Ac excision from an NPTII gene in which excisions of Ac result in calli resistant to the antibiotic kanamycin (Baker et al., 1987). Here we show that deletion of the Ac DNA which encodes the untranslated leader of the Ac transcript does not prevent Ac excision. A deletion which removes 110 bp including the first 75 bp of long open reading frame prevents Ac excision in tobacco cells. However, it will excise in tobacco cells previously transformed with Ac indicating that deletion of the region prevents the synthesis of a product required for Ac excision. Deletion of the Ac sequences between bp 44 and bp 92 or from bp 75 to bp 181 abolishes, or strongly reduces, transposition in cells which are already transgenic for an active Ac element. This indicates that these deleted elements have lost sequences which are required for the transposon to respond to the transposase, when the enzyme is produced in trans. We also describe a tobacco strain transformed with a Ds element stably inserted wtihin an NPTII gene. This strain is Kms and was retransformed with a construct containing the open reading frame (ORF) of the 3.5-kb Ac transcript expressed from a plant promoter. Expression of the cDNA construct promotes excision of the Ds element. These data suggest that the 3.5-kb transcript of Ac encodes the only Ac product required for transposition, i.e. the transposase function.
Keywords: maize transposon, transgenic tobacco, transposase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Coupland G., Fedoroff N., Starlinger P., Schell J. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 1987 Jun;6(6):1547–1554. doi: 10.1002/j.1460-2075.1987.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Dooner H., English J., Ralston E., Weck E. A single genetic unit specifies two transposition functions in the maize element activator. Science. 1986 Oct 10;234(4773):210–211. doi: 10.1126/science.234.4773.210. [DOI] [PubMed] [Google Scholar]
- Döring H. P., Starlinger P. Barbara McClintock's controlling elements: now at the DNA level. Cell. 1984 Dec;39(2 Pt 1):253–259. doi: 10.1016/0092-8674(84)90002-3. [DOI] [PubMed] [Google Scholar]
- Döring H. P., Starlinger P. Molecular genetics of transposable elements in plants. Annu Rev Genet. 1986;20:175–200. doi: 10.1146/annurev.ge.20.120186.001135. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wessler S., Shure M. Isolation of the transposable maize controlling elements Ac and Ds. Cell. 1983 Nov;35(1):235–242. doi: 10.1016/0092-8674(83)90226-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M. Demonstration that a chemically synthesized BPV1 oncoprotein and its C-terminal domain function to induce cellular DNA synthesis. Cell. 1987 Dec 4;51(5):795–802. doi: 10.1016/0092-8674(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Kunze R., Stochaj U., Laufs J., Starlinger P. Transcription of transposable element Activator (Ac) of Zea mays L. EMBO J. 1987 Jun;6(6):1555–1563. doi: 10.1002/j.1460-2075.1987.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLINTOCK B. Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol. 1951;16:13–47. doi: 10.1101/sqb.1951.016.01.004. [DOI] [PubMed] [Google Scholar]
- Pohlman R. F., Fedoroff N. V., Messing J. The nucleotide sequence of the maize controlling element Activator. Cell. 1984 Jun;37(2):635–643. doi: 10.1016/0092-8674(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D., Gerlach W. L., Peacock W. J., Schwartz D. Molecular analysis of ds controlling element mutations at the adh1 locus of maize. Science. 1984 Mar 23;223(4642):1265–1268. doi: 10.1126/science.223.4642.1265. [DOI] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Velten J., Velten L., Hain R., Schell J. Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1984 Dec 1;3(12):2723–2730. doi: 10.1002/j.1460-2075.1984.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wessler S. R., Baran G., Varagona M., Dellaporta S. L. Excision of Ds produces waxy proteins with a range of enzymatic activities. EMBO J. 1986 Oct;5(10):2427–2432. doi: 10.1002/j.1460-2075.1986.tb04517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]