Abstract
Background
Synucleinopathy is any of a group of age-related neurodegenerative disorders including Parkinson's disease, multiple system atrophy, and dementia with Lewy Bodies, which is characterized by α-synuclein inclusions and parkinsonian motor deficits affecting millions of patients worldwide. But there is no cure at present for synucleinopathy. Rapamycin has been shown to be neuroprotective in several in vitro and in vivo synucleinopathy models. However, there are no reports on the long-term effects of RAPA on motor function or measures of neurodegeneration in models of synucleinopathy.
Methods
We determined whether long-term feeding a rapamycin diet (14 ppm in diet; 2.25 mg/kg body weight/day) improves motor function in neuronal A53T α-synuclein transgenic mice (TG) and explored underlying mechanisms using a variety of behavioral and biochemical approaches.
Results
After 24 weeks of treatment, rapamycin improved performance on the forepaw stepping adjustment test, accelerating rotarod and pole test. Rapamycin did not alter A53T α-synuclein content. There was no effect of rapamycin treatment on midbrain or striatal monoamines or their metabolites. Proteins adducted to the lipid peroxidation product 4-hydroxynonenal were decreased in brain regions of both wild-type and TG mice treated with rapamycin. Reduced levels of the presynaptic marker synaptophysin were found in several brain regions of TG mice. Rapamycin attenuated the loss of synaptophysin protein in the affected brain regions. Rapamycin also attenuated the loss of synaptophysin protein and prevented the decrease of neurite length in SH-SY5Y cells treated with 4-hydroxynonenal.
Conclusion
Taken together, these data suggest that rapamycin, an FDA approved drug, may prove useful in the treatment of synucleinopathy.
Keywords: rapamycin, synucleinopathy, 4-hydroxynonenal (4-HNE), synaptic injury, synaptophysin, motor function
Synucleinopathy is any of a group of age-related neurodegenerative disorders characterized by α-synuclein inclusions in brain. Synucleinopathy including Parkinson's disease (PD), multiple system atrophy (MSA), and dementia with Lewy bodies (DLB) affects millions of patients worldwide (1). Parkinsonian-like motor symptoms, including tremor, rigidity, and slowness of movement, are common symptoms of synucleinopathy. There is no effective treatment at present for these conditions.
There is evidence for a role of elevated mammalian target of rapamycin (mTOR) in synucleinopathy. For example, mTOR activity is elevated in postmortem brain from DLB patients compared to control patients. Rapamycin (RAPA), an allosteric inhibitor of mTOR, reduces neuronal toxicity induced by PD mimetics in vitro and in vivo (2–4) and reverses the neurodegenerative phenotype in neuronal cells overexpressing human α-synuclein (5, 6). Short-term intra-cerebral infusion of RAPA ameliorates neurodegenerative alterations in mice overexpressing human wild-type α-synuclein (7). However, there are no reports on the long-term effects of RAPA on motor function or measures of neurodegeneration in models of synucleinopathy.
The A53T mutation in human α-synuclein was the first mutation of α-synuclein reported to be associated with PD, affecting families in Italy, Greece, and Australia (8–10). It has also been detected in sporadic PD (11). Therefore, we investigated the effects of chronic feeding of a RAPA-containing diet in a mouse model of synucleinopathy which overexpress the human A53T α-synuclein specifically in neurons (TG) and exhibits motor dysfunction (12).
Materials and methods
Animals
TG mice (12) were obtained from National Institute on Aging contract colony. Wild-type (WT) genetic controls on the same C57Bl/C3H background were obtained from the Jackson Laboratory. Mice unable to perform the righting reflex were excluded from behavioral testing. Mouse diet containing microencapsulated RAPA (Southwest Research Institute, San Antonio TX; 14 ppm in diet; 2.25 mg/kg body weight/day) or the Eudragit S100 (Rohm Pharma) microencapsulation material (13) was fed to age-matched TG and WT of both sexes (n=24–25; 2 cohorts) from 13 weeks of age for 24 weeks. This dose of RAPA was chosen because it increased longevity in old mice (13), and the increase in survival conferred by RAPA treatment is hypothesized to be mediated by delaying age-related diseases, including neurodegenerative diseases (14). Animal experiments were conducted according to the National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’ and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Behavioral tests
Pole test
Mice were placed with the head oriented upward at the top of a vertical wooden pole 50 cm long (1 cm in diameter) which was placed in the home cage. Mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) are slower to orient downward in the pole test compared with controls (15). Mice received 2 days of training that consisted of three trials for each session. On the test day, mice received three trials, and the average time to orient downward, that is, to reverse orientation, was calculated.
Forepaw stepping adjustment test
The forepaw stepping adjustment test is a reliable approach to detect forelimb akinesia in MPTP-induced Parkinsonism (16). The experimenter lifts the hind legs of each mouse by pulling up on the tail leaving only the forepaws touching the table. At a steady pace of approximately 1 m in 3–4 s, the mouse was pulled backwards by the tail for 1 m. The test was recorded by video, and the number of adjusting steps from both forepaws was counted.
Accelerating rotarod test
The animals were pre-trained for 5 days on a rotarod apparatus (Rotamex 4/8; Columbus Instruments, Columbus, OH). Three trials were performed on each day. On each trial, the rotarod started at an initial speed of 4 RPM and then accelerated to 40 RPM within 300 s. On the test day, the average latency to fall from the rod during three trials was calculated.
Western blot analyses of proteins in brain mouse tissue
Following behavioral tests, the mice were sacrificed under anesthesia, and the brain was rapidly removed, placed on an ice-cold glass plate, and dissected into regions. Each region was immediately frozen in a dry ice and propanol mixture before being stored at −80°C.
Protein from the above brain regions was extracted with 1×RIPA buffer with 1×Calbiochem Protease Inhibitor Cocktail Set I and 1×Halt* Phosphatase Inhibitor Cocktail (Thermo Scientific). Samples were homogenized at 50 Hz using the TissueLyser LT (Invitrogen) with 5 mm steel bead tissue lysate was then centrifuged at 13,000 rpm for 15 min at 4°C. Supernatant was stored at −80°C freezer. Bicinchoninic acid protein assay reagents were used to determine protein concentration. Equal amounts of protein were subjected to 4–12% Criterion SDS-PAGE gel (Bio-Rad) and transferred onto nitrocellulose membrane. Proteins were detected with antibodies directed to human α-synuclein (Abcam), synaptophysin (EMD Millipore), 4-HNE adducted protein (R&D), and beta-actin (CST), respectively. Secondary antibodies (LiCor) were used to visualize the bands which were scanned and analyzed by the Odyssey® Imaging System (LiCor).
Cell culture
SH-SY5Y is a human cell line subclone of SK-N-SH cell, which was isolated from a bone marrow biopsy taken from a 4-year-old neuroblastoma patient. SH-SY5Y cells express dopaminergic markers and are widely used to study PD. We chose to use SH-SY5Y cells, based on a study of synaptophysin protein expression in different dopaminergic cell lines (17) and the presence of relatively long neurites. SH-SY5Y cells (passage 9–11) were incubated in a humidified, 5% CO2, 37°C incubator. The medium used was Advanced DMEM:F12 1:1 (Gibco) supplemented with 5% fetal bovine serum (FBS, Hyclone), 50 unit/ml Penicillin/Streptomycin (Gibco), and 2 mM Glutamax (Invitrogen).
Western analyses of synaptophysin in SH-SY5Y cells
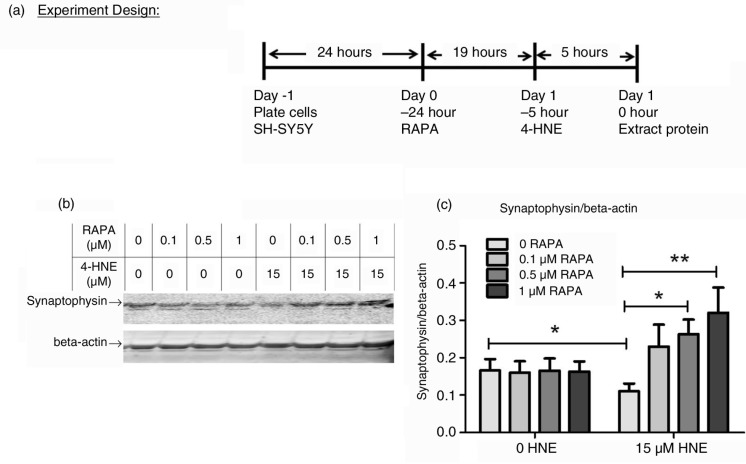
SH-SY5Y cells (2×106/well) were plated on six-well plates. Different doses of RAPA (LC Laboratories, Woburn, MA) and corresponding volumes of vehicle (Ethanol) were added to each well. Cells were treated with 4-HNE (15 µM) 19 h later for 5 h as illustrated by Fig. 4a. The doses of RAPA and 4-HNE and the treatment duration were determined from preliminary studies. Protein from cells was extracted, and synaptophysin was detected and quantified as described in 2.3.
Fig. 4.
Effect of RAPA on 4-HNE-induced loss of synaptophysin protein in SH-SY5Y cells. (a) experiment design. RAPA at different doses and corresponding volumes of Vehicle was added to SH-SY5Y cells. Cells were then treated with 4-HNE (15 µM) 19 h later for 5 h in the same medium before protein extraction. (b) representative immunoblot. Each well of 4–12% Criterion gel was loaded with 100 µg cell lysate. (c) quantification of immunoblot by Odyssey software. Each group contained nine replicates. Data were analyzed by a two-way ANOVA followed by Bonferonni post hoc n=9, p<0.05, **p<0.01.
Measurement of neurite length in SH-SY5Y cells
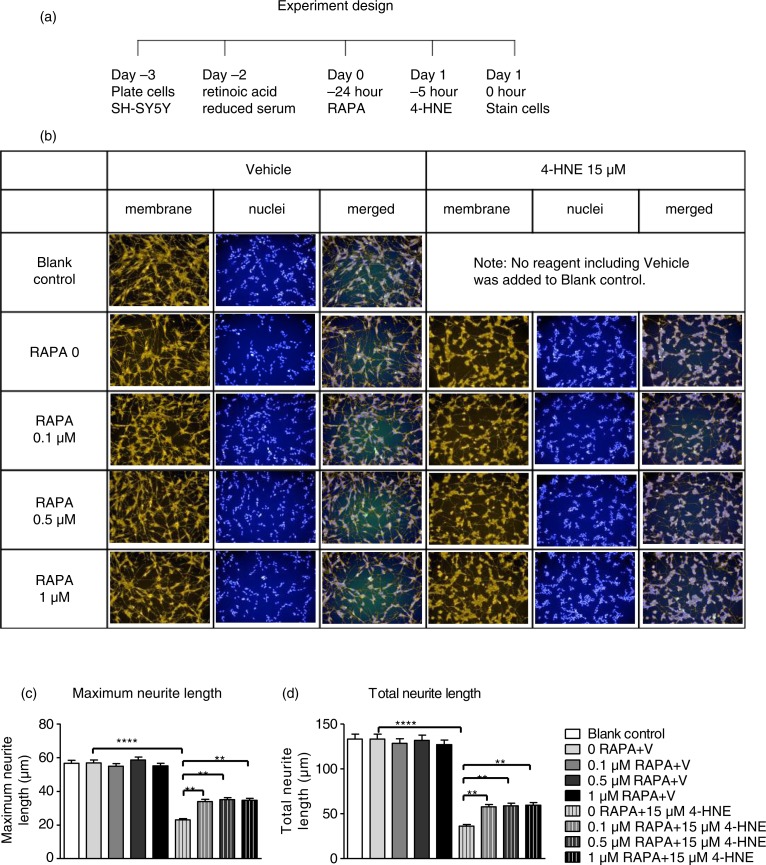
SH-SY5Y cells (1.4×105/well) were seeded on 12-well plates. Culture medium was replaced the next day with differentiation medium containing 2.5% FBS serum and 10 mM retinoic acid. The differentiation medium was replaced with normal culture medium 2 days later. Different doses of RAPA and corresponding volumes of vehicle were added to each well. Cells then were treated with 4-HNE (15 µM) 19 h later for 5 h in the same medium as shown in Fig. 5a. A non-treated control consisted of cells without any added reagent.
Fig. 5.
Effect of RAPA on 4-HNE-induced loss of neurites in SH-SY5Y cells. (a) experiment design. SH-SY5Y cells were plated in normal culture medium which was replaced the next day with differentiation medium which contained reduced serum (2.5% FBS) and 10 mM retinoic acid. Two days later, different doses of RAPA and corresponding volume of vehicle in normal culture medium was added to cells, followed by 4-HNE (15 µM) treatment 19 h later for another 5 h followed by protein extraction. Blank controls had no reagent, including vehicle, added to cells. (b) representative images captured using PerkinElmer High Content Screen Imaging system (20X). Orange staining is for cell membrane (neurite), blue staining is for nuclei. (c,d) effect of RAPA on 4-HNE-induced decrease in maximum neurite length (c) and total neurite length (d) in SH-SY5Y cells. Cells (n=1119±94.6, mean±SEM) in 3–4 images per experiment were analyzed using Columbus Image Data Storage and Analysis System. Data were analyzed by two-way ANOVA with followed by Tukey post hoc tests to compare individual means. ****p=0.0000, **p<0.01. Experiments were repeated three times.
Neurites were monitored using Molecular Probes® Neurite Outgrowth Staining Kit (Molecular Probes). Hoechst 33342 blue (Molecular Probes) was used to stain nuclei. Nuclear staining was combined with cell membrane staining in 4% PFA in DBPS.
An Operetta® High Content Imaging System (PerkinElmer) was used to capture multiple images of cells in the plate. Cells (n>1,059) in 3–4 fields per experiment were analyzed. Briefly, the nuclei were identified under the Hoechst channel, and the number of cells was recorded. Then neurites were identified under the Alexa 546 channel. Maximum neurite length and total neurite length per cell were analyzed by the CSIRO Neurite Analysis 2 default method.
Statistical Analyses
The results of behavioral tests were statistically analyzed using a mixed-effects regression model to dissect the effect of cohort, sex, genotype, RAPA treatment, age, and interactions among these factors. Latency to fall from the accelerating rotarod and time to reverse from the pole test were treated as time-to-event data; that is, the data represent the time elapsed until an event occurs (falling from the rotarod or reversing direction from the top of the pole). A parametric, accelerated failure time model was used to dissect the effect of cohort, sex, genotype, RAPA, age, and interactions among these factors on these two measures.
A two-way ANOVA was used to analyze effects of genotype and RAPA on levels of synaptophysin and 4-HNE protein adducts in brain regions of either sex. To analyze effects of 4-HNE and RAPA on synaptophysin protein or neurite length, a two-way ANOVA with Bonferonni or Tukey posttest was used, respectively. Data in the figures were plotted as the mean±SEM. Statistical significance was set to p<0.05.
Results
Effect of RAPA on motor function
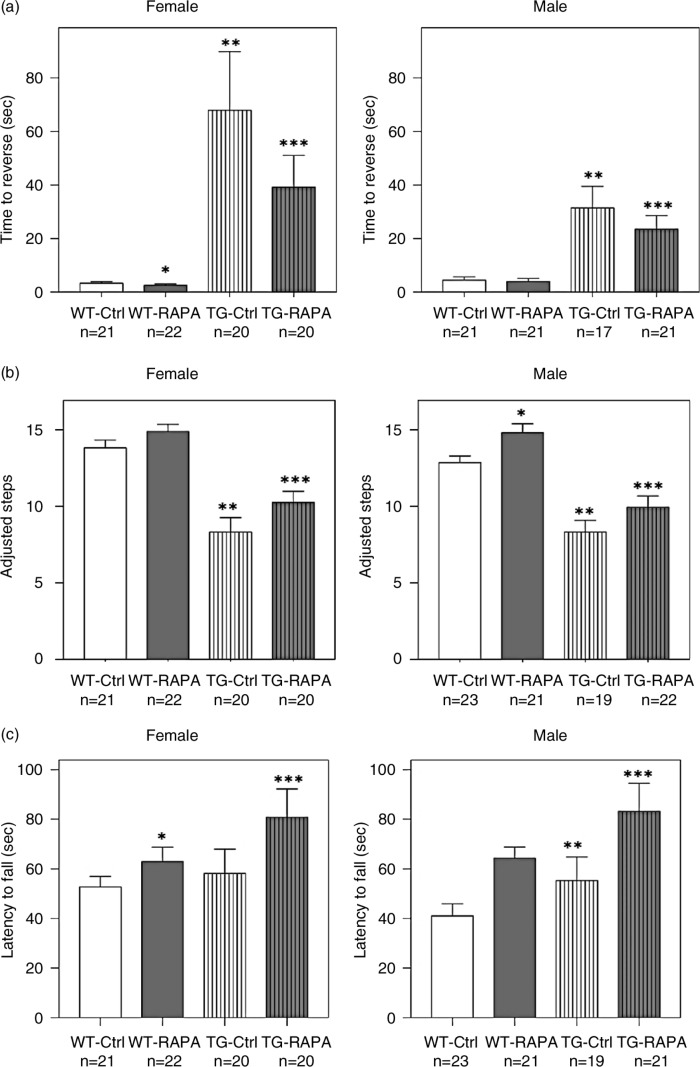
Female TG had a log-ratio of 2.992±0.319 (20-fold increase) for the time to reverse from the top of the pole compared to female WT (p<0.0001) (log-ratio is the logarithm base e of the fold-change=loge[Reversal Time of TG/Reversal Time of WT] and [e2.99≈20-fold increase]),RAPA treatment significantly decreased the log-ratio (log-ratio=loge[Reversal Time of RAPA/Reversal Time of Untreated]) of the time to reverse by 0.5505±0.1948 (1.7 fold increase) in female TG (Fig. 1a, p<0.005). Likewise, male TG had a log-ratio of 1.67±0.2038 (5.3 fold increase) for the time until reversing compared to male WT (p<0.0001), and RAPA treatment significantly decreased the log-ratio by 0.3143±0.0591 (1.4 fold increase) in male TG (Fig. 1a, p<0.0001). RAPA also significantly decreased the log-ratio time until reversing from the top of the pole in female WT by 0.1944±0.04486 (1.2-fold increase) (p<0.0001) but not in male WT (p>0.05) compared to mice treated with control diet.
Fig. 1.
Effect of RAPA on performance in tests of motor function. (a) pole test; (b) forepaw stepping adjustment test; (c) accelerating rotarod test. Age-matched WT and TG mice were fed mouse diet containing microencapsulated RAPA or the microencapsulation material Eudragit S100 (Vehicle Control) from 13 weeks of age for 24 weeks. Data represent mean±SEM. Data were analyzed using a mixed-effect regression model. *p<0.05, WT control versus WT RAPA; **p<0.05, WT control versus TG control; ***p<0.05, TG control versus TG RAPA.
Female TG made 5.597±0.7379 significantly fewer adjustment steps (p<0.0001), and male TG made 4.421±0.7916 fewer adjustment steps (p<0.0001) in the forepaw stepping adjustment test compared to their respective WT groups. RAPA treatment significantly increased 1.932±0.7467 adjustment steps in female TG (p<0.01) and 1.671±0.7994 in male TG (Fig. 1b, p<0.05) compared to either sex group treated with control diet, respectively. RAPA also significantly increased 1.717±0.772 adjustment steps in male WT (p<0.05) but not female WT (p>0.05, Fig. 1b) compared to the corresponding group of mice treated with control diet.
Although female TG spent a similar amount of time on the accelerating rotarod compared to female WT (genotype effect p>0.05), RAPA treatment significantly increased the latency to fall from the accelerating rotarod by a log-ratio of 0.1954±0.0202 (1.2-fold increase) in female WT (p<0.0001) and by a log-ratio of 0.2813±0.0162 (1.3-fold increase) in female TG (p<0.0001) compared to mice treated with control diet, respectively (Fig. 1c). The effect of RAPA in female TG was significantly greater than in female WT (RAPA/genotype interaction effect p<0.05). Male TG mice had a log-ratio 0.3639±0.1826 increase (1.4-fold increase) in latency to fall from the accelerating rotarod compared to male WT mice (genotype effect p<0.05), while RAPA treatment further increased the latency to fall by a log-ratio of 0.3612±0.1052 (1.4-fold increase) in male TG compared to male TG control (p<0.0001, Fig. 1c). That the TG did not perform worse on the accelerating rotarod as compared to WT mice is consistent with previous reports (12, 18).
Effect of RAPA on brain regional 4-HNE protein adducts
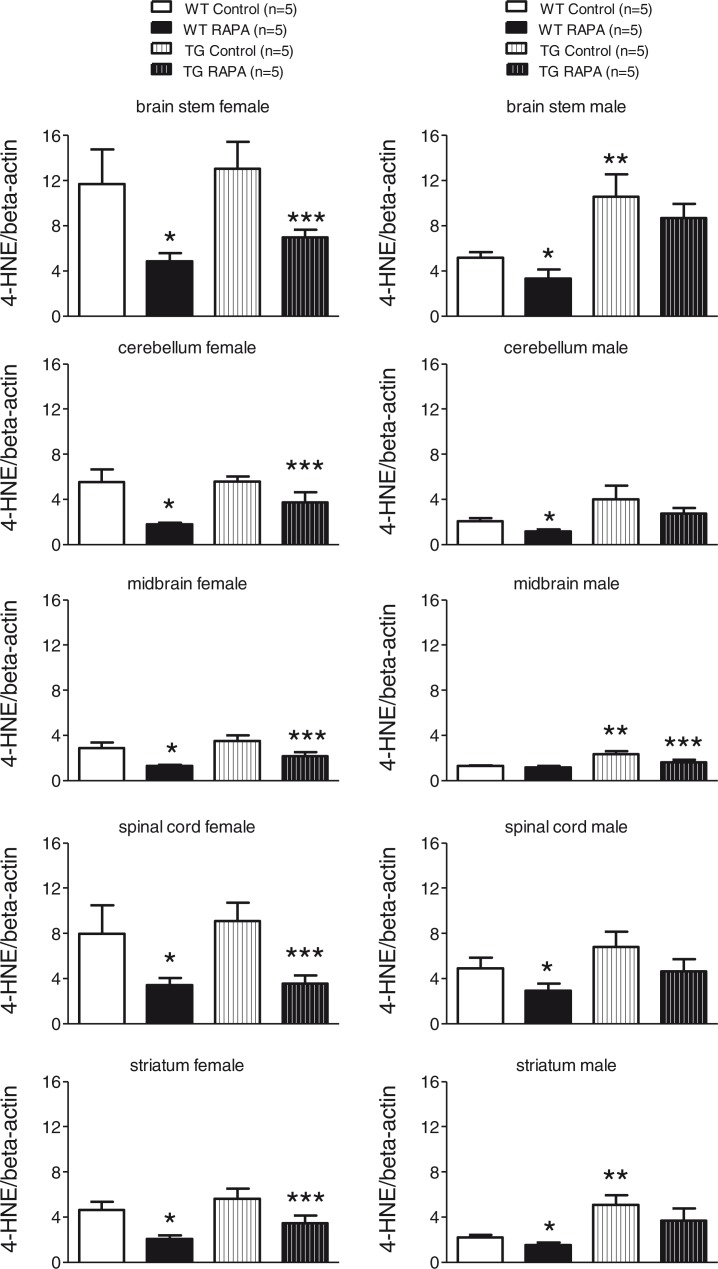
The level of 4-HNE-adducted proteins (Fig. 2, Supplementary Fig. 4) was significantly increased in the cerebellum, midbrain, and striatum of male TG compared to WT (p<0.05). RAPA treatment was associated with significant (p<0.05) decreases in 4-HNE-protein adducts in each of the five brain regions tested from both WT and TG female. There were significant (p<0.05) decreases in 4-HNE-protein adducts in four of the five brain regions from RAPA-treated male WT. There was also a trend toward decreases in the mean content of 4-HNE-protein adducts in each of the five brain regions in RAPA-treated male TG, achieving significance (p<0.05) in the midbrain.
Fig. 2.
Effect of RAPA on 4-HNE protein adducts in midbrain, striatum, brain stem, cerebellum, and spinal cord. Age-matched female WT and TG mice were fed mouse diet incorporated with microencapsulated RAPA or the microencapsulation material Eudragit S100 (Control) from 13 weeks of age for 24 weeks. Each well of a 4–12% Criterion gel was loaded with 40 µg brain tissue lysate. Immunoreactive bands were quantified by Odyssey software. Data represent the mean±SEM. Two-way ANOVAs followed by post hoc Bonferonni tests were used to analyze effects of genotype and RAPA on levels of synaptophysin in either sex. *p<0.05, WT control versus WT RAPA; **p<0.05, WT control versus TG control; ***p<0.05, TG control versus TG RAPA.
Effect of RAPA on brain regional synaptophysin
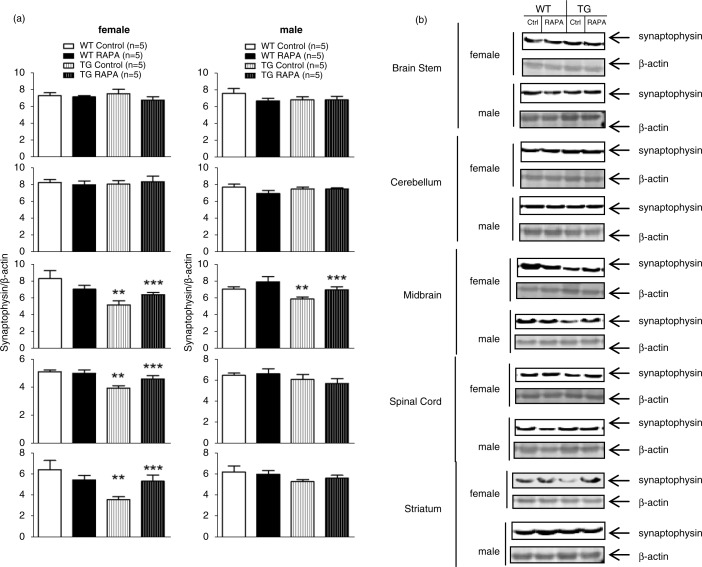
As shown in Fig. 3, synaptophysin was significantly decreased in the midbrain of male and female TG mice compared to WT, and RAPA significantly attenuated the decrease (p<0.05). Synaptophysin was also significantly decreased in the spinal cord, and striatum of female TG mice compared to WT and RAPA significantly attenuated these differences (p<0.05).
Fig. 3.
Effect of RAPA on synaptophysin protein level in midbrain, striatum, brain stem, cerebellum, and spinal cord. (a) quantification of Western blot; (b) representative immunoblots. Age-matched female WT and TG mice were fed mouse diet incorporated with microencapsulated RAPA and the microencapsulation material Eudragit S100 (vehicle control) from 13 weeks of age for 24 weeks. Each well of a 4–12% Criterion gel was loaded with 40 µg brain tissue lysate. Immunoreactive bands were quantified by Odyssey software. Data represent the mean±SEM. Two-way ANOVA with post hoc Bonferonni t-tests were used to analyze the effects of genotype and RAPA on levels of synaptophysin in either sex. *p<0.05, WT control versus WT RAPA; **p<0.05, WT control versus TG control; ***p<0.05, TG control versus TG RAPA.
Effect of RAPA and 4-HNE on synaptophysin in SH-SY5Y cells
As shown in Fig. 4, synaptophysin was significantly reduced in SH-SY5Y cells treated for 5 h with 15 µM 4-HNE (p<0.05). Pretreatment with 0.5 µM (p<0.05) and 1 µM (p<0.01) but not by 0.1 µM RAPA significantly attenuated the decrease in synaptophysin. There was no effect of RAPA alone on synaptophysin.
Effect of RAPA on 4-HNE-induced loss of neurite length in SH-SY5Y cells
As shown in Fig. 5 and Supplementary Table 1, the vehicle did not change maximum or total neurite length. Treatment with 4-HNE significantly decreased maximum neurite length from 63.97±2.26 to 25.22±1.31 µM and total neurite length from 150.43±6.84 to 36.30±2.18 µM (p<0.0001). RAPA alone did not alter maximum or total neurite length but increased maximum neurite length to more than 1.3-fold and total neurite length to more than 1.7-fold compared to 4-HNE-treated cells (p<0.01).
Discussion
We have found that RAPA treatment for 24 weeks improved motor performance in A53T transgenic mice in the accelerating rotarod, pole, and forepaw stepping adjustment tests. This is the first report that rapamycin rescues motor performance in A53T TG mice. The effects of RAPA on motor function were not related to effects of RAPA on grip strength or front stride length because RAPA had no beneficial effect on the two measures (Supplementary Fig. 1).
To further study the mechanism that mediates effects of RAPA, we measured soluble and total human α-synuclein protein levels in five brain regions (midbrain, striatum, brain stem, cerebellum, and spinal cord) that are important for motor function and motor coordination, but found they were not altered by RAPA (Supplementary Fig. 2). These results did not support our original hypothesis that RAPA improves motor function of TG by decreasing human α-synuclein. Although it is possible that higher concentration of RAPA may decrease α-synuclein protein concentrations in this mouse model, the improvement of motor function induced by the current dose of RAPA directed us to examine other mechanisms that might be involved in the beneficial effects of RAPA, that is, increases in dopamine and metabolites, reduction in 4-HNE-modified proteins, and preservation of synapses as measured by synaptophysin protein.
Previously published studies reported selected changes in catecholamine function (19) in TG mice. RAPA has been reported to increase dopamine content in aging C57BL/6 mice (20) and MPTP-treated mice (21). Therefore, we tested whether RAPA increases dopamine and its metabolites in striatum or midbrain. We found no effect of the A53T transgene (consistent with previous studies (19)) or RAPA on dopamine and metabolites in either brain region (Supplementary Fig. 3). The cause for the differences in our results, showing no effect of RAPA on dopamine content, and those previously reported are unclear, but the differences may be due to the strain of mice employed and/or in the acute nature of the MPTP treatment.
We found that RAPA decreased the levels of 4-HNE-protein adducts in brain regions of both WT and TG, which might partially explain the effects of RAPA on improved motor function that we observed in some tests in both WT and TG. Although the levels of 4-HNE protein adducts were similar between WT and TG fed the control diet, TG develop motor deficits but WT do not. This may be because A53T α-synuclein overexpression increases susceptibility to 4-HNE-induced oxidative damage, thus leading to enhanced susceptibility to synaptic degeneration. It has been reported that cells overexpressing A53T human α-synuclein exhibit mitochondrial dysfunction and increased mitochondrial production of reactive oxygen species (22) and are more susceptible to 4-HNE-induced oxidative insults (23). Thus, we hypothesize that RAPA treatment protects against 4-HNE-induced loss of synapses.
To test that hypothesis, we examined the effects 4-HNE on synaptophysin protein content and neurite length in the SH-SY5Y dopaminergic cell line. We found that RAPA attenuated 4-HNE-induced loss of synaptophysin and reduction in neurite length. The exact molecular mechanism mediating this effect of RAPA is unclear. Previous reports have shown that RAPA-induced inhibition of mTOR signaling, which occurs by binding to FKBP12, is neuroprotective in cultured cells (3–6, 24). Additionally, RAPA binding to FKBP52 has also been shown to enhance neurite growth in cultured neurons (25). Furthermore, available evidence from the literature supports the idea that RAPA's neuroprotective effects may be mediated by one or more mTOR downstream mechanisms including inhibition of protein translation and stimulation of autophagy. For example, RAPA prevents dopaminergic neuron loss and motor deficits in drosophila models of PD through activation of 4E-BP to inhibit protein translation (26). RAPA is also reportedly neuroprotective in MPTP-treated mice by inhibiting translation of the pro-cell death protein RTP801 (3) or by enhancing lysosome biogenesis and autophagosome clearance (4). RAPA reduces paraquat toxicity in vivo (2), rotenone toxicity in vitro and the accumulation of α-synuclein and neurodegenerative phenotype in α-synuclein overexpressing cells (5, 6, 24) and mice (7) by inducting autophagy (27). Although we did not address these mechanisms in the studies reported here, the available evidence supports the idea that inhibition of protein translation and/or stimulation of autophagy might play an important role in decreasing 4-HNE-adducted protein in different brain regions and conferring neuroprotective effects in the TG mice used here. Based on our results and evidence from literature, we hypothesize that such an effect of RAPA on 4-HNE might be related to suppression of oxidative stress and augmentation of oxidative defenses. In that regard, oxidative stress response genes including superoxide dismutase1 and glutathione reductase have been shown to be significantly upregulated by RAPA in adult stems cells derived from C57BL/6J mice (28). RAPA also activates the protein kinase GCN2, which plays an important role in oxidant defenses and response to oxidative insults in the yeast Saccharomyces cerevisiae (29).
In summary, we for the first time have shown that long-term RAPA treatment improves motor function in a mouse model of synucleinopathy, that is, mice overexpressing mutant A53T human α-synuclein in neurons. Our results indicate that RAPA reduces lipid peroxidation and synaptic injury and ameliorates motor dysfunction without altering human α-synuclein protein levels. Thus, targeting downstream consequences of human α-synuclein accumulation instead of, or in addition to, human α-synuclein accumulation may be effective in treatment of synucleinopathy. The presence of the lipid peroxidation product 4-HNE in Lewy bodies in PD and DLB patients (30), as well as in glia and neuronal inclusions in MSA patients (31) provides evidence supporting the idea that 4-HNE and human α-synuclein accumulation are strongly linked to each other. In addition, cells overexpressing A53T human a-synuclein are more susceptible to oxidative insults including H2O2 and 4-HNE (23). Modification of human α-synuclein by 4-HNE induces formation of soluble oligomers and contributes to oxidative damage of neurons and increases dopaminergic neurotoxicity (32–34). Therefore, genetic or pharmacological interventions to decrease 4-HNE and consequent synaptic injury may be potentially effective therapeutic approaches to the treatment of synucleinopathy.
Supplementary Material
Acknowledgements
We are grateful to Mrs. Vanessa Martinez for technical assistance and Ms. Vivian Diaz at the Nathan Shock Aging Animal and Longevity Assessment Core (San Antonio) for maintenance of the mice. The study is supported by NIH grants AG022307, AG013319, and VA grant BX001641 (RS) and a Translational Science Pre-doctoral Training Grant (XB) from University of Texas Health Science Center at San Antonio, University of Texas System Graduate Training Initiative.
Conflict of interest and funding
Dr. Strong is an unpaid member of the Scientific Advisory Board for Rapamycin Holdings, Inc. None of the other authors have conflicts of interest to disclose.
Author's contributions
XB, CSSR, and RS were responsible for drafting/revision of the manuscript for content including medical writing for content. XB, MCYW, EF, and RS conceived study concept and design. XB and RS performed analysis and interpretation of data. MJH and RS were responsible for vital reagents/tools/patents. XB, MCYW, MJH, and RS were responsible for acquisition of data. XB, JG, AFB, and RS performed statistical analysis. XB, EF, and RS were responsible for study supervision or coordination. XB and RS were responsible for obtaining funding.
References
- 1.Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 2.Ravikumar B, Berger Z, Vacher C, O'Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006;15:1209–16. doi: 10.1093/hmg/ddl036. [DOI] [PubMed] [Google Scholar]
- 3.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci. 2010;30:1166–75. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–44. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 6.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, et al. Aggregate-prone proteins are cleared from the cytosol by autophagy: Therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 7.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Golbe LI, Di Iorio G, Lazzarini A, Vieregge P, Gershanik OS, Bonavita V, et al. The Contursi kindred, a large family with autosomal dominant Parkinson's disease: Implications of clinical and molecular studies. Adv Neurol. 1999;80:165–70. [PubMed] [Google Scholar]
- 10.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–9. [PubMed] [Google Scholar]
- 11.Michell AW, Barker RA, Raha SK, Raha-Chowdhury R. A case of late onset sporadic Parkinson's disease with an A53T mutation in alpha-synuclein. J Neurol Neurosurg Psychiatry. 2005;76:596–7. doi: 10.1136/jnnp.2004.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp ZD, Strong R. The role of mTOR signaling in controlling mammalian life span: What a fungicide teaches us about longevity. J Gerontol A Biol Sci Med Sci. 2010;65:580–9. doi: 10.1093/gerona/glp212. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–8. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- 16.Blume SR, Cass DK, Tseng KY. Stepping test in mice: A reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp Neurol. 2009;219:208–11. doi: 10.1016/j.expneurol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Bai X, Strong R. Expression of synaptophysin protein in different dopaminergic cell lines. J Biochem Pharmacol Res. 2014;2(4):185–90. [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DR, Sidhu A. Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res. 2010;88:1777–83. doi: 10.1002/jnr.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiriou E, Vassilatis DK, Vila M, Stefanis L. Selective noradrenergic vulnerability in alpha-synuclein transgenic mice. Neurobiol Aging. 2010;31:2103–14. doi: 10.1016/j.neurobiolaging.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–13. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Shi N, Sun Y, Zhang T, Sun X. Therapeutic effects of rapamycin on MPTP-induced Parkinsonism in mice. Neurochem Res. 2013;38:201–7. doi: 10.1007/s11064-012-0909-8. [DOI] [PubMed] [Google Scholar]
- 22.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009;41:2015–24. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 24.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, et al. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci USA. 2008;105:33–8. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12:1129–35. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–51. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Kofman AE, McGraw MR, Payne CJ. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging (Albany NY) 2012;4:279–89. doi: 10.18632/aging.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascarenhas C, Edwards-Ingram LC, Zeef L, Shenton D, Ashe MP, Grant CM. Gcn4 is required for the response to peroxide stress in the yeast saccharomyces cerevisiae. Mol Biol Cell. 2008;19:2995–3007. doi: 10.1091/mbc.E07-11-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellani RJ, Perry G, Siedlak SL, Nunomura A, Shimohama S, Zhang J, et al. Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett. 2002;319:25–8. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- 31.Shibata N, Inose Y, Toi S, Hiroi A, Yamamoto T, Kobayashi M. Involvement of 4-hydroxy-2-nonenal accumulation in multiple system atrophy. Acta Histochem Cytochem. 2010;43:69–75. doi: 10.1267/ahc.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282:5862–70. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- 33.Nasstrom T, Fagerqvist T, Barbu M, Karlsson M, Nikolajeff F, Kasrayan A, et al. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of alpha-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic Biol Med. 2011;50:428–37. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Xiang W, Schlachetzki JC, Helling S, Bussmann JC, Berlinghof M, Schaffer TE, et al. Oxidative stress-induced posttranslational modifications of alpha-synuclein: specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity. Mol Cell Neurosci. 2013;54:71–83. doi: 10.1016/j.mcn.2013.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.