Abstract
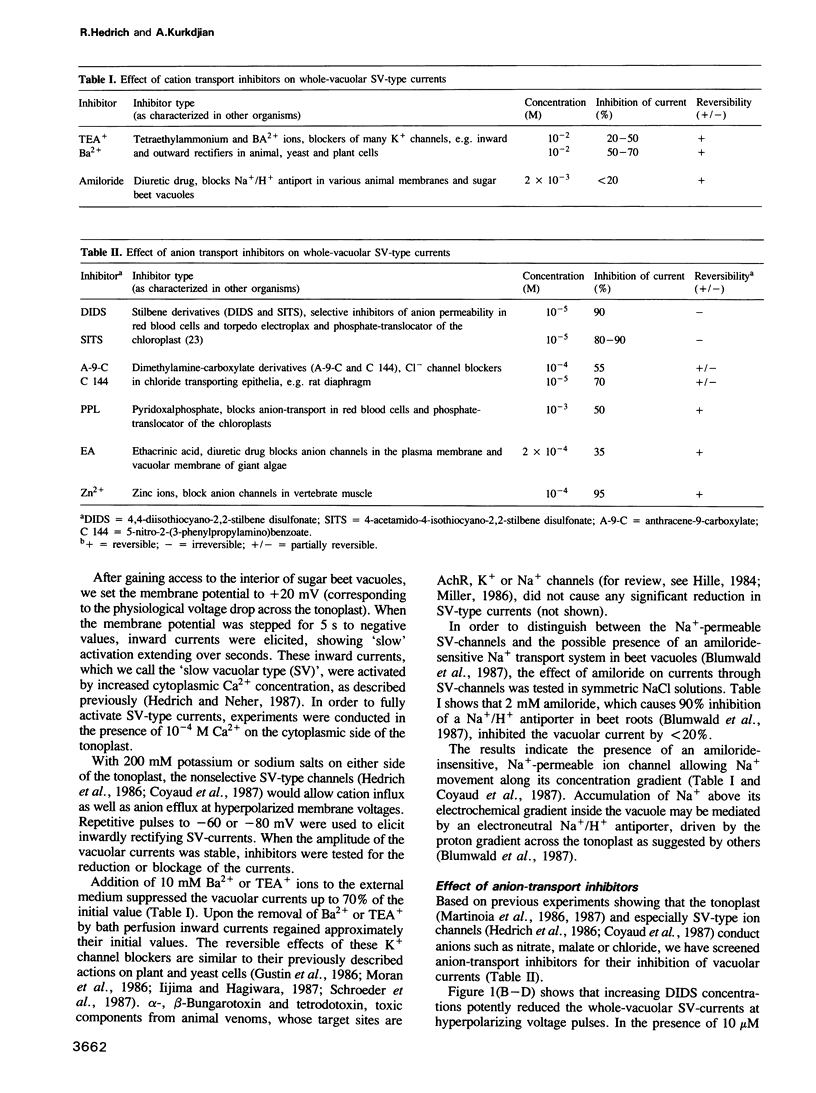
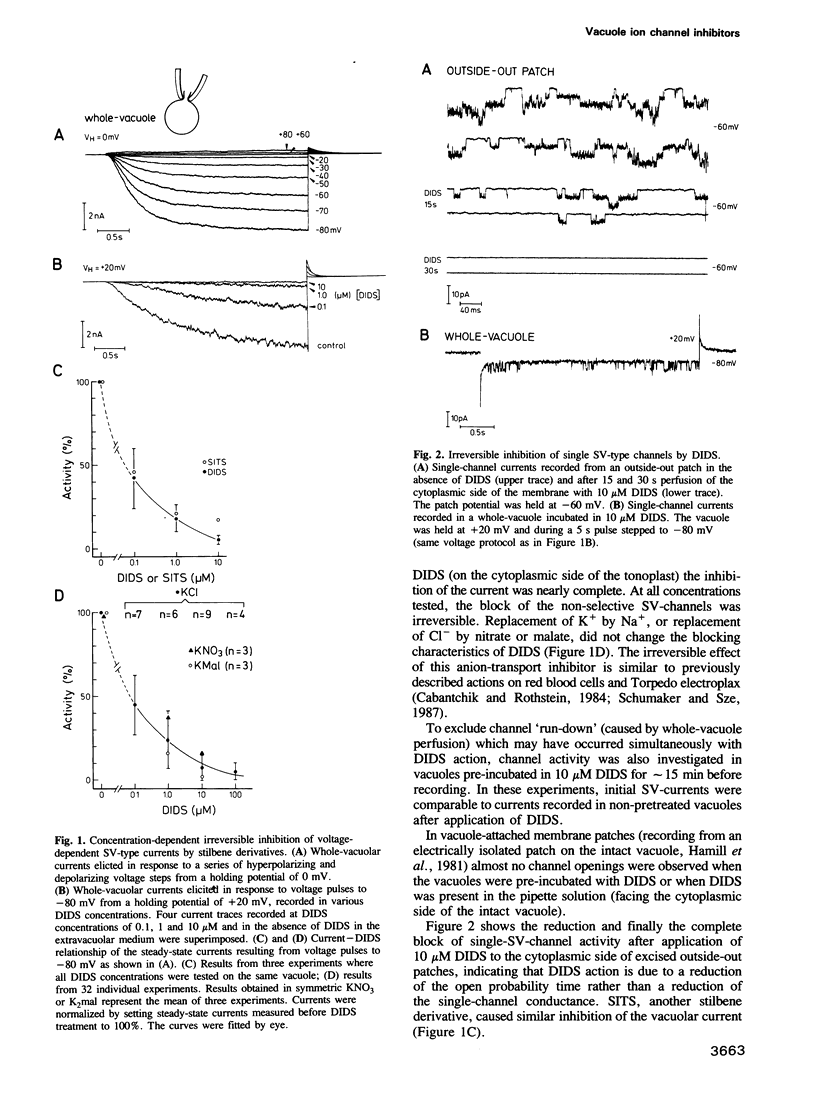
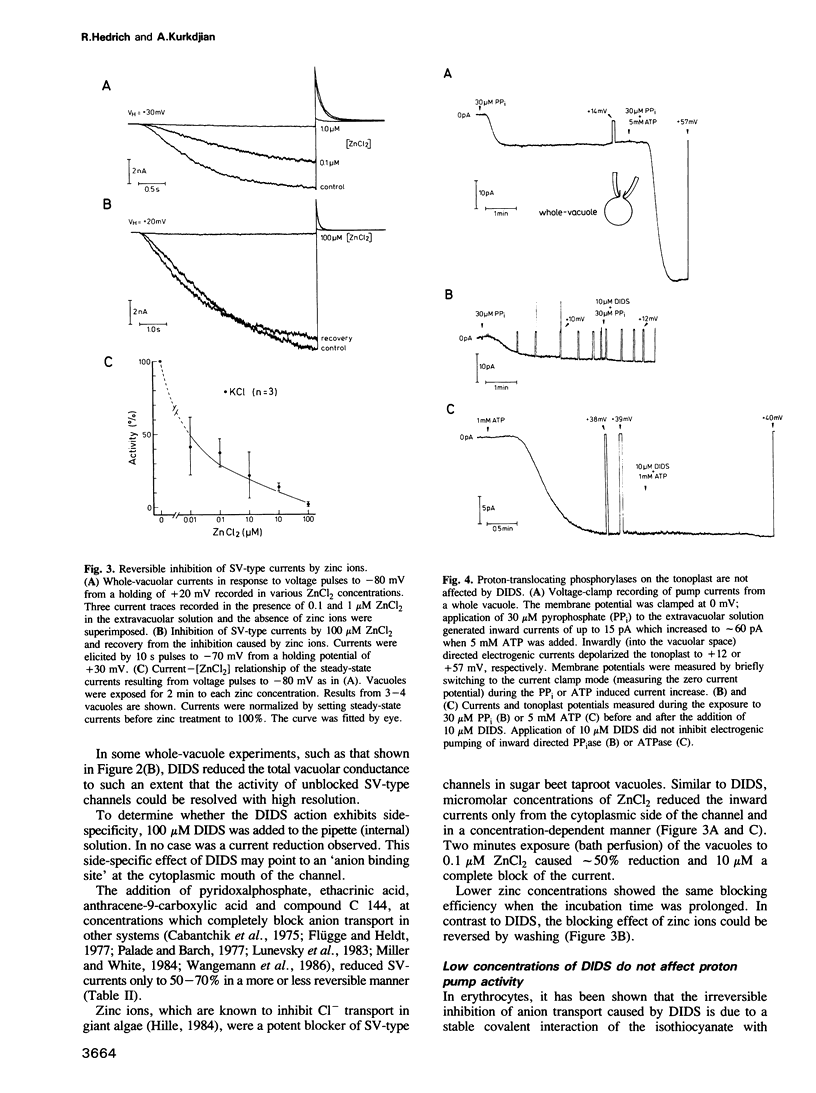
The vacuole occupies 25-95% of the plant cell volume and plays an essential role in maintaining cytoplasmic homeostasis of nutrients and ions. Recent patch-clamp studies identified ion channels and electrogenic pumps as pathways for the movement of ions and metabolites across the vacuolar membrane (tonoplast). At high cytoplasmic Ca2+ (>10-6 M) and negative potentials (inside the vacuole) non-selective channels of the `slow-vacuolar (SV)-type' were activated resulting in anion release or cation influx. In the present study these vacuolar channels were characterized pharmacologically by ion channel inhibitors. The cation-transport inhibitors Ba2+, TEA+ and amiloride caused only partial and reversible block of the `SV-type'channels, whereas anion-transport inhibitors strongly affected the vacuolar channels. Pyridoxalphosphate and the dimethylaminecarboxylate derivates anthracene-9-carboxylic acid and C 144 reversibly blocked the channels up to 70% and Zncl2 up to 95%. DIDS and SITS inhibited this channel irreversibly up to 95%. The block developed under a variety of experimental conditions using solutions containing combinations of permanent cations and anions. The DIDS binding site is located on the cytoplasmic surface of the tonoplast, as intravacuolar DIDS did not block the channels. DIDS concentrations in the micromolar range, efficient in blocking 70—80% of the `SV-type' channels did not significantly affect ATP-induced or pyrophosphate-induced proton-pumps. Stilbene derivatives may therefore be useful tools for studies on the substrate binding site on this vacuolar channel and for channel isolation.
Keywords: patch clamp, ion channels, inhibitors, vacuoles, sugar beet
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Cragoe E. J., Poole R. J. Inhibition of na/h antiport activity in sugar beet tonoplast by analogs of amiloride. Plant Physiol. 1987 Sep;85(1):30–33. doi: 10.1104/pp.85.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik I. Z., Balshin M., Breuer W., Rothstein A. Pyridoxal phosphate. An anionic probe for protein amino groups exposed on the outer and inner surfaces of intact human red blood cells. J Biol Chem. 1975 Jul 10;250(13):5130–5136. [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Heldt H. W. Specific labelling of a protein involved in phosphate transport of chloroplasts by pyridoxal-5'-phosphate. FEBS Lett. 1977 Oct 1;82(1):29–33. doi: 10.1016/0014-5793(77)80878-8. [DOI] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iijima T., Hagiwara S. Voltage-dependent K channels in protoplasts of trap-lobe cells of Dionaea muscipula. J Membr Biol. 1987;100(1):73–81. doi: 10.1007/BF02209142. [DOI] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., White M. M. Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1984 May;81(9):2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977 Jun;69(6):879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Decrease of pH Gradients in Tonoplast Vesicles by NO(3) and Cl: Evidence for H-Coupled Anion Transport. Plant Physiol. 1987 Mar;83(3):490–496. doi: 10.1104/pp.83.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]


