Abstract
The anti-cancer actions of vitamin D and its hormonally active form, calcitriol, have been extensively documented in clinical and pre-clinical studies. However, the mechanisms underlying these actions have not been completely elucidated. Here we examined the effect of dietary vitamin D and calcitriol on mouse breast tumor-initiating cells (TICs, also known as cancer stem cells). We focused on MMTV-Wnt1 mammary tumors, for which markers for isolating TICs have previously been validated. We confirmed that these tumors expressed functional vitamin D receptors (VDRs) and estrogen receptors (ERs) and exhibited calcitriol-induced molecular responses including ER down-regulation. Following orthotopic implantation of MMTV-Wnt1 mammary tumor cells into mice, calcitriol injections or a vitamin D-supplemented diet caused a striking delay in tumor appearance and growth while a vitamin D-deficient diet accelerated tumor appearance and growth. Calcitriol inhibited TIC tumor spheroid formation in a dose-dependent manner in primary cultures and inhibited TIC self-renewal in secondary passages. A combination of calcitriol and ionizing radiation inhibited spheroid formation more than either treatment alone. Further, calcitriol significantly decreased TIC frequency as evaluated by in vivo limiting dilution analyses. Calcitriol inhibition of TIC spheroid formation could be overcome by the overexpression of β-catenin, suggesting that the inhibition of Wnt/β-catenin pathway is an important mechanism mediating the TIC inhibitory activity of calcitriol in this tumor model. Our findings indicate that vitamin D compounds target breast TICs reducing tumor-initiating activity. Our data also suggest that combining vitamin D compounds with standard therapies will enhance anti-cancer activity and may improve therapeutic outcomes.
Keywords: Breast cancer, vitamin D, calcitriol, MMTV-Wnt1, tumor initiating cells (TICs), Wnt/β-catenin
INTRODUCTION
Breast cancer (BCa) is the most common cancer and the second most common cause of cancer death in women. Breast tumors are composed of heterogeneous subpopulations of cells and are maintained by tumor-initiating cells [TICs; also called cancer stem cells (CSCs)] that can initiate new tumors, which recapitulate the heterogeneity of the original mass (1). TICs can self-renew to generate more TICs or can differentiate into progeny called non-tumorigenic cells (NTCs) that often make up the bulk of a tumor (2). Since TICs maintain tumors, elimination of TICs is critical for achieving cure. Previous studies have found that cells resembling TICs are relatively resistant to radiotherapy and chemotherapy compared to NTCs (3,4), suggesting that improved outcomes for BCa patients will require direct targeting of TICs or overcoming their resistance mechanisms.
Calcitriol (1,25-dihydroxyvitamin D3), the hormonally active form of vitamin D, is a potent steroid hormone that acts through its cognate nuclear receptor, the vitamin D receptor (VDR), and regulates the expression of target genes in most tissues of the body in a context specific manner (5). In addition to its classical actions on calcium homeostasis and bone mineralization, calcitriol has multiple non-skeletal actions, and exhibits direct antiproliferative, pro-differentiating and anti-inflammatory activities in multiple cancer cells including BCa cells (6–11). A recent study of human pancreatic tumors shows that calcitriol exerts inhibitory and anti-inflammatory activities on the cancer-associated fibroblast-like cells in the tumor stroma that promote the initiation and progression of pancreatic cancer, revealing another important mechanism of the anti-cancer activity of calcitriol (12). In preclinical mouse studies, calcitriol also inhibits estrogen synthesis as well as estrogen receptor (ER) expression and estrogen signaling, and shows beneficial effects especially in the treatment of ER-positive BCa (13). Dietary vitamin D supplementation has been shown to exhibit equivalent anti-cancer actions to calcitriol in a mouse BCa model due to intra-tumoral conversion of circulating 25-hydroxyvitamin D [25(OH)D] to calcitriol (14,15). However, the mechanisms by which vitamin D and calcitriol inhibit the initiation and progression of BCa have not been completely elucidated.
Recent studies have suggested that calcitriol regulates the self-renewal and differentiation of several types of normal stem cells as well as TIC-like cells found in established cell lines. In one study, calcitriol treatment promoted cell cycle arrest, senescence, and the differentiation of a cell line model of normal prostate progenitor/stem cells into luminal cell fate through IL-1α (16). Previous studies of TIC-like cells found that the vitamin D analog BXL0124 repressed the expression of CD44, a TIC marker, in a BCa cell line (17). Silencing of the VDR gene increased the expression of genes related to epithelial-mesenchymal transition (EMT) and mammosphere formation in TN and SKBR3 cells (18). Another study reported the repression of markers associated with stem cell–like phenotype as well as pluripotency markers in MCF10DCIS cell line treated with calcitriol or a vitamin D analog (19). These data suggest that vitamin D may inhibit normal stem cell function and may target TIC-like cells.
While these findings using TIC-like cells are intriguing, significant uncertainty remains regarding how well these cells approximate TICs from primary tumors. We therefore hypothesized that vitamin D and calcitriol target primary breast cancer TICs and set out to test this hypothesis using TICs from MMTV-Wnt1 tumors, for which markers for isolating TICs have previously been validated (14,20). We discovered that vitamin D and calcitriol inhibited the growth of MMTV-Wnt1 mammary tumors in mice and calcitriol decreased TIC proliferation and self-renewal, measured both ex vivo and in vivo. Calcitriol treatment inhibited Wnt-target gene expression in the tumors as well as in primary cultures of the TICs. Constitutive activation of the Wnt/β-catenin pathway in the TICs abrogated calcitriol’s inhibitory effect on TIC proliferation ex vivo. Further, a combination of calcitriol and ionizing radiation inhibited TIC proliferation more than either treatment alone. Our findings suggest that vitamin D compounds target breast TICs and therefore have potential therapeutic utility in BCa prevention and treatment.
MATERIALS AND METHODS
MATERIALS
1,25-dihydroxyvitamin D3 (calcitriol) was a kind gift from Milan Uskokovic (BioXell Company, Nutley NJ). The rodent diets were from Research Diets Inc. (New Brunswick, NJ). [H3]-labeled 17-β estradiol (E2) and [H3]-1,25-dihydroxyvitamin D3 were obtained from Amersham – GE Healthcare Life Sciences (Buckinghamshire, UK). Tissue culture media, supplements and fetal bovine serum (FBS) were obtained from GIBCO BRL (Grand Island, NY) and Mediatech Inc. (Herndon, VA). Matrigel was obtained from BD Biosciences (San Jose, CA). Female FVB/NJ female mice used for tumor implantation and testing the effects of the vitamin D compounds were obtained from the Jackson Laboratory (Bar Harbor, Maine).
METHODS
Breast tumor dissociation, flow cytometry, and cell isolation
Mammary tumors from FVB/NJ female mice bearing MMTV-Wnt1 tumor orthografts (FVB.Cg-Tg(Wnt1)1Hev/J) (21) were minced with a razor blade and suspended in 10 ml of L-15 Leibovitz medium (Thermo Fisher Scientific Inc., Waltham, MA) supplemented with 0.5 mL of collagenase/hyaluronidase (Stem Cell Technologies, Vancouver, BC, Canada). Tumors were digested to completion for 1.5–2 h at 37 °C and 5% CO2 with manual dissociation by pipetting every 30 min. Once digested, 20 ml of Hank’s balanced salt solution (HBSS) with 2% bovine calf serum (BCS) was added and tumor cells were collected by centrifugation. Tumor cells were resuspended in 5 ml of trypsin/0.05% EDTA for 5 min and centrifuged. The cell pellet was resuspended in HBSS with 2% BCS and incubated with 100 Kunitz units of DNase I (Sigma) and Dispase (Stem Cell Technologies) for 5 minutes at 37 °C and centrifuged again with the addition of HBSS with 2% BCS. Once digested, tumor cells were treated with ACK (Ammonium-Chloride-Potassium) lysis buffer to lyse the red blood cells and filtered through a 40 μm cell strainer (BD Biosciences). After centrifugation, tumor cells were resuspended in HBSS with 2% BCS, blocked with rat IgG for 10 min, and stained with rat anti-mouse CD31 (Biolegend, San Diego CA), anti-mouse CD45 (Biolegend), anti-mouse CD140a (eBioscience, San Diego, CA), rat anti-mouse EpCAM (Biolegend), and rat anti-human/mouse CD49f (BD Biosciences, Franklin Lakes, NJ). Lineage negative, viable, EpCAM+CD49fhigh cells were sorted for further analysis. A minimum three tumors from different mice were used to generate the tumor spheroid assay results described below and the numbers of replicates are indicated in each figure legend.
Ex vivo tissue slice culture assays
300 μm sections were precision cut from MMTV-Wnt1 tumor orthografts to generate tissue slices. The slices were transferred in a sterile manner to titanium mesh inserts in sterile six-well plates containing culture media mounted on a rotating platform set at a 30° angle in a tissue culture incubator at 37°c with 95% air and 5% CO2 as described before (22,23). The tumor tissue slices were incubated in phenol-red free DMEM-F12 media containing 5% charcoal-stripped FBS containing vehicle, calcitriol (100 nM), E2 (10 nM) or a combination of both for 5 h following which RNA was isolated from the tissue slices for the measurement of estrogen receptor α (Erα), progesterone receptor (Pr), 25-hydroxyvitamin D3-24 hydroxylase (Cyp24), 25-hydroxyvitamin D3-1a hydroxylase (Cyp27B1), Vdr and aromatase (Cyp19) mRNA expression. At least three tumors from different mice were used to generate the tissue slices.
[3H]-Estradiol and [3H]-1,25(OH)2D3 binding assays
The expression of ER and VDR in high salt homogenates of MMTV-Wnt1 tumor tissue was determined by [3H]-estradiol and [3H]-1,25(OH)2D3 binding respectively as described before (24,25). Protein concentrations of tissue homogenates were measured by the method of Bradford (26).
Mouse Studies
All animal procedures were performed in compliance with the guidelines approved by Stanford University Administrative Panels on Laboratory Animal Care. Five to six week-old female FVB/N mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in a designated pathogen-free area in a facility at Stanford University School of Medicine accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Dietary manipulations and calcitriol treatment
The mice were randomly assigned to different experimental groups and fed various diets for a period of 12 weeks prior to tumor inoculation. The mice on the standard diet received vehicle or calcitriol injections 8 weeks prior to tumor inoculation. The four experimental groups were as follows: (1) mice fed a standard diet (AIN76, Research Diets Inc, 1000 IU of vitamin D3/kg diet) receiving injections of vehicle, (2) mice fed standard diet receiving injections of calcitriol (50 ng/mouse three times/week), (3) mice fed a vitamin D-supplemented diet (5000 IU of vitamin D3/kg of diet) and (4) mice fed a vitamin D-deficient diet (100 IU of vitamin D3/kg of diet). Stock solutions of calcitriol were made in 100% ethanol and stored at −20°C. Appropriate dilutions were made in sterile phosphate-buffered saline (PBS) and were administered by intraperitoneal (i.p.) injections 3 times a week (on Mondays, Wednesdays and Fridays). The mice in the control group received i.p. injections of 0.1% ethanol in PBS (vehicle). The dosage of calcitriol and the intermittent administration regimen were based on our prior work (15,27).
Establishment and growth of MMTV-Wnt1 orthotopic tumors
Tumors were established in FVB/NJ female mice by injecting MMTV-Wnt1 tumor cell suspensions (~ 150,000 cells suspended in 100 μl of a 1:1 mixture of the culture medium and Matrigel). Orthotopic implantation was done using a midline abdominal skin incision to visualize the fourth mammary fat pads (inguinal glands) and cell suspensions were injected directly into the mammary fat pad on the left side. For tumor cell implantation, mice were manipulated in surgical aseptic conditions under isoflurane anesthesia and received carprofen (5 mg/kg) for analgesia. The experimental diets and calcitriol injections were continued over the next 7 weeks. Body weights and tumor sizes were measured weekly after tumor inoculation and continued throughout the experiment. Tumor volumes were calculated from two tumor diameter measurements using a vernier caliper and using the formula: tumor volume = (length x width2)/2 (15,27). At the end of the study, 14 hours after the final calcitriol injections, mice were euthanized according to APLAC guidelines using CO2. Blood samples were collected by cardiac puncture while under CO2 anesthesia causing exsanguinations and serum samples were prepared and frozen. Tumors were harvested, measured, weighed and snap frozen in liquid nitrogen for subsequent analysis.
Tumor spheroid culture assay
FACS-sorted MMTV-Wnt1 tumor cells were cultured in the presence of vehicle or calcitriol in DMEM/F12 medium with 2% B27, 20 ng/ml mouse EGF, 20 ng/mL human FGF (BD, #354060), and 1% antibiotics, and plated on top of solidified Matrigel (28) for 10–14 days. Spherical colonies (> 50 μm in diameter) were counted using Clono-counter or manually. For the secondary colony formation assay, spheroids were dissociated with 1 mg/mL dispase (Invitrogen, #17105-041) for 30 minutes, digested with trypsin/0.05% EDTA for 5 minutes, and passaged through a 27-G needle five times to dissociate into single cells (29). After centrifugation, cells were resuspended and cultured as described above. For radiosensitization experiments, sorted TICs were irradiated by 2 Gy and cultured in media containing vehicle or calcitriol for 10–14 days.
Transplantation and Limiting Dilution Analysis
Dissociated spheroid cells were resuspended in culture medium with 25% Matrigel and injected subcutaneously into mice in the vicinity of the mammary fat pads. After injections, mice were examined weekly for up to 4–5 months for tumor formation. Tumor initiating cell frequencies were calculated using L-Calc (Vancouver, BC, Canada, http://www.stemcell.com).
Statistical Analysis
Statistical analyses were performed in GraphPad Prism using Student’s t-test for single comparisons and ANOVA for multiple comparisons, with Bonferroni correction for post hoc analyses. The limiting dilution analysis experiments were analyzed using L-Calc (Vancouver, BC, Canada, http://www.stemcell.com). Data are presented as mean ± standard error of the mean (S.E.M.) using at least 3 independent experiments. P < 0.05 was considered significant.
Detailed methods are available in Supplementary Materials and Methods.
RESULTS
Estrogen- and calcitriol-mediated bio-responses in MMTV-Wnt1 tumors
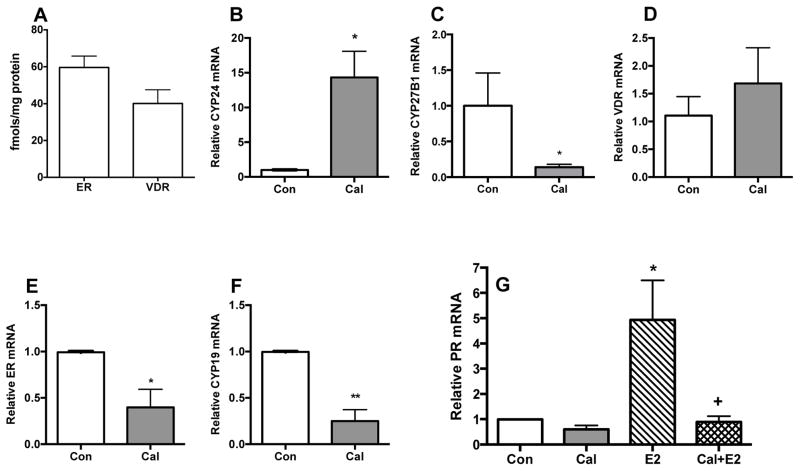
To explore the effect of vitamin D or calcitriol treatment on breast TICs, we focused on MMTV-Wnt1 mammary tumors, because they have well-characterized TIC subpopulation (14,20,30). In initial experiments we used tissue slice cultures that provide realistic preclinical models of diverse tissues and organs. The use of tissue slice cultures to investigate hormone dependence and cancer-specific responses has been validated in studies of benign and malignant human prostate tissue (23). [3H]-labeled ligand binding assays in ex vivo cultures of MMTV-Wnt1 tumor tissue slices demonstrated that these tumors expressed functional ER and VDR proteins (Fig. 1A). Calcitriol treatment of tissue slices induced Cyp24 mRNA expression (Fig 1B) and repressed that of Cyp27B1 (Fig 1C), while Vdr mRNA showed no changes (Fig 1D). Calcitriol also repressed the expression of Erα (Fig 1E) and aromatase (Cyp19) mRNA (Fig. 1F). Estradiol (E2) treatment of tissue slice cultures caused a significant induction of Pr mRNA expression, confirming ER function (Fig. 1G) and co-treatment with calcitriol attenuated PR induction by E2 (Fig. 1G). These data demonstrate calcitriol inhibition of estrogen synthesis and signaling as previously described in a different breast cancer model (13). These data also demonstrate the presence of both ER and VDR in MMTV-Wnt1 tumor cells and their functional responses to hormone.
Fig. 1. Presence and functional activity of ER and VDR in tissue slice cultures of MMTV-Wnt1 tumors.
MMTV-Wnt1 tumor orthografts were used to generate tissue slices. The slices were cultured and the expression of ER and VDR and their functional responses were determined as described in Methods Section. A, Basal ER and VDR levels. [3H]-labeled ligand binding assays revealed the expression of ER and VDR proteins in the tumor slices. B–F, VDR functional responses. Tissue slice cultures were treated with 0.1% ethanol vehicle or 100 nM calcitriol (Cal) for 5 h and the mRNA levels of the Cyp24, Cyp27B1, Vdr, Erα and Cyp19 were determined by qRT-PCR (n = 4; * p<0.05 and ** p<0.01 as compared to the Std group). G, ER functional response. Tissue slice cultures in phenol red-free culture media were exposed to 0.2% ethanol vehicle (control, Con), 100 nM calcitriol (Cal), 10 nM E2 (E2) or a combination of both (Cal+E2) for 5 h and PR mRNA levels were determined by qRT-PCR (n = 4; * p<0.05 as compared to Con and + p < 0.05 as compared to E2). At least three different orthografts were used to generate the tissue slices and each experiment was conducted in duplicate. Values represent mean ± SEM.
Dietary vitamin D3 status and calcitriol effect tumor appearance and growth
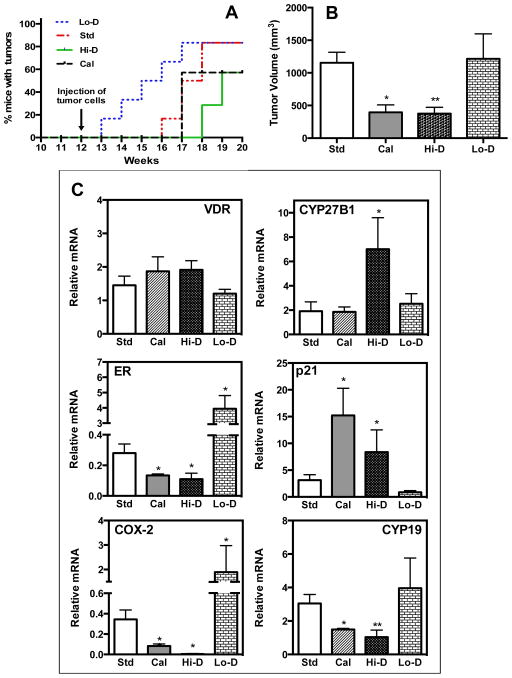
We next investigated the in vivo effects of diets supplemented with vitamin D3 and calcitriol injections on the growth of MMTV-Wnt1 mammary tumors. Mice in the various experimental groups were monitored for the appearance of palpable tumors. As shown in Fig. 2A, within a week of tumor cell implantation (experimental week 13), palpable tumors appeared in 20% of the mice fed the vitamin D-deficient diet while no tumors were noted in the mice in other experimental groups. Palpable tumors appeared in 20% of the mice fed the standard diet 4 weeks after tumor inoculation (experimental week 16) at which time point ~66% of the mice on the vitamin D-deficient diet exhibited tumors. However, in calcitriol-treated mice and in mice fed the vitamin D-supplemented diet, tumor appearance was first noted at 5 and 6 weeks after tumor inoculation (experimental weeks 17 and 18), respectively (Fig. 2A). The time to appearance of tumors in 50% of mice in the vitamin D-deficient group (3.5 weeks after tumor inoculation) was significantly shorter (p = 0.05) than in the control group receiving the standard diet (5.5 weeks after tumor inoculation). In contrast, the time to appearance of tumors in 50% of mice in the vitamin D-supplemented group (7 weeks after tumor inoculation) was significantly delayed (p = 0.016) compared to the group fed the standard diet. There were no significant differences between the standard diet group and the calcitriol-treated group in the time taken for the appearance of tumors in 50% of the mice but calcitriol treatment reduced the total number of tumors that appeared. At the end of the study, 20% of the mice were tumor-free in the vitamin D-deficient and the standard diet groups, while 40% of the mice were tumor-free in the calcitriol and vitamin D-supplemented groups.
Fig. 2. Effect of calcitriol and dietary vitamin D3 levels on the appearance and growth of MMTV-Wnt1 tumors.
FVB/N mice were fed the standard diet (Std), a vitamin D-deficient diet (Lo-D) or a vitamin D-supplemented diet (Hi-D) for 12 weeks (Experimental weeks 0–12). A parallel experimental group consisted of mice on the standard diet receiving calcitriol injections (Cal) in the last seven weeks of the 12-week period (Experimental weeks 6–12). On Experimental week 12 MMTV-Wnt1 tumor cell suspensions were implanted in the left inguinal mammary fat pads of the mice. Diets and calcitriol treatments were continued for the next 7 weeks (Experimental weeks 12–19) and tumor appearance and growth were monitored.
A, Tumor appearance. Kaplan-Meier analysis of the occurrence of palpable tumors in mice in the various experimental groups: Std (red line), LO-D (blue line), Cal (green line) and Hi-D (magenta line). B, End-point tumor volumes. Tumor volumes in the various experimental groups at the end of the study are shown (The experiment was performed twice with half the number of mice each time. Total number of mice in each group was as follows: Std diet =10; Cal=6; Hi-D=6; and Lo-D=6.; * p<0.05 and ** p < 0.001 as compared to the Std group). C, Changes in mRNA expression of Vdr, CYP27B1, Erα, p21, Cox-2 and aromatase (Cyp19) in MMTV-Wnt1 tumors due to calcitriol and dietary vitamin D status. Relative mRNA expression of each gene is shown with the expression in tumors from mice on the standard diet (Std) set at 1. (n = 6–10 determinations; * p < 0.05 as compared to Std). Values represent mean ± SEM.
We further observed that the mean tumor volume in mice receiving calcitriol injections or fed the vitamin D3-supplemented diet was significantly decreased compared to mice on the standard or vitamin D-deficient diet at the end point of the study (experimental week 19; Fig. 2B). In the group that received the standard diet, the tumors achieved a mean volume of ~1,150 mm3 while in mice receiving either calcitriol injections or the vitamin D3-supplemented diet mean end point tumor volumes were ~66% and ~67% lower (p < 0.05 – p < 0.01). Although a significant acceleration in the time of appearance of tumors was seen in mice fed the vitamin D-deficient diet, the mean end-point tumor volume in these mice was similar to that seen in the mice fed the standard diet. Thus, vitamin D deficiency accelerated tumor appearance and dietary vitamin D supplementation and calcitriol treatment inhibited both tumor appearance and the growth of tumors.
Effects of dietary D3 and calcitriol on body weight and serum calcium and vitamin D metabolites
Blood samples were drawn at the time of sacrifice 14 h after the final calcitriol injections for the measurement of serum calcium and vitamin D metabolites. As expected, administration of the vitamin D-supplemented diet caused a significant elevation (~72% increase, p<0.001) and the vitamin D-deficient diet caused a significant decrease (~63% decrease, p<0.001) in the mean serum levels of 25(OH)D, in comparison to the mice fed the standard diet (Table 1). In the mice receiving calcitriol, the mean serum 25(OH)D level was significantly decreased (Table 1) as expected after calcitriol treatment due to the induction of CYP24 and degradation of 25(OH)D (5). Also, in the mice receiving calcitriol injections, the mean serum 1,25(OH)2D level showed an expected decrease due to induction of Cyp 24 (Table 1). It is important to note that the blood samples were drawn 14 hours after the final calcitriol injections and the data from mice receiving calcitriol therefore represent the nadir values of serum 1,25(OH)2D concentrations that peak between 1 and 3 hours after injections (31). The mice receiving the vitamin D-deficient diet also registered a significant decrease in the mean serum 1,25(OH)2 D level. Interestingly mice ingesting the vitamin D-supplemented diet displayed a substantial and statistically significant elevation in the mean serum 1,25(OH)2D level, a phenomenon we previously described in tumor-bearing mice in other mouse models of breast and prostate cancers and is thought to be due to intra-tumoral synthesis of 1,25(OH)2D and its release into the circulation (15). Although the mean serum calcium level in the calcitriol treated group was slightly higher than the mean serum calcium in the group receiving the standard diet, this increase was not statistically significant (Table 1). There were no statistically significant differences in mean serum calcium levels between the other experimental groups (Table 1). There were no significant differences in body weights of the mice in the various experimental groups (Table 1).
TABLE 1.
Body Weights and Serum measurements
| GROUPS | Body Weight (g) | Serum 25-Hydroxy-vitamin D (ng/ml) | Serum 1,25-dihydroxy-vitamin D (pg/ml) | Serum Calcium (mg/dl) |
|---|---|---|---|---|
| Std | 23±0.8 | 47±3 | 91±10 | 10.5±2 |
| Cal | 21±0.8 | 35±11 | 46±8* | 11.2±3 |
| Hi-D | 22±0.7 | 74±5* | 197±33* | 10.5±1 |
| Lo-D | 24±0.6 | 10±4* | 43±3** | 9.5±3 |
p < 0.05;
p < 0.01 when compare to Std diet. Values are represented as Mean ± SEM of 5–8 determinations for each group.
Abbreviations: Std = Std diet, Cal = Std diet+calcitriol, Hi-D = High vitamin D diet, Lo-D = Low vitamin D diet.
Changes in gene expression due to dietary vitamin D3 and calcitriol in MMTV-Wnt1 tumors
We next examined the effect of dietary vitamin D supplementation and calcitriol administration on gene expression in orthotopic MMTV-Wnt1 tumors as a measure of their abilities to inhibit estrogen synthesis (Aromatase) and signaling (Erα) as well as to exert anti-inflammatory (Cox-2) and anti-proliferative (p21) activities. As shown in Fig. 2C, MMTV-Wnt1 tumors expressed Vdr and CYP27B1 mRNA at levels that were not altered by calcitriol or dietary vitamin D except for the increase seen in CYP27B1 mRNA in mice receiving the vitamin D-supplemented diet, a phenomenon we have described previously in other mouse models of BCa (15). Calcitriol and dietary vitamin D administration resulted in significantly decreased tumor mRNA expression of Erα, aromatase (Cyp19) and Cox-2 compared to mice fed the standard diet, while the mRNA levels of the cell cycle inhibitor p21 was increased by calcitriol and dietary vitamin D supplementation (Fig 2C). Dietary vitamin D deficiency had the opposite effects causing increased expression of Erα, aromatase and Cox-2 mRNA and decreased p21 mRNA in the tumors. Thus, the dietary content of vitamin D and the dose of calcitriol that we used were sufficient to lead to vitamin D signaling-related gene expression changes in the tumors while the vitamin D deficient diet caused a reversal of these expression patterns.
Calcitriol targets tumor-initiating cells
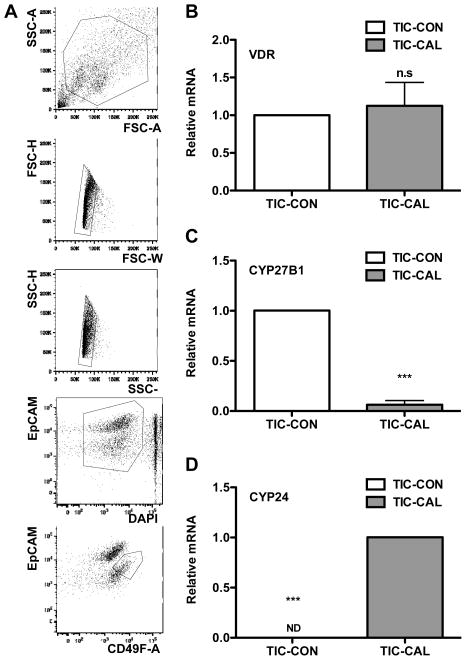
Given the ability of dietary vitamin D and calcitriol to inhibit tumor growth and previous observations of effects of vitamin D compounds on normal stem cells and TIC-like cells from cell lines (16–19), we hypothesized that these agents target TICs in MMTV-Wnt1 mammary tumors. To functionally test this hypothesis, first we sorted the CD49fhighEpcamlow population of cells, that has been shown to be significantly enriched for TICs (14,20), from freshly dissociated tumors (Fig. 3A) and treated them with calcitriol in vitro overnight. While calcitriol treatment did not change Vdr gene expression, it decreased the expression of Cyp27b1 mRNA and increased the expression of Cyp24a1 mRNA, demonstrating that TICs in MMTV-Wnt1 mammary tumors express VDR, which is functionally responsive to calcitriol (Fig. 3B-D).
Fig. 3. Breast cancer tumor-initiating cells express functionally active vitamin D receptor.
A, FACS sorting schema for purification of CD49fhiEpCamlow MMTV-Wnt-1 TIC-enriched cells. B–D, VDR functional responses in breast cancer TICs. Sorted CD49fhiEpCamlow TIC cells were treated with 1 nM calcitriol (Cal) for 16 h and mRNA levels of Vdr, Cyp24, and Cyp27b1 were determined by qRT-PCR (n = 3; *** p<0.01 as compared to the CON group; ND, not detected). Values represent mean ± SEM.
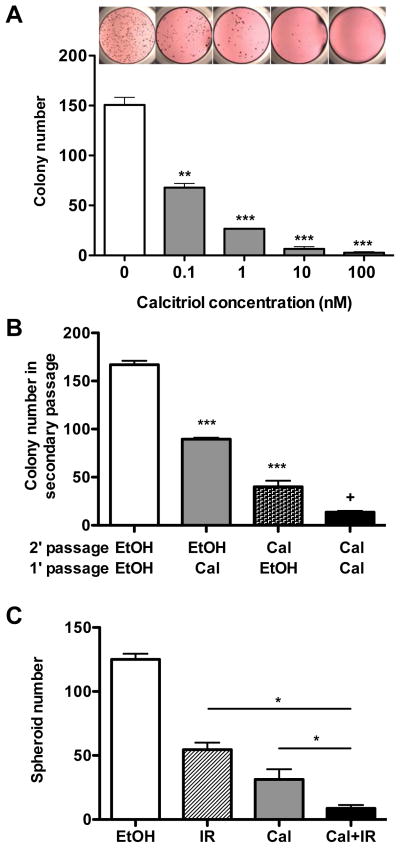
To further examine whether vitamin D compounds functionally target TICs, we plated TICs from MMTV-Wnt1 mammary tumors in a previously described 3-dimensional tumor spheroid culture assay (14). Treatment with calcitriol decreased TIC spheroid formation in a dose dependent manner (Fig. 4A) and calcitriol-treated TICs formed fewer spheroids than vehicle-treated TICs in secondary passage without further calcitriol addition, suggesting that calcitriol inhibits both TIC-initiated spheroid formation and the ability of TICs to self-renew (Fig. 4B).
Fig. 4. Calcitriol inhibits the self-renewal of breast cancer tumor-initiating cells.
A, Representative images and bar graphs showing that calcitriol treatment (vehicle or 0.1, 1, 10, and 100 nM) decreased the spheroid forming abilities of TICs in a dose dependent manner (n = 3; ** P < 0.01 and *** P < 0.001 as compared to the control). B, Calcitriol treatment (at 1 nM) in primary culture reduced the spheroid formation in secondary culture (n = 3; *** P < 0.001 as compared to white bar and + P < 0.001 as compared to dotted bar). C, Analysis of combined treatment of TICs with calcitriol (10 nM) and 2 Gy of ionizing radiation (n = 3; P < 0.05). Values represent mean ± SEM.
Previous studies from several labs, including ours, have shown that TICs are more resistant to ionizing radiation and chemotherapy than non-tumorigenic cancer cells (3,4). Since calcitriol was able to target TICs and inhibit their proliferation, we tested whether calcitriol could also enhance the inhibitory effect of ionizing radiation on TICs. As shown in Fig. 4C, calcitriol in combination with a clinically relevant dose of ionizing irradiation (2 Gy) showed an additive effect in inhibiting TIC spheroid formation.
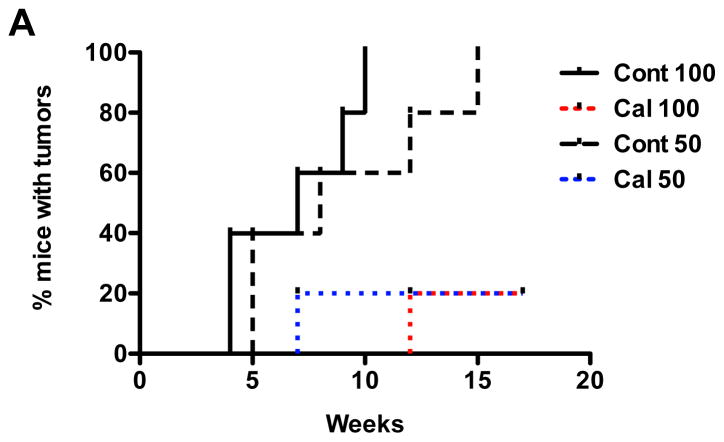
In order to demonstrate that calcitriol treatment inhibits not only the proliferation of TICs but also their tumor initiating activity, we tested whether calcitriol could suppress in vivo tumor forming ability of TICs. Purified CD49fhighEpcamlow TIC-enriched cells were treated with vehicle (ethanol) or calcitriol, dissociated into single cell suspensions, and orthotopically implanted into syngeneic mice in limiting dilution fashion. As shown in Table 2, calcitriol treatment decreased the frequency of TICs by greater than 3-fold (control group: 1/98 (1/66 – 1/147), calcitriol treated group: 1/320 (1/207 – 1/495), P < 0.0046). In addition, the appearance of palpable tumors was significantly delayed by calcitriol pre-treatment (Fig. 5A). Taken together, these data demonstrate that calcitriol targets TICs and inhibits their tumor initiating ability.
TABLE 2.
Limiting Dilution Analysis
| Cell number implanted | Vehicle-treatment | Calcitriol-treatment |
|---|---|---|
| 500 | 5/6 | 4/5 |
| 100 | 5/5 | 1/5 |
| 50 | 4/5 | 1/5 |
|
| ||
| TIC frequency (p < 0.01) | 1/98 (1/66 – 1/147) | 1/320 (1/207 – 1/495) |
Number of tumors formed/number of injections
Limiting dilution analysis of purified CD49fhighEpCamlow TIC-enriched cells from MMTV-Wnt1 mammary tumors. TICs were cultured in the presence of calcitriol (1 nM) or vehicle (ethanol). After 2 weeks, breast tumor spheroids were dissociated into single cells, and varying numbers of viable tumor cells were orthotopically implanted into the mammary fat pad and assayed for tumor initiation. TIC frequencies and p-value were calculated using L-Calc. B,
Fig. 5. Limiting dilution analysis of calcitriol-treated TICs.
A, Kaplan-Meier plot of tumor appearance. Time taken for gross tumor appearance was traced after orthotopic implantation of 50 and 100 control- (ethanol) or calcitriol- treated cells.
Inhibition of Wnt/β-catenin signaling pathway contributes to the anti-TIC effect of calcitriol and vitamin D
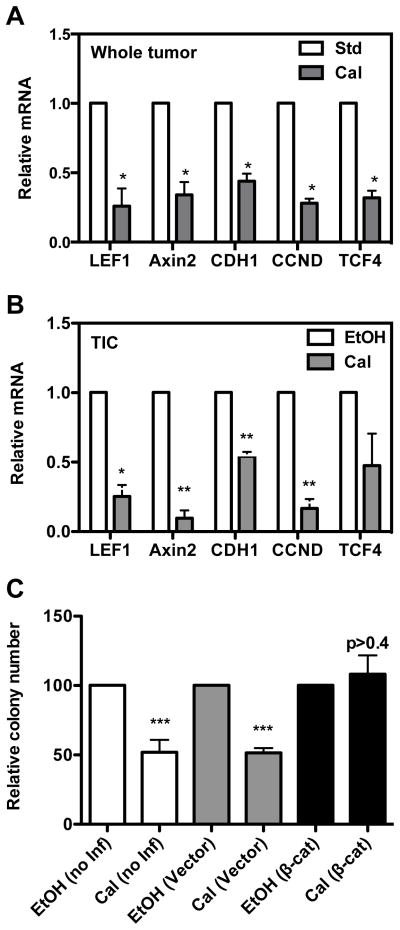
Wnt/β-catenin signaling plays an important role in normal and cancer stem cell function (32,33). It is activated in triple negative breast cancers, and has been shown to be associated with poor survival in these patients (34,35). We therefore hypothesized that calcitriol inhibits the Wnt/β-catenin pathway in TICs from MMTV-Wnt1 tumors. We first examined the effect of calcitriol in vivo on the expression of the Wnt target genes (Lef1, Axin2, Cdh1, Ccnd and Tcf4) in MMTV-Wnt1 tumors. As shown in Fig. 6A, calcitriol administration to mice significantly reduced expression of the Wnt target genes in MMTV-Wnt1 orthotopic tumors compared to the mice on the standard diet. Consistently, in vitro treatment of TIC cultures with calcitriol for 16 h also decreased the expression of these Wnt target genes (Fig. 6B). However, Wnt1 gene expression was not significantly affected by calcitriol treatment (Supplemental Fig. 1), showing that calcitriol inhibits Wnt/β-catenin pathway without inhibiting expression of Wnt1.
Fig. 6. Effect of calcitriol on Wnt signaling and downstream target genes.
A, Changes in the expression of Wnt target genes in MMTV-Wnt1 mammary tumors harvested from mice on the standard diet (Std) and those receiving calcitriol (Cal). Relative mRNA expression of each gene is shown with the expression in tumors from mice on the standard diet (Std) normalized to 1 (n = 6–10; *, P < 0.05). B, Changes in the expression of Wnt target genes in CD49fhiEpCAMlow TIC-enriched cells treated with ethanol or calcitriol (1 nM) for 16 hrs. Relative mRNA expression of each gene is shown (n = 3; ***, P < 0.05). C, Effect of constitutively active β-catenin on calcitriol sensitivity of TICs. Spheroid formation by CD49fhiEpCamlow cells was assayed after treatment with vehicle (ethanol) or calcitriol (1 nM) following transduction with control or constitutively active β-catenin expressing lentiviruses (n = 5–6; P < 0.05). Values represent mean ± SEM.
Finally, to examine the role of the repression of Wnt signaling in calcitriol’s inhibitory effect on TICs, we tested whether the constitutive expression of β-catenin could overcome calcitriol’s inhibitory effect on TIC spheroid formation. A constitutively active β-catenin expression plasmid was introduced into CD49fhighEpcamlow TIC-enriched cells using lentiviral transduction (36). As shown in Fig. 6C, the overexpression of β-catenin completely abrogated the inhibitory effect of calcitriol on TIC spheroid formation. These data indicate that inhibition of the Wnt/β-catenin signaling pathway is essential for calcitriol regulation of TICs.
DISCUSSION
Intriguing but inconsistent epidemiological data have generally supported the hypothesis that vitamin D may improve breast cancer risk and prognosis (7,37). Consistent and compelling data from cultured cell experiments have demonstrated a substantial inhibitory effect of calcitriol on BCa cell growth, suggesting the epidemiologic benefits result from a direct causative effect of vitamin D signaling on BCa (7,10,11). In vivo experiments using a variety of mouse tumor models have likewise shown tumor regression in response to calcitriol and vitamin D (6–11). Additionally, Vdr-null mice show increased susceptibility to carcinogen-induced BCa (11). At a mechanistic level, many genes relevant to cancer progression are regulated by calcitriol and a number of molecular pathways appear to be involved in the anti-cancer effects of calcitriol (5–7). However, the essential molecular and cellular mechanisms underlying the effects of vitamin D signaling on BCa remain incompletely understood.
New insight into cancer biology has identified a subpopulation of cells in certain tumors, including BCa, as competent to regenerate entire tumors and therefore targeting these cells is critical for curing patients. Identification of existing and novel agents that target breast TICs promises to reduce BCa incidence and improve disease outcomes. If calcitriol inhibited breast TIC activity as a part of its effect on BCa, its potential for BCa prevention and treatment would be more promising. Recently, vitamin D and calcitriol were shown to regulate a cell line model of prostate progenitor/stem cells and mesenchymal stem cells (16,38,39), suggesting the possibility that vitamin D could also inhibit breast TICs. Furthermore, treatment of BCa cell lines with calcitriol or an analogue suppressed CD44 expression (17) and repressed pluripotency markers and markers associated with stem cell–like phenotypes (19), while VDR silencing enhanced mammosphere formation and expression of genes involved in EMT (18). However, these studies focused on TIC-like cells in long-term cultured BCa cell lines whose relationship to TICs in primary tumors remains unclear. Additionally, these studies did not directly examine whether a change in TIC frequency occurs after calcitriol treatment.
In our study, we have demonstrated that calcitriol treatment of MMTV-Wnt1 tumor tissue slices resulted in increased expression of Cyp24 mRNA, a marker for the actions of calcitriol. This was accompanied by decreases in Erα and Cyp19 mRNA suggesting decreased estrogen synthesis and estrogen signaling. Tissue slices contain an unsorted population of cells representing a mixture of both TICs and non-TICs and it is not possible to conclude whether the calcitriol responsiveness was due to TICs, non-TICs or both from these experiments alone. We therefore purified TICs from tumor orthografts in order to specifically analyze effects of calcitriol on this subpopulation. We demonstrated that calcitriol treatment not only suppressed TIC-initiated tumor spheroid formation in primary culture but also decreased spheroid formation in the secondary passage, even without further calcitriol treatment of cells in the secondary passage. Furthermore, we corroborated these findings by limiting dilution analysis in mice. These results suggest that calcitriol targets the ability of BCa TICs to self-renew. Additionally, we found that the combination of calcitriol and a clinically relevant dose of ionizing radiation eliminated more TICs than either treatment alone. Finally, we provide evidence that the mechanism of action is at least partly due to inhibition of the Wnt pathway and that constitutive Wnt pathway activation can overcome the TIC inhibitory effect of calcitriol.
Combinations of vitamin D compounds and anti-cancer drugs have been evaluated in experimental models of a variety of cancers as well as in a few clinical trials (7,40). The rationale behind these studies is that the combination approach could reduce toxicity by allowing the use of lower doses of the individual agents to still elicit significant anti-cancer activity. Combinations of calcitriol and cytotoxic chemotherapy or aromatase inhibitors have been shown to elicit enhanced anti-proliferative and apoptotic effects in BCa cell lines (41,42) and enhanced anti-tumor effects in mouse models (27,43,44). However, a phase II clinical trial evaluating the combination of a high dose of calcitriol with docetaxel in advanced prostate cancer patients yielded negative findings (45). These results have been criticized as having been impacted by trial design issues and the advanced stage of cancer in the study population (40). Importantly, our observation that vitamin D compounds target TICs suggests that future clinical studies should incorporate analysis of TICs as endpoints since therapeutic effects on TICs could be missed if only tumor size-based response metrics are considered.
Our study also provides evidence that the addition of calcitriol may be a useful strategy for enhancing anti-TIC effects of ionizing radiation. We, and others, have previously shown that TICs are relatively resistant to radiation therapy and chemotherapy compared to NTCs (3,46) and this likely contributes to treatment failure. Our data revealed that calcitriol in combination with irradiation demonstrated an additive effect on the suppression of TIC-initiated spheroid formation, suggesting that these therapies likely target different pathways and provide a rationale for considering combining the two agents in clinical trials. Our findings are in agreement with previous reports documenting a sensitizing or additive effect of calcitriol with ionizing radiation (47–49). It will be interesting to further explore the molecular effects of combined treatment with calcitriol and radiation and whether calcitriol can also enhance the anti-TIC effects of chemotherapy.
Recent studies show that vitamin D compounds exert inhibitory effects on TIC signaling pathways such as Notch, Hedgehog, Wnt and TGF-β. Our data indicate that the mechanism of the inhibition of TICs from MMTV-Wnt1 tumors by calcitriol is at least in part mediated by the inhibition of the Wnt/β-catenin signaling pathway. Wnt/β-catenin signaling has previously been implicated in various cancers, including colorectal cancer, hepatocellular carcinoma, acute myeloid leukemia, and BCa and plays an important role in the self-renewal and maintenance of TICs in breast and colorectal cancers (4,32,50,51). β-catenin signaling has been shown to promote EMT (52) and is implicated in stem/progenitor cell survival (2,53). Furthermore, recent studies have provided evidence for the activation of β-catenin signaling in triple negative breast cancers and shown that β-catenin activation portends poor survival (34,35). Similar to our findings in breast TICs, studies in colon cancer cells have shown that calcitriol represses the Wnt/β-catenin signaling pathway (9). Vdr-null colon cancers show higher expression of Wnt/β-catenin target genes and enhanced nuclear translocation of β-catenin (54). Calcitriol has also been shown to induce the expression of the Wnt antagonist Dickkopf-1 (55). In our experiments, calcitriol decreased the expression of Wnt target genes in breast TICs both in vivo and in vitro while not affecting the expression of Wnt1 itself. Importantly, constitutive activation of β-catenin counteracted calcitriol’s TIC inhibitory effect, indicating that vitamin D compounds can inhibit TICs by suppressing Wnt/β-catenin. Further studies are needed to elucidate the mechanisms underlying calcitriol inhibition of Wnt/β-catenin signaling in TICs. Future studies also should evaluate if this mechanism is active in other BCa models as well as patient-derived xenografts and whether measurement of baseline Wnt/β-catenin activity may allow selection of patients who will be most likely to benefit from calcitriol treatment.
In conclusion, we have demonstrated that vitamin D compounds, both vitamin D dietary supplements and calcitriol, inhibit MMTV-Wnt1 tumor growth and that vitamin D deficiency accelerates tumor growth. In mechanistic studies we showed that calcitriol targets breast cancer TICs by suppressing the Wnt/β-catenin signaling pathway. Developing a deeper understanding of vitamin D signaling in TICs will likely elucidate additional pathways that may be important in self-renewal and survival of these cells and could thus lead to new strategies for their elimination. Furthermore, our findings suggest that vitamin D compounds represent potential therapeutic agents for targeting breast cancer TICs and that avoidance of vitamin D deficiency might improve breast cancer risk and prognosis. Future clinical trials employing vitamin D compounds in breast cancer will likely benefit from combining these agents with other therapies and incorporating translational endpoints focused on quantifying TICs.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by grants from CIRM (TG2-01159; Y. Jeong), California Breast Cancer Research Program (17OB-0071 D. Feldman and 19IB-0103 B. Feldman), American Institute of Cancer Research (208633 D. Feldman), the NIH (T32 CA09302; J. Williams, P30CA147933 and P01CA139490; M. Diehn), Nadia’s Gift (M. Diehn), and the Virginia and D.K. Ludwig Foundation (M. Diehn).
We thank Dr. Milan Uskokovic (BioXel) for the kind gift of calcitriol. Control and β-catenin expression plasmids were a generous gift from Dr. Roel Nusse (Stanford University). The authors thank Phoebe Um (currently a freshman at University of Pennsylvania, Philadelphia) and Ramya Swami (currently a sophomore at Revelle College, University of California, San Diego) for their assistance with the in vivo mouse experiments and qPCR data.
Footnotes
Conflicts of interest: None to declare
References
- 1.Angeloni V, Tiberio P, Appierto V, Daidone MG. Implications of stemness-related signaling pathways in breast cancer response to therapy. Seminars in cancer biology. 2014 doi: 10.1016/j.semcancer.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 5.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcified tissue international. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 6.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annual review of pharmacology and toxicology. 2011;51:311–36. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 9.Pereira F, Larriba MJ, Munoz A. Vitamin D and colon cancer. Endocrine-related cancer. 2012;19:R51–71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 10.Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Archives of biochemistry and biophysics. 2012;523:107–14. doi: 10.1016/j.abb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh J. Vitamin D and cancer: integration of cellular biology, molecular mechanisms and animal models. Scandinavian journal of clinical and laboratory investigation Supplementum. 2012;243:103–11. doi: 10.3109/00365513.2012.682870. [DOI] [PubMed] [Google Scholar]
- 12.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin d receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77:1107–12. doi: 10.1016/j.steroids.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng W, Gentles A, Nair RV, Huang M, Lin Y, Lee CY, et al. Targeting Unique Metabolic Properties of Breast Tumor Initiating Cells. Stem cells. 2014;32:1734–45. doi: 10.1002/stem.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, et al. Dietary vitamin D(3) and 1,25-dihydroxyvitamin D(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153:2576–87. doi: 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maund SL, Barclay WW, Hover LD, Axanova LS, Sui G, Hipp JD, et al. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer research. 2011;71:5276–86. doi: 10.1158/0008-5472.CAN-10-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, et al. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Molecular pharmacology. 2011;79:360–7. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pervin S, Hewison M, Braga M, Tran L, Chun R, Karam A, et al. Down-regulation of vitamin D receptor in mammospheres: implications for vitamin D resistance in breast cancer and potential for combination therapy. PloS one. 2013;8:e53287. doi: 10.1371/journal.pone.0053287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahler J, So JY, Cheng LC, Maehr H, Uskokovic M, Suh N. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J Steroid Biochem Mol Biol. 2015;148:148–55. doi: 10.1016/j.jsbmb.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CY, Lin Y, Bratman SV, Feng W, Kuo AH, Scheeren FA, et al. Neuregulin autocrine signaling promotes self-renewal of breast tumor-initiating cells by triggering HER2/HER3 activation. Cancer research. 2014;74:341–52. doi: 10.1158/0008-5472.CAN-13-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–25. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 22.Krumdieck CL. Development of a live tissue microtome: reflections of an amateur machinist. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43:2–7. doi: 10.3109/00498254.2012.724727. [DOI] [PubMed] [Google Scholar]
- 23.Maund SL, Nolley R, Peehl DM. Optimization and comprehensive characterization of a faithful tissue culture model of the benign and malignant human prostate. Lab Invest. 2014;94:208–21. doi: 10.1038/labinvest.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan AV, Cramer SD, Bringhurst FR, Feldman D. Regulation of 1,25-dihydroxyvitamin D3 receptors by parathyroid hormone in osteoblastic cells: role of second messenger pathways. Endocrinology. 1995;136:705–12. doi: 10.1210/endo.136.2.7835303. [DOI] [PubMed] [Google Scholar]
- 25.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–9. [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Swami S, Krishnan AV, Wang JY, Jensen K, Peng L, Albertelli MA, et al. Inhibitory Effects of Calcitriol on the Growth of MCF-7 Breast Cancer Xenografts in Nude Mice: Selective Modulation of Aromatase Expression in vivo. Horm Cancer. 2011;2:190–202. doi: 10.1007/s12672-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nature protocols. 2010;5:702–13. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem cells. 2008;26:364–71. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 31.Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66:62–6. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- 32.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 33.Mohinta S, Wu H, Chaurasia P, Watabe K. Wnt pathway and breast cancer. Frontiers in bioscience: a journal and virtual library. 2007;12:4020–33. doi: 10.2741/2368. [DOI] [PubMed] [Google Scholar]
- 34.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, et al. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:209–31. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 35.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. The American journal of pathology. 2010;176:2911–20. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PloS one. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giovannucci E. Epidemiology of vitamin d and colorectal cancer. Anti-cancer agents in medicinal chemistry. 2013;13:11–9. [PubMed] [Google Scholar]
- 38.Piek E, Sleumer LS, van Someren EP, Heuver L, de Haan JR, de Grijs I, et al. Osteo-transcriptomics of human mesenchymal stem cells: accelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone. 2010;46:613–27. doi: 10.1016/j.bone.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Curtis KM, Aenlle KK, Roos BA, Howard GA. 24R,25-dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells. Molecular endocrinology. 2014;28:644–58. doi: 10.1210/me.2013-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinology and metabolism clinics of North America. 2010;39:401–18. doi: 10.1016/j.ecl.2010.02.011. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7:1043–51. [PubMed] [Google Scholar]
- 42.Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151:32–42. doi: 10.1210/en.2009-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koshizuka K, Koike M, Asou H, Cho SK, Stephen T, Rude RK, et al. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res Treat. 1999;53:113–20. doi: 10.1023/a:1006123819675. [DOI] [PubMed] [Google Scholar]
- 44.Wietrzyk J, Nevozhay D, Filip B, Milczarek M, Kutner A. The antitumor effect of lowered doses of cytostatics combined with new analogs of vitamin D in mice. Anticancer Res. 2007;27:3387–98. [PubMed] [Google Scholar]
- 45.Scher HI, Jia X, Chi K, de Wit R, Berry WR, Albers P, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:2191–8. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 46.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 47.Sundaram S, Gewirtz DA. The vitamin D3 analog EB 1089 enhances the response of human breast tumor cells to radiation. Radiation research. 1999;152:479–86. [PubMed] [Google Scholar]
- 48.Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Molecular cancer therapeutics. 2006;5:2786–97. doi: 10.1158/1535-7163.MCT-06-0316. [DOI] [PubMed] [Google Scholar]
- 49.Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, Maiti A, et al. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy. 2012;8:739–53. doi: 10.4161/auto.19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer biology & therapy. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 51.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 52.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debeb BG, Lacerda L, Xu W, Larson R, Solley T, Atkinson R, et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/beta-catenin signaling. Stem cells. 2012;30:2366–77. doi: 10.1002/stem.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larriba MJ, Ordonez-Moran P, Chicote I, Martin-Fernandez G, Puig I, Munoz A, et al. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PloS one. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–84. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.