Abstract
Introduction
Oral delivery of therapeutics, particularly protein-based pharmaceutics, is of great interest for safe and controlled drug delivery for patients. Hydrogels offer excellent potential as oral therapeutic systems due to inherent biocompatibility, diversity of both natural and synthetic material options and tunable properties. In particular, stimuli-responsive hydrogels exploit physiological changes along the intestinal tract to achieve site-specific, controlled release of protein, peptide and chemotherapeutic molecules for both local and systemic treatment applications.
Areas covered
This review provides a wide perspective on the therapeutic use of hydrogels in oral delivery systems. General features and advantages of hydrogels are addressed, with more considerable focus on stimuli-responsive systems that respond to pH or enzymatic changes in the gastrointestinal environment to achieve controlled drug release. Specific examples of therapeutics are given. Last, in vitro and in vivo methods to evaluate hydrogel performance are discussed.
Expert opinion
Hydrogels are excellent candidates for oral drug delivery, due to the number of adaptable parameters that enable controlled delivery of diverse therapeutic molecules. However, further work is required to more accurately simulate physiological conditions and enhance performance, which is important to achieve improved bioavailability and increase commercial interest.
Keywords: controlled release, drug delivery, hydrogel, intelligent system, oral delivery
1. Introduction
The primary goals in development of drug delivery systems are to protect an active therapeutic molecule from premature degradation, enhance drug efficacy and minimize side effects. Ideally, controlled release systems can meet the criteria by maintaining the drug concentration within a therapeutic window over an extended period of time, minimizing dosage and frequency of administration [1,2]. Stimuli-responsive hydrogel systems have been extensively studied for therapeutic delivery applications as they respond to changes in environmental conditions. The diversity of materials and unique biological and physiochemical characteristics provide a variety of potential for pharmaceutical applications.
Oral drug delivery is the ideal administration route for many therapeutic molecules, particularly for chronic diseases. The oral route is simpler, improves patient compliance and comfort and can potentially reduce cost compared to injection-based administration [3,4]. However, oral delivery is primarily limited to small-molecule drugs. Other therapeutic molecules of interest include peptides, proteins and chemotherapeutics [3–5]. Oral delivery of these agents has been limited by low bioavailability due to enzymatic degradation and poor penetration of the intestinal membrane into the bloodstream [6–8]. Successful delivery requires innovative drug delivery techniques to overcome these obstacles, such as protection by hydrogel networks. This review focuses on hydrogel formulations for oral administration, with an emphasis on protein and peptide therapeutics, and in vitro and in vivo techniques to evaluate performance of oral delivery systems.
2. Hydrogels
Hydrogels are three-dimensional, polymeric networks consisting of crosslinked hydrophilic components. In certain environmental conditions, hydrogels can imbibe large amounts of water or biological fluids, while remaining insoluble. Physical integrity in aqueous media is maintained by physical crosslinks (e.g., entanglements, crystallites) and/or chemical crosslinks (e.g., tie-points, junctions) [4,9–11]. High affinity for water absorption gives hydrogels physical properties resembling living tissues, such as a soft consistency and low interfacial tension with aqueous media. These properties match living tissues more than any other class of synthetic biomaterial and are therefore highly biocompatible for biological applications [12]. Hydrogel use thus extends beyond the drug delivery applications discussed here to tissue engineering, surface coating, contact lenses and diagnostics among others [2,12–18].
Hydrogel systems can be classified by a variety of characteristics: nature of polymer side groups (neutral or ionic), mechanical and structural characteristics (affine or phantom networks), as well as preparation methods (homo- or copolymer), physical structure (amorphous, semi-crystalline, hydrogen-bonded, supermolecular structures and hydrocolloidal) and physiological response to environmental stimuli (physical or chemical) [10]. Hydrogels may be composed of synthetic or natural polymers or a combination of both. Synthetic polymers have well-defined structures and allow for highly tunable mechanical properties, degradation and release kinetics. Naturally occurring polymers may have suboptimal mechanical stability and could evoke an immunogenic or inflammatory response. However, they offer the advantages of generally being nontoxic, biocompatible and have suitable physiochemical properties due to their natural origin [19,20].
Hydrogels possess a diversity of tunable features of the bulk structure that can be tailored for a specific therapeutic. Crosslinked hydrogel networks can protect drugs from harmful environments, such as enzymes and low pH in the stomach [21]. Chemical structure and density of a crosslinking agent determines the mesh size, ξ, and can be optimized for loading and controlled diffusion of water soluble drugs in or out of the network [10,22]. For example, higher crosslinking density hinders polymer chain mobility, lowering the swelling ratio compared to a hydrogel of the same type with a lower crosslinking density. Incorporation of hydrophilic groups in the crosslinking agent can cause higher degree of swelling compared to those containing hydrophobic groups that collapse in the presence of water, thereby reducing hydrogel swelling. Mesh size of the swollen network affects the physical properties of the gel, such as mechanical strength, degradation and diffusion of captured molecules [10]. Reported mesh size of hydrogels in the swollen state typically ranges from 5 to 100 nm and can be optimized for sustained release of macromolecules according to their hydrodynamic radii [23–25]. Mesh size can be determined experimentally via swelling studies or by theoretical calculation [26–28].
Stimuli-responsive hydrogels are of particular interest for oral delivery as they can respond to environmental changes to alter network structure, swelling behavior, permeability or mechanical strength and can control drug release [29]. Many different physical and chemical stimuli have been applied to smart hydrogel systems. Physical stimuli include temperature, electric field, light and solvent composition. Chemical and biochemical stimuli, such as pH, ionic strength and molecular recognition events, are more commonly exploited in oral delivery [10].
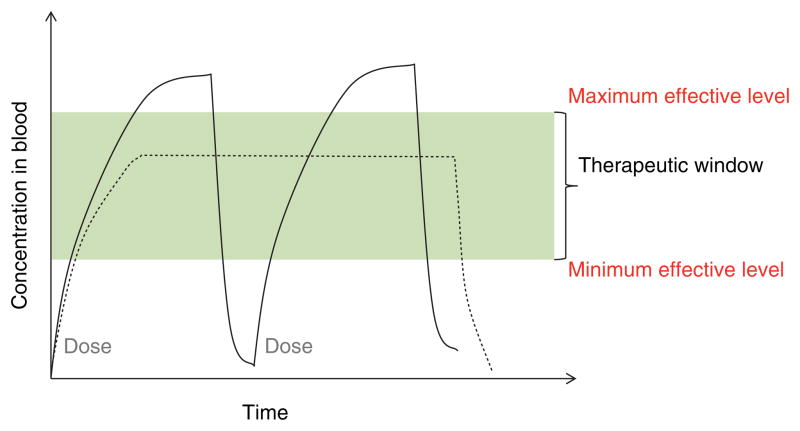
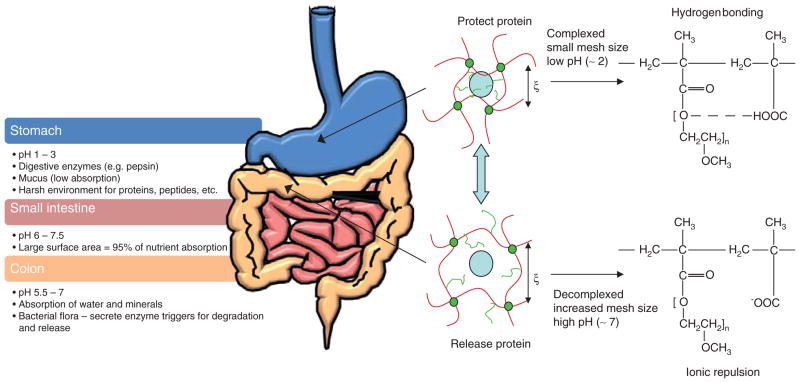
Controlled drug delivery aims at achieving sustained concentration of a molecule, within the therapeutic window, in contrast to traditional administrations that result in a sharp rise and fall leading to toxic and ineffective levels (Figure 1). Hydrogels provide a platform for protecting the therapeutics through the complex environment of the gastrointestinal (GI) tract and achieve site-specific delivery utilizing fundamental physiological changes (Figure 2). The primary barriers to oral delivery of therapeutics, particularly protein and peptide drugs, are: i) inactivation in the GI tract due to denaturation by acidic pH or digestive enzymes; and ii) poor permeability through the epithelial membrane into the bloodstream [2]. The mouth, esophagus, small intestine, colon and rectum are all portions of the GI tract that have been investigated as target sites for drug delivery.
Figure 1.
Representation of drug levels in the blood with traditional repeated dosing (solid line) and controlled delivery dosing (dashed line).
Figure 2. The complex physiology of the gastrointestinal tract poses challenges for oral delivery but can be exploited to achieve controlled drug release.
Complexation hydrogels can deliver a therapeutic through the harsh environment of the stomach, protecting it from denaturation by acidic pH or digestive enzymes. Drug is released in the upper small intestine, which has a lower population of enzymes, neutral pH and a large surface area accounting for 95% of nutrient absorption, due to decomplexation and an increase in mesh size triggered by ionic repulsion and swelling of the polymer at a high pH. The colon is another commonly targeted site due to neutral pH and lower enzymatic activity.
Adapted with permission from [4].
The small intestine is the most common target site due to shortest transit time and a large surface area of specialized cells, microvilli, and associated microvessels for material absorption and transportation to the bloodstream. For example, M cells are known for high transcytotic capacity and low lysosomal activity, transporting foreign material from the intestinal lumen to the immune cells of the lamina propria, and have been evaluated as optimal delivery sites and cellular targets for DNA, protein or peptide antigen vaccines [30,31]. The potential of microvasculature to directly absorb materials for deliver to the bloodstream and for systemic delivery or transport to diseased cells is promising for a variety of diseases, such as diabetes and hemophilia. The colon has also been investigated due to lower activity of proteolytic enzymes than the small intestine. Colonic delivery has been evaluated for both local disease treatment, such as colorectal cancer [32], and systemic drug delivery [33]. However, the colon poses additional barriers over the small intestine, such as bacterial activity, longer transit times and potential interference by fecal matter [34].
Hydrogels provide a platform for achieving controlled therapeutic delivery to specific sites within the GI tract. Our laboratory has successfully developed complexation, pH-responsive copolymer systems that address the aforementioned barriers with immense potential in oral therapeutic delivery. These systems consist of a family of grafted copolymers that include PEG grafted on poly(methacrylic acid) (PMAA), designated as P(MAA-g-EG) and other polyacids. These complexation pH-sensitive hydrogels respond to the surrounding environment, protecting the drugs from the harsh environment of the stomach and releasing them in the small intestine.
3. Therapeutics
Oral delivery is desirable for a diversity of therapeutics for treatment of both systemic and local diseases. However, many drugs are not feasibly delivered in the same method as conventional small-molecule drugs. For example, protein and peptide drugs suffer poor stability in the GI tract, being subject to enzymatic degradation, acidic denaturation, low solubility and absorption [35]. Hydrogels are well suited for the oral delivery of small, hydrophilic molecules, and macro-molecular drugs (~ 400 Da to 30 kDa) [36]. Molecular size dictates diffusion of drug payload through a hydrogel network and can be addressed by engineering the network pore size, discussed in Section 2. Protein and peptide drugs are increasingly important therapeutics for a wide range of diseases due to their high selectivity and potent action. Additionally, proteins have the potential to cure diseases by replacing the missing or malfunctioning proteins that are characteristic of various diseases rather than by simply alleviating the symptoms [3].
Both pharmaceutical industry and academic laboratories have identified therapeutic proteins where oral delivery is extremely desirable, particularly for chronic conditions. A commonly investigated protein is insulin for the treatment of diabetes. Diabetes is a disease in which the body does not produce or properly use insulin, a hormone required to convert sugars, starches and other food into energy. It affects 25.8 million people in the USA, most of whom are required to self-administer at least two daily injections of insulin to control blood glucose levels [37]. Injection has proved an effective therapy for diabetes but causes immense discomfort and noncompliance in patients, making oral delivery a desirable alternative [8]. Other proteins evaluated for oral delivery include salmon calcitonin, a model analog for human calcitonin – a hormone treatment for postmenopausal osteoporosis [36,38] and growth hormone for treating diseases associated with stunted development and decelerated growth [36].
Hydrophobic molecules present a different set of challenges to be addressed when designing hydrogel carriers. For example, many cancer drugs are hydrophobic, small-molecule drugs, limiting favorable interaction with hydrophilic hydrogel complexes. Oral chemotherapy treatment is extremely desirable in order to improve patients’ quality of life by minimizing side effects of intravenous administration and increasing drug effectiveness [39–41]. Intravenous treatment achieves systemic delivery to both healthy and diseased tissues in an unbiased manner. Studies have demonstrated orally delivered chemotherapeutics to be effective in comparison to conventional intravenous administration [39,42,43]. In addition to protecting the chemotherapeutics from the GI tract, an effective strategy must also be considered for protecting the GI tract from potentially toxic effects of the drug. One strategy has been to incorporate hydrophobic moieties into pH-responsive polymer matrices to enable efficient loading and release of hydrophobic drugs, such as doxorubicin and fluorouracil [39]. Another method is to incorporate hydrophobic interpenetrating networks or nanoparticles to develop an amphiphilic polymer structure conducive to encapsulation of chemotherapeutic agents for oral delivery systems [44].
4. Hydrogel oral delivery systems
Despite the challenges of oral delivery, many hydrogel systems have been developed to exploit the physiological changes through the GI tract to achieve controlled drug release. The most common strategies use the pH-gradient and/or enzyme localization for site-specific delivery. Example systems are summarized in Table 1.
Table 1.
Categories of hydrogel systems with example polymers and applications for oral drug delivery.
| Formulation | Polymer type | Delivery site | Therapeutic and ref. | |
|---|---|---|---|---|
| Intestinal delivery systems | ||||
| Anionic | P(MAA-g-EG) | Synthetic | Small intestine | Insulin [63,64], calcitonin [28,65], IFN-α [65] |
| Alginate-based | Natural | Small intestine and colon | Heparin [72], hemoglobin [73], melatonin [74], vaccines [75,76], peptides [77], probiotic yeast [78], cedroxil [71] | |
| Hyaluronic acid-based | Natural | Small intestine | Insulin [82], thrombin [80], α-chymotrypsin [81] | |
| Cationic | Chitosan-based | Natural | Small intestine | Salmon calcitonin [91], peptides [92], BSA [69,93] |
| Amphiphilic | P(MAA-g-EG) with PMMA nanoparticles | Synthetic | Colon | Doxorubicin [44] |
| Degrading formulations | Dextran-based | Natural | Colon | Hydrocortisone [105], salmon calcitonin [106] |
| Azoaromatic crosslinks | Synthetic | Colon | [107–109] | |
| Intracellular delivery systems | ||||
| Cationic | Dimethylaminoethyl methacrylate-based | Synthetic | Endosome | [95] |
| Chitosan-based | Natural | Endosome | Methotrexate disodium [96] | |
| Chitosan-based | Natural | Cytosol | [101] | |
| Poly-L-lysine containing | Synthetic | Cytosol | [102] | |
| Poly(γ-benzyl-L-glutamate) containing | Synthetic | Cytosol | [103] | |
| Acid-sensitive bonds | Containing oxime bonds | Synthetic | Endosome | Doxorubicin [97] |
| Containing acetal bonds | Synthetic | Endosome | Doxorubicin [98] | |
| Acid-degradable crosslinker | Crosslinked with 2,2-dimethacroyloxy-1-ethozypropane | Synthetic | Endosome | [99] |
| Enzyme-degradable crosslinker | Peptide crosslinker | Synthetic | Lysosome | DNA, antibodies [110] |
BSA: Bovine serum albumin; PMMA: Poly(methyl methacrylate); P(MAA-g-EG): Poly(methacrylic acid) grafted with poly(ethylene glycol).
4.1 pH-Responsive hydrogels
The pH-responsive hydrogels have become popular platforms for the oral delivery of drugs. Such systems can be tailored for drug delivery to specific organs (e.g., small intestine, colon) or intracellular vesicles (e.g., endosomes, lysosomes). For intestinal delivery, particles on the micron scale (1 – 1000 μm) offer a larger surface area and are not taken up by M cells [45]. Particles on the nanoscale (50 – 200 nm) are used for intestinal delivery for cellular internalization [46,47]. Particles < 10 nm are cleared by lymph drainage [46]. Additionally, pH differences in microenvironments associated with pathological conditions, such as cancer and inflammation, can be used to trigger drug release. The two basic strategies for imparting pH-responsive behavior are incorporating: i) ionizable groups with solubility and/or conformational changes in response to environmental pH; and ii) acid-sensitive bonds that cleave to release molecules anchored into the backbone [48]. Ionizable polymeric systems are pH-sensitive due to the basic or acidic pendant groups of the polymer network. Ionization of the pendant groups results in a net charge in the polymer network. Due to the electrostatic repulsions of the charged polymer network, the pores increase in size, allowing for the influx of water and increased swelling.
The pH-responsive hydrogels can be classified as anionic or cationic. Anionic hydrogels are ionized, and thus swollen, at a pH higher than the pKa of the polymer network [11,49]. Intestinal drug delivery systems take advantage of pH-responsive anionic hydrogels to protect drugs from gastric degradation and denaturation at low pH and release drugs in specific locations, such as the upper small intestine and colon, further in the GI tract. Ionic strength of the solution also affects the swelling of pH-responsive hydrogels [50,51]. At pH below the pKa, there is minimal effect of ionic strength on swelling, since the hydrogel is in the collapsed state. Experimental observations found that as the ionic strength increases, the degree of swelling decreases for anionic hydrogels at a pH above the pKa of the polymer network [51,52]. Increasing the ionic strength of the solution leads to ion shielding which diminishes the degree of electrostatic repulsion of the negative carboxylic acid groups [53].
In contrast to anionic hydrogels, cationic hydrogels are ionized at a pH lower than the pKa of the polymer network [54]. Cationic hydrogels are suited for drug release in the stomach or intracellular environments. Amino acid groups of cationic polymers impart high water solubility at acidic pH and low water solubility at neutral pH. In an oral delivery system, cationic polymers provide protection of the drug in the oral cavity (pH 5.8 – 7.4) [55], while releasing the drug in the stomach (pH 1 – 3.5) [56]. Due to the low solubility at neutral pH, suppressing drug release, cationic polymers often serve as taste-masking formulations [56–59].
4.1.1 pH-Responsive hydrogels for intestinal delivery
Our laboratory largely focuses on anionic pH-responsive polymeric systems for the delivery of proteins to the upper small intestine. As previously noted, our laboratory has successful investigated a complexation hydrogel, PMAA grafted with PEG tethers, denoted as P(MAA-g-EG). Methacrylic acid (MAA) imparts pH-sensitivity due to the ionizable carboxyl pendant groups. Carboxylic acids begin to deprotonate at pH values above its pKa of 4.8, developing a negative charge in the network. The ratio of deprotonated to protonated carboxylic acid groups can be determined using the Henderson-Hasselbalch equation.
| (1) |
where pH is the environmental pH, pKa is that of the acid group, [A−] is the concentration of deprotonated acid groups, and [HA] is the concentration of protonated acid groups. Interpolymer complexation occurs when protons of the carboxylic acid groups of PMAA backbone form hydrogen bonds with the etheric oxygen of the PEG tethers [60].
At low pH, P(MAA-g-EG) networks collapse due to complexation. As the environmental pH increases above the pKa of 4.8, deprotonation causes disruption of the polymer complexes and ionization and electrostatic repulsion of the acid groups of the MAA backbone. The polymer network swells and the mesh size, ξ, increases due the effects of deprotonation, as demonstrated in Figure 2. The mesh size, or the network correlation length, is the end-to-end distance of the polymer chains between junction points. The complexed, or collapsed, network has a mesh size of 70 Å, whereas that of the decomplexed, or swollen, network is 210 Å[61]. In the oral delivery route, this network is collapsed in the low pH environment of the stomach, providing protection to the drugs, and then swells in the increased pH environment of the upper small intestinal allowing for drug release.
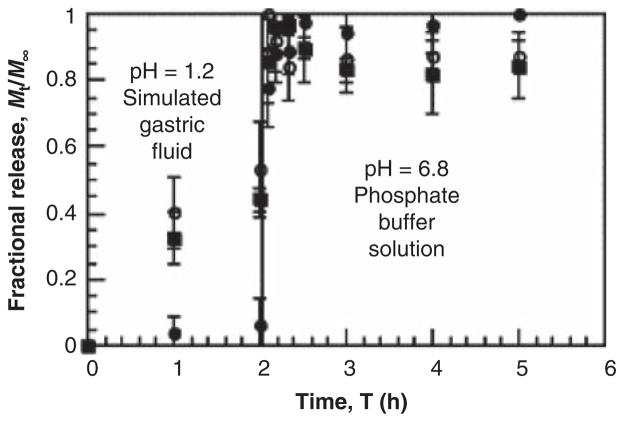
The pH-responsiveness of P(MAA-g-EG) was first investigated by Klier et al. and Peppas and Klier further studied this polymer network for applications in oral drug delivery systems [62,63]. An evaluation of grafted PEG chain lengths determined that PEG chains with a molecular weight of 1000 exhibited the highest degree of complexation in low pH conditions [64]. Equimolar amounts of carboxylic acid groups of MAA and etheric oxygen molecules of PEG result in the largest amount of complexation [65]. Adjusting the amount of carboxylic acid groups or other substituent groups tailors the hydrogel system for a specific pH value and, therefore, the site of drug release. Our laboratory has optimized the pH-dependent swelling behavior of P(MAA-g-EG) hydrogel systems for release targeted in the upper small intestine. Previous studies have demonstrated the successful use of this hydrogel system for the oral delivery of proteins, such as insulin [66,67], calcitonin [68,69] and IFN-α [69]. Representative in vitro release of insulin from P(MAA-g-EG) microparticles can be found in Figure 3 [70].
Figure 3. Representation of in vitro release of insulin from P(MAA-g-EG) microparticles in simulated gastric conditions (pH 1.2) and intestinal conditions (pH 6.8).
The formulations vary in ratios of MAA:PEG where (■) is 4:1, (●) is 1:1 and (○) is pure PMAA.
Reproduced with permission from [70].
Another important feature that takes advantage of the pH-responsive behavior of the P(MAA-g-EG) system is release of PEG tethers. In the decomplexed state, the grafted PEG tethers are no longer hydrogen bonding with carboxylic acids of the PMAA backbone and act as mucoadhesive promoters on the surface of the polymer network. Tethered PEG chains interpenetrate the mucus layer of the small intestine, participating in physical entanglement and hydrogen bonding with the polysaccharide components [71]. Mucoadhesion increases the residence time of the carrier at the site of absorption, which promotes increased bioavailability [72]. It is important to note that pH-sensitivity is designed for targeted release of drugs in the upper small intestine, as well as triggering the PEG tethers to promote mucoadhesion at the target absorption site.
The P(MAA-g-EG) system has been successful in the delivery of hydrophilic molecules; however, modifications are necessary for the delivery of hydrophobic molecules, such as chemotherapeutics. Schoener et al. developed novel amphiphilic polymeric carriers for oral delivery of hydrophobic molecules, specifically doxorubicin, with targeted release in the colon. This system combines the pH-responsive behavior of anionic complexation hydrogels with hydrophobic poly (methyl methacrylate) (PMMA) nanoparticles incorporated into P(MAA-g-EG) networks. Increased PMMA incorporation resulted in increased doxorubicin loading levels and extended release for improved delivery to the colon [44].
As previously mentioned, natural polymers, such as alginate and chitosan, are attractive matrices for oral drug delivery due to their biocompatibility, physiochemical properties and mild gelation conditions [73]. Alginate is a linear polysaccharide extracted from brown seaweed and is composed of alternating blocks of 1 – 4 linked α-L-guluronic (G-block) and β-D-mannuronic (M-block) acid residues. The ability to form gels, particularly under mild conditions, is important for the applications of alginates as drug delivery systems. Alginates can form gels by reacting with divalent cations, such as Ca2+. The divalent cations bind to the negative charges of the G-blocks, resulting in a crosslinked matrix. As an anionic polymer, alginate shrinks in a low pH environment, forming an insoluble alginic acid skin [74,75]. The alginic skin changes into a soluble viscous layer when exposed to higher pH conditions of the intestinal tract.
Modifications to alginate matrices, such as forming complexes with other polymers and coating beads, allow for greater control over the drug release profile by preventing rapid degradation of alginate at high pH [73]. Interpenetrating networks of alginate with egg albumin and gelatin crosslinked with glutaraldehyde have shown prolonged control release of cedroxil in in vitro studies [76], and such networks have also shown promise for protein oral delivery [73]. Various studies have also used alginate as plain beads, coated beads and microcapsules for entrapping various biological molecules, including heparin [77], hemoglobin [78], melatonin [79] and vaccines [80,81]. Alginate microspheres coated in serum albumin-alginate membrane were investigated in vitro for the sustained release of peptides [82]. Hébrard et al. developed alginate/whey protein microparticles coated with alginate for the intestinal delivery of probiotic yeast based on pH variations [83]. Coated beads and microcapsules are more effective as oral delivery systems [73], and modifying the alginate matrices allows for greater control of drug release.
Another natural polymer commonly used in drug delivery formulations is hyaluronic acid (HA), which is an anionic glycosaminoglycan [84,85]. The presence of one carboxylic group per repeat unit imparts a pH-responsiveness, and such behavior is enhanced in crosslinked hydrogel network [86]. Pitarresi et al. evaluated the pH-responsive behavior of photo-crosslinked HA hydrogels for the release of thrombin [85]. Recent research found pH-responsive HA nanoparticles as a viable option for oral insulin delivery systems, showing enhanced delivery via transcellular pathway found in both in vitro and in vivo studies [87]. Fiorica et al. developed a novel derivative of HA with increased carboxylic groups to optimize the system for delivery to the colon and demonstrated pH-sensitive release using α-chymotrypsin. Future work aims to combine the pH-responsiveness of this system with HA’s biological properties, such as interacting with CD44 receptors overexpressed on colon tumoral cells [86].
Chitosan is another natural polymer that has been extensively used for drug delivery systems. Chitosan is a cationic polymer extracted from crustacean chitin [88]. Like alginate, chitosan is an attractive polymer due to its inherent biocompatibility [89], pH-sensitivity [90] and mild gelation conditions. Due to the amino groups on polymer chain, at low pH, chitosan is protonated and dissolves easily, whereas at high pH, it is insoluble. As a result of its pH-responsiveness, chitosan has been widely studied as a delivery vehicle for chemical drugs to the stomach. However, modifications have been investigated to improve chitosan as a drug delivery system for proteins [73].
Although chitosan can be ionically crosslinked with tripolyphosphate [91], covalent crosslinking with dialdehydes, such as glyoxal [92,93] and glutaraldehyde [94,95], chemically and mechanically reinforces the matrix. Covalently crosslinked chitosan hydrogels are more stable and more suitable for intestinal protein delivery. Further chemical modifications, such as thiolated chitosan, trimethylated chitosan, carboxymethyl chitosan and N-(2-hydroxyl) proyl-3-trimethyl ammonium chitosan, have been evaluated for oral delivery of salmon calcitonin [96], various peptides [97] and bovine serum albumin [74,98], respectively. Polyelectrolyte complexes, such as chitosan–alginate, have been investigated as pH-responsive hydrogels for the oral delivery of peptides and proteins, such as hemoglobin [99]. The complexation of chitosan and alginate results in decreased porosity, which is typical of alginate-only systems, and thus reduces drug leakage [73].
4.1.2 pH-responsive hydrogels for intracellular delivery
The pH-responsive hydrogels, specifically nanocarriers, can also be tuned for intracellular release of drugs. Nanocarriers are often internalized by cells through endocytosis, which confines the particles in endosomes. The acidification of endosomes reduces the pH from ~ 7 to ~ 5, and late endosomes can also fuse with lysosomes (pH ~ 4 – 5), which provides another pH variation to trigger drug release [48]. At the cellular level, nanocarriers have been designed to release drugs under endosomal and/or lysosomal pH conditions or to escape such compartment for drug release in the cytosol. Swelling can be tuned for release in the endosomal pH range, as demonstrated with a dimethylaminoethyl methacrylate-based nanocarrier for the release of DNA in the endosome [100]. A dual release system, where the nanocarriers are encapsulated in anionic microparticles, can be used for oral delivery applications.
Similarly, Zhang et al. developed a chitosan-based nanocarrier for anticancer agent methotrexate (MTX) disodium which exhibited increased swelling at endosomal pH and thus increased drug release [101]. An alternative approach using pH-responsiveness to achieve drug release in the endosome employs acid-cleavable bonds and crosslinkers. Hydrogel nanocarriers with acid-sensitive bonds in the polymer backbone, such oxime [102] and acetals [103], have been studied for the endosomal delivery of doxorubicin, whereas use of acid-degradable crosslinkers, such as 2,2-dimethacroyloxy-1-ethozypropane [104], have targeted release of DNA and enzymes to the endosome.
Intracellular delivery in the endosomal and lysosomal compartments can be harmful to some therapeutic agents due to the low pH and enzymatic activity. Recent studies have focused on designing systems that can escape endosomes and lysosomes to allow for drug delivery in the cytosol. One key approach employs the proton sponge effect in order to increase the osmotic pressure inside such compartments leading to swelling and rupture [48,54]. Endosomal escape has been demonstrated with nanocarriers incorporating protonatable polyhistidine segments [105] as well as chitosan-based nanocarriers due to ionization at decreased pH [106]. However, rupture of endosomes and lysosomes may potentially trigger autophagy and cell death due to leakage of enzymes [48]. Intracellular delivery systems have also been designed to buffer endosomal and lysosomal pH by incorporating amine-containing polymers such as poly-L-lysine [107] and poly(γ-benzyl-L-glutamate) [108].
4.2 Environmentally triggered degrading hydrogels
Enzymatically sensitive systems exploit differing enzymatic populations in physiological environments or in pathological conditions (e.g., cancer, inflammation) with altered enzyme expression profiles [48]. As mentioned previously, colon-specific delivery can be desirable for local and systemic therapeutic treatments. A common strategy to achieve colonic delivery exploits microbial enzymes predominantly found in the colon, such as reductive (e.g., azoreductases) and hydrolytic (e.g., glycosidases) enzymes [109]. Dextran hydrogels have potential in colonic-specific delivery by undergoing degradation by a dextranase, allowing release in the presence of the colon’s microbial enzyme. Hovgaard et al. demonstrated the potential of dextran hydrogels for oral delivery by successfully releasing the anti-inflammatory agent, hydrocortisone [110]. Dextran hydrogels continue to be explored for delivery of peptide and protein drugs, such the peptide hormone salmon calcitonin [111].
Incorporation of cleavable crosslinking agents is another strategy to trigger site-specific degradation. Azoaromatic bonds that act as crosslinking agents, which can be degraded by azoreductases, also targets colon-specific delivery. By synthesizing hydrogels that contain both pH-sensitive monomers and azoaromatic crosslinkers, Kopecek et al. created a system that began to swell in the small intestine, making crosslinks accessible to azoreductases by the time the particles reach the colon for protein drug release [112–114]. Similar approaches incorporate enzyme-specific peptides as crosslinking agents to achieve disease-triggered drug release for particular tissues or pathophysiological conditions. For example, cathepsin B is a protease over-expressed in tumor cells. PEG diacrylate crosslinked with cathepsin-specific diacrylated peptide was used in achieving enzyme-triggered release of the model antibodies and nucleic acids [115]. The system demonstrates potential for incorporation of other enzymatically specific peptide linkers for disease-controlled biomolecule delivery. Similar strategies can be employed for site-specific delivery in the intestine.
5. Evaluation of oral drug delivery systems
Systems designed to improve drug delivery through oral administration can be studied for their efficacy both in vitro and in vivo. Animal studies are essential to more closely replicate the body’s complex environment, but in vitro methods provide an initial evaluation of permeation characteristics in a manner that is more cost-effective and has higher throughput, while still providing predictive power of the system’s potential. A variety of in vitro and in vivo studies to evaluate drug permeability are discussed here.
5.1 In vitro evaluation
Initial in vitro analysis of hydrogels is performed to investigate and confirm desired behavior, often of the system in gastric fluids. As mentioned previously, the GI tract encompasses fluids containing different enzymes, ionic strength and pH that affect both therapeutics and the systems that deliver them. To confirm desired protection, release or degradation behavior in these harsh environments, bench-top analysis can be performed in simulated fluids that follow a standard such as those from the US Pharmacopeia [116]. Therapeutic-containing hydrogels are suspended in either simulated gastric or intestinal fluid, pH ~ 1.2 and ~ 7.5 respectively, and the amount of therapeutic or degradation products present in the supernatant is measured over time [68]. Concentration can be determined by techniques such as high-performance liquid chromatography, enzyme-linked immunosorbent assay, spectrometry, scintillation counter and others, depending on the target. To further emulate the GI environment, the experiments are often performed at physiological temperature and some are agitated [117].
The everted sac is an ex vivo technique that has been used for measuring mucoadhesion [118] and transport [119]. In both cases, the intestine is removed, segmented, everted and filled with buffer or cell media after being sutured closed. After incubation in a suspension, the number of particles adhered and the concentration of drug in the serosal fluid are quantified for mucoadhesion and transport, respectively. This technique is limited by differences in the extracellular environment, such as lack of enzymatic activity, and poor viability of the intestine in vitro that prevents experiments longer than 2 h [120].
Although the everted sac technique adds the intestine as a model, the viability issues and presence of attached smooth muscle prevent it from representing a healthy monolayer of cells. Utilization of cell culture offers a number of advantages: estimation of monolayer permeability, cellular metabolism, determination of transport mechanism or pathway, use of human tissue and minimization of animal studies through initial screening. These advantages have led in vitro cell studies to become one of the major analysis methods for therapeutic transport.
Therapeutics can be transported across the intestinal epithelium monolayer either paracellularly or transcellularly. In paracellular transport, molecules diffuse between the cells but are limited by intercellular contacts called tight junctions that seal the epithelial layer together. The tight junctions are located at the apical (luminal) side of adjacent cells and consist of occludins, claudins and junctional adhesion molecules, which are three families of transmembrane proteins. A more detailed review of tight junctions is available [121], but the regulation of these proteins plays a role in the properties of paracellular permeability. The main barriers to paracellular transport are size and charge restrictions that limit diffusive transport to only small molecules and particular ions. Paracellular, as well as transcellular, pathways are unavailable to protein therapeutics due to their large size and charged nature. A number of approaches to disrupt tight junctions and thus increase paracellular transport of large molecules have been investigated. Examples include calcium-binding agents [67], surfactants [122], polysaccharides [123] and poly(acrylic acid) derivatives [124], among others [125]. These methods often increase cytotoxicity and reduce cell monolayer integrity [126]. Additionally, they are not selective for the therapeutic of interest, potentially allowing other unwanted molecules to enter systemic circulation.
Alternatively, the surface area of the cellular brush border membrane is much greater than the paracellular region, which makes it a prime target for transport. There are several transcellular pathways through the epithelium, all falling under the umbrella of transport through the cell. The simplest is passive diffusion of small hydrophobic molecules through the cell membrane and can be predicted based on the molecular weight and degree of hydrophobicity [127]. Important participants in the diffusion of these therapeutics are membrane transporters, such as P-glycoproteins, that are apically polarized efflux pumps that expel these molecules back into the lumen. P-glycoproteins have been shown to interact with antibiotics, cancer therapeutics and hormones, among others [128]. Similarly, some small molecules (i.e., glucose, amino acids, di/tripeptides) are taken up from the lumen using active carrier-mediated transport by membrane proteins for transport into the cell [129]. The last type of transport is transcytosis in which molecules of interest, often large and hydrophilic proteins, are endocytosed by the cell into vesicles that are transported to the basolateral side and merge with the membrane to dump their cargo. The protein transferrin, which binds iron for uptake, is commonly used to exploit this type of transport for therapeutics or nanoparticles as it binds to a common cell surface receptor that results in endocytosis [130,131].
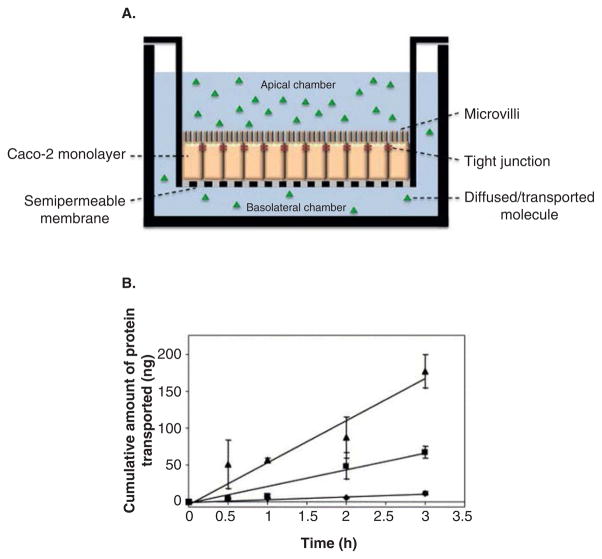
The most common cell model of the intestine utilizes Transwell® systems that consist of an insert with a porous 10 μm thick membrane that separates two chambers. The membrane is made of polycarbonate or polyester that is cell culture treated with a range of pore sizes available to ensure that the membrane is not the main diffusion barrier. An intestinal cell line is grown on the insert’s membrane to form a polarized confluent monolayer so that it is the main diffusion barrier separating the apical and basolateral chambers, as seen in Figure 4A.
Figure 4. Typical Transwell® transport study setup and experimental data is shown.
(A) Diagram of a Caco-2 monolayer grown onto a Transwell system to study the transport of therapeutics. The drug is delivered apically and samples are collected from the basolateral chamber. (B) Typical data from an experiment aiming to increase protein transport across the epithelium are presented. Insulin (●) is transported less effectively than insulin in the presence of P(MAA-g-EG) microparticles (■), whereas insulin conjugated to transferrin (▲) outperformed both due to transferrin receptor-mediated transcytosis.
Reproduced with permission from [130].
P(MAA-g-EG): Poly(methacrylic acid) grafted with poly(ethylene glycol).
The most common cell line used in intestinal Transwell models is human epithelial colorectal adenocarcinoma (Caco-2). These cells represent the main cell type in the intestine and are widely used in evaluating the potential bioavailability of drugs in academia and industry [132]. A typical experimental result is shown in Figure 4B [130]. Monolayers are grown on the insert for approximately 21 days after seeding and before experimentation to allow differentiation into cells representative of the intestine with a brush border, directional transport and tight junctions [133]. Caco-2 monolayer development is monitored by transepithelial electrical resistance (TEER) using a chopstick electrode. The values increase and then plateau between 300 and 1000 Ω/cm2 depending on laboratory protocols, such as media composition and variability between cell batches [134]. A higher TEER value is representative of a tighter monolayer that will reduce paracellular transport of large molecules, whereas values much lower than this range indicate monolayer disturbance or cell toxicity [135].
Caco-2 monolayers, although widely used in modeling small intestine transport, are actually more restrictive to paracellular transport than in vivo, demonstrated by colon TEER values of 300 Ω/cm2 [132,136] However, a strong correlation between the apparent permeability coefficient and actual in vivo absorption indicate that accurate predictions can be made [137]. Additionally, the in vivo environment includes many other types of cells, such as the M cells found in Peyer’s patches and mucus secreting cells. These cells result in a more complex transport environment with the mucus layer forming another diffusion barrier and the M cells taking up large particulates, including nanoparticles [138]. To address this deficiency, coculture Transwell systems have been developed. The human adenocarcinoma cell line HT29 is seeded onto Transwells with Caco-2 cells to produce a mucus layer [139]. HT29s differentiate into mature mucus secreting goblet cells in the presence of MTX. The viability of the coculture is most easily assessed with TEER; however, increasing ratios of HT29s cells inherently reduce TEER values compared to Caco-2 monolayers. Different HT29 subclones can be used in obtaining varying mucus layer depths [140]. However, this coculture model regularly underestimates the bioavailability of drugs when compared to in vivo results [141].
The fusion of microfluidics and cell culture, which has resulted in ‘organs-on-a-chip’, promises to revolutionize many areas of biotechnology. An intestine-on-a-chip more accurately represents the intestinal environment with fluid flow over the cells grown on the porous membrane and has demonstrated additional benefits of faster Caco-2 monolayer development and inclusion of human intestine microbial flora [142]. In future, these types of systems will likely be better models of the complex physiological environment.
5.2 In vivo evaluation
Often the best representation of how a particular system will perform is in vivo. The most common animal models used are mice, rats or rabbits in a closed-loop intestine in situ model [143] or an in vivo oral dosage [66]. In the closed-loop model, the animal is fasted before being placed under anesthesia for the experiment. The small intestine is exposed through an incision in the abdomen, and the targeted segment is ligated to form a sealed intestinal tube without damaging the blood supply or muscle movement [144]. Alternatively, some procedures ligate the segment ends together to form a continuous loop to evaluate transport or uptake into Peyer’s patches [80]. The hydrogel therapeutic system is then injected into the sealed intestine, which is then returned into the body cavity for the duration of the experiment (up to 4 h), while the animal remains under anesthesia [143].
During the study, a simple analysis is the collection of blood samples to measure drug or protein bioavailability at desired time points. Additionally, the intestinal loop and other organs can be harvested and flash frozen for further analysis such as histology after sacrificing the animal [80,145]. Microscopy can determine the system’s effect on intestinal morphology as well as assess transport into intestinal tissue and systemic distribution in organs. Some therapeutics can be monitored for symptom reversal, such as insulin with blood glucose concentration [146], hemophilia with blood clotting assays [147] and calcitonin with blood calcium levels [69]. For immunology applications, both oral tolerance and vaccination success can be evaluated by plasma antibody titers, with high levels indicating an immune response and low levels indicating tolerance [148]. Finally, radio- or fluorescent-labeling of the therapeutic or hydrogel particles allows real-time noninvasive imaging to evaluate absorption or distribution using single-photon emission computed tomography/computed tomography [149], positron emission topography [150] and fluorescent in vivo imaging systems [151].
6. Expert opinion
From the previous analysis of the recent literature, it is evident that hydrogels have a special position as oral drug delivery systems. They are excellent candidates as carriers for drug, peptide and protein delivery because: i) their three-dimensional structure allows for topological control of the solute transport; and ii) their ability to be modified by hydrophilic/hydrophobic balance allows for better control and delay or acceleration of the drug transport. Hydrogels are particularly important as carriers for oral delivery because they can be rendered anionic, cationic or amphiphilic by appropriate copolymerization processes with ionic components. Although this incorporation of ionic moieties leads to environmentally sensitive structures, and therefore often intelligent systems, there are numerous unanswered questions concerning the hydrogels’ use as oral delivery vehicles. Below, we summarize a number of important problems that need to be addressed and solved in the next few years of drug delivery research.
Although design of hydrogel carriers is based on a three-component thermodynamic behavior (hydrogel/drug/water), design equations must incorporate the importance of other components in the studied systems, such as electrolytes.
Intelligent systems are often designed without consideration of possible interaction of the solute (drug/protein) with other secondary components such as electrolytes, etc. The importance of changing microenvironment pH values is usually neglected or presented only in very general terms with ‘spot’ pH verification, without much analysis in terms of the importance of local change of the pH on transport. This is often the result of the use of standard US Pharmacopeia methods of analysis and evaluation that do not require variation of the pH, as well as FDA guidelines that recommend one specific pH where the testing is done at a representative value. It is important that studies are performed under conditions where common electrolytes and the like are present at physiologically relevant levels as swelling and release behavior will vary depending on the ionic strength and type of ions available.
Phenomena of segregation or agglomeration of hydrogel particles are not taken into consideration, when studying drug delivery.
Significant changes in local osmotic effects due to associated changes in salt concentration and ionic strength variations are rarely taken into consideration during design.
Other well-known gel-related phenomena are not taken into consideration, such as Donnan equilibrium, electroneutrality of charges, and the like.
Our knowledge of the mechanisms of drug/protein/peptide transport across an underlying mucosal or cellular structure after initial drug release is still not well understood for a large number of large molecular weight solutes.
For these reasons, future research on the development and understanding of solute transport through hydrogels under ‘real’ conditions must be further understood. Still, the previous analysis has shown that the so-called environmentally sensitive or intelligent hydrogels are very useful in a large number of applications and will continue to be important, especially for protein delivery.
Article highlights.
Tunable intelligent hydrogel systems used in oral delivery applications are introduced, including general features and advantages with particular emphasis on their use for protein therapies.
Characteristics and examples of pH-responsive and environmentally triggered degrading hydrogels for oral delivery are provided.
Examples of specific therapeutic molecules addressed with oral hydrogel systems, including protein, peptide and chemotherapeutic drugs are provided.
In vitro and in vivo techniques used in evaluating the systems for important parameters such as mucoadhesion, therapeutic integrity, release, cytotoxicity and transport are surveyed.
Future research directions for better understanding the hydrogel behavior in physiological conditions are discussed.
This box summarizes key points contained in the article.
Acknowledgments
We acknowledge that SD Horava and AM Daily are recipients of the NSF GRF in addition to the other stated interests.
Footnotes
Declaration of interest
This work is supported by a grant from the National Institutes of Health (5-R01-EB-000246-20) and the Fletcher S. Pratt Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Liechty WB, Kryscio DR, Slaughter BV, et al. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010;1:149–73. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem Rev. 2012;112(5):2853–88. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 3.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006;11(19):905–10. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4(6):1–7. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 5.Blanchette J, Peppas NA. Oral Chemotherapeutic delivery: design and cellular response. Ann Biomed Eng. 2005;33(2):142–9. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 6.Shofner JP, Phillips MA, Peppas NA. Cellular evaluation of synthesized insulin/transferrin bioconjugates for oral insulin delivery using intelligent complexation hydrogels. Macromol Biosci. 2010;10(3):299–306. doi: 10.1002/mabi.200900223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morishita M, Peppas N, Sant S, et al. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 2012;64(6):496–507. doi: 10.1016/j.addr.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antosova Z, Mackova M, Kral V, et al. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–35. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Blanchette J, Park K, Peppas NA. Oral administration of chemotherapeutic agents using complexation hydrogels. MRS Proc. 2002;724:N10.4–N.4. [Google Scholar]
- 10••.Peppas NA, Bures P, Leobandung W, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. Comprehensive review of hydrogel applications, characterization and analysis of drug release. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Peppas NA. Poly(ethylene glycol)-containing hydrogels for oral protein delivery applications. Biomed Microdevices. 2003;5(4):333–41. [Google Scholar]
- 12.Ratner BD, Hoffman AS, Schoen FJ, et al. Biomaterials science: an introduction to materials in medicine. 2. Academic Press; San Diego, CA: 2004. [Google Scholar]
- 13.Arora R, Jain S, Monga S, et al. Efficacy of continuous wear PureVision contact lenses for therapeutic use. Contact Lens Anterior Eye. 2004;27(1):39–43. doi: 10.1016/j.clae.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011;111(8):4453–74. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–80. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Yang Y, Qian P, et al. Drug carrying hydrogel base wound dressing. Radiat Phys Chem. 1993;42(4):915–18. [Google Scholar]
- 18.Hoffman AS. Hydrogels for biomedical applications. Ann NY Acad Sci. 2006;944(1):62–73. doi: 10.1111/j.1749-6632.2001.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 19.Peppas NA, Hilt JZ, Khademhosseini A, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60. [Google Scholar]
- 20.Coviello T, Matricardi P, Marianecci C, et al. Polysaccharide hydrogels for modified release formulations. J Control Release. 2007;119(1):5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim SW, Bae YH, Okano T. Hydrogels: swelling, drug loading, and release. Pharm Res. 1992;9(3):283–90. doi: 10.1023/a:1015887213431. [DOI] [PubMed] [Google Scholar]
- 23.Mason MN, Metters AT, Bowman CN, et al. Predicting controlled-release behavior of degradable PLA- b -PEG-b -PLA hydrogels. Macromolecules. 2001;34(13):4630–5. [Google Scholar]
- 24.Amsden B. Solute diffusion within hydrogels. Mechanisms and models Macromolecules. 1998;31(23):8382–95. [Google Scholar]
- 25.Lustig SR, Peppas NA. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J Appl Polym Sci. 1988;36(4):735–47. [Google Scholar]
- 26.Canal T, Peppas NA. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res. 1989;23(10):1183–93. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 27.Robinson DN, Peppas NA. Preparation and characterization of pH-responsive poly(methacrylic acid- g -ethylene glycol) nanospheres. Macromolecules. 2002;35(9):3668–74. [Google Scholar]
- 28.Torres-Lugo M, García M, Record R, et al. Physicochemical behavior and cytotoxic effects of p(methacrylic acid–g-ethylene glycol) nanospheres for oral delivery of proteins. J Control Release. 2002;80(1–3):197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 29.Mastropietro DJ, Omidian H, Park K. Drug delivery applications for superporous hydrogels. Expert Opin Deliv. 2012;9(1):71–89. doi: 10.1517/17425247.2012.641950. [DOI] [PubMed] [Google Scholar]
- 30.des Rieux A, Fievez V, Théate I, et al. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30(5):380–91. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill MJ, Bourre L, Melgar S, et al. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011;16(5):203–18. doi: 10.1016/j.drudis.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Jain SK. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur J Pharm Sci. 2008;35(5):404–16. doi: 10.1016/j.ejps.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Bayat A, Dorkoosh FA, Dehpour AR, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: ex vivo and in vivo studies. Int J Pharm. 2008;356(1–2):259–66. doi: 10.1016/j.ijpharm.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein A, Haupt SM. The colon as a possible target for orally administered peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2002;19(6):499–552. doi: 10.1615/critrevtherdrugcarriersyst.v19.i6.10. [DOI] [PubMed] [Google Scholar]
- 35•.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. Barrier properties of the gastrointestinal tract and strategies to improve drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr DA, Gómez-Burgaz M, Boudes MC, et al. Complexation hydrogels for the oral delivery of growth hormone and salmon calcitonin. Ind Eng Chem Res. 2010;49(23):11991–5. doi: 10.1021/ie1008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 38.Gupta V, Hwang BH, Lee J, et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release. 2013;172(3):753–62. doi: 10.1016/j.jconrel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Hennink W, Liechty WB, Caldorera-Moore M, et al. Advanced molecular design of biopolymers for transmucosal and intracellular delivery of chemotherapeutic agents and biological therapeutics. J Control Release. 2011;155(2):119–27. doi: 10.1016/j.jconrel.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparreboom A, de Jonge MJA, Verweij J. The use of oral cytotoxic and cytostatic drugs in cancer treatment. Eur J Cancer. 2002;38(1):18–22. doi: 10.1016/s0959-8049(01)00322-7. [DOI] [PubMed] [Google Scholar]
- 41.Kanard A, Jatoi A, Castillo R, et al. Oral vinorelbine for the treatment of metastatic non-small cell lung cancer in elderly patients: a phase II trial of efficacy and toxicity. Lung Cancer. 2004;43(3):345–53. doi: 10.1016/j.lungcan.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Pescovitz MD, Rabkin J, Merion RM, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44(10):2811–15. doi: 10.1128/aac.44.10.2811-2815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neill VJ, Twelves CJ. Oral cancer treatment: developments in chemotherapy and beyond. Br J Cancer. 2002;87(9):933–7. doi: 10.1038/sj.bjc.6600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoener CA, Hutson HN, Peppas NA. pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics. J Biomed Mater Res A. 2013;101(8):2229–36. doi: 10.1002/jbm.a.34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreuter J. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189:503–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 47.Arruebo M, Fernandez-Pacheco R, Ibarra MR, et al. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32. [Google Scholar]
- 48••.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. Survey of stimuli-responsive nanoscale systems. [DOI] [PubMed] [Google Scholar]
- 49.Kim B, La Flamme K, Peppas NA. Dynamic swelling behavior of pH-sensitive anionic hydrogels used for protein delivery. J Appl Polym Sci. 2003;89(6):1606–13. [Google Scholar]
- 50.Siegel RA, Firestone BA. pH-dependent equilibrium swelling properties of hydrophobic polyelectrolyte copolymer gels. Macromolecules. 1988;21(11):3254–9. [Google Scholar]
- 51•.Khare AR, Peppas NA. Swelling/deswelling of anionic copolymer gels. Biomaterials. 1995;16(7):559–67. doi: 10.1016/0142-9612(95)91130-q. Controlling swelling properties of ionizable gels. [DOI] [PubMed] [Google Scholar]
- 52.De SK, Aluru NR, Johnson B, et al. Equilibrium swelling and kinetics of pH-responsive hydrogels: models, experiments, and simulations. J Microelectromech Syst. 2002;11(5):544–55. [Google Scholar]
- 53.Elliott JE, Macdonald M, Nie J, et al. Structure and swelling of poly(acrylic acid) hydrogels: effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer (Guildf) 2004;45(5):1503–10. [Google Scholar]
- 54.Raemdonck K, Demeester J, De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5(4):707–15. [Google Scholar]
- 55.Randale SA, Dabhi CS, Tekade AR, et al. Rapidly disintegrating tablets containing taste masked metoclopramide hydrochloride prepared by extrusion-precipitation method. Chem Pharm Bull. 2010;58(4):443–8. doi: 10.1248/cpb.58.443. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida T, Lai TC, Kwon GS, et al. pH- and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv. 2013;10(11):1497–513. doi: 10.1517/17425247.2013.821978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8(5):665–75. doi: 10.1517/17425247.2011.566553. [DOI] [PubMed] [Google Scholar]
- 58.Douroumis D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin Drug Deliv. 2007;4(4):417–26. doi: 10.1517/17425247.4.4.417. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto Y, Tanaka M, Kishimoto H, et al. Preparation, characterization and taste-masking properties of polyvinylacetal diethylaminoacetate microspheres containing trimebutine. J Pharm Pharmacol. 2002;54(10):1323–8. doi: 10.1211/002235702760345383. [DOI] [PubMed] [Google Scholar]
- 60.Bell CL, Peppas NA. Modulation of drug permeation through interpolymer complexed hydrogels for drug delivery applications. J Control Release. 1996;39(2):201–7. [Google Scholar]
- 61.Lowman AM, Peppas NA. Analysis of the complexation/decomplexation phenomena in graft copolymer networks. Macromolecules. 1997;30(17):4959–65. [Google Scholar]
- 62.Klier J, Scranton AB, Peppas NA. Self-associating networks of poly (methacrylic acid-g-ethylene glycol) Macromolecules. 1990;23(23):4944–9. [Google Scholar]
- 63.Peppas NA, Klier J. Controlled release by using poly(methacrylic acid-g-ethylene glycol) hydrogels. J Control Release. 1991;16(1):203–14. [Google Scholar]
- 64.Bell CL, Peppas NA. Swelling/syneresis phenomena in gel-forming interpolymer complexes. J Biomater Sci Polymer Ed. 1996;7(8):671–83. doi: 10.1163/156856296x00444. [DOI] [PubMed] [Google Scholar]
- 65.Lowman AM, Peppas NA. Molecular analysis of interpolymer complexation in graft copolymer networks. Polymer (Guildf) 2000;41(1):73–80. [Google Scholar]
- 66.Lowman AM, Morishita M, Kajita M, et al. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88(9):933–7. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 67.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: in vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J Biomed Mater Res A. 2003;67(2):609–17. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torres-Lugo M, Peppas NA. molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules. 1999;32(20):6646–51. [Google Scholar]
- 69.Kamei N, Morishita M, Chiba H, et al. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. J Control Release. 2009;134(2):98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peppas NA. Devices based on intelligent biopolymers for oral protein delivery. Int J Pharm. 2004;277(1–2):11–17. doi: 10.1016/j.ijpharm.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Peppas NA, Huang YB. Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev. 2004;56(11):1675–87. doi: 10.1016/j.addr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Leobandung W, Foss A, et al. Molecular aspects of muco- and bioadhesion. J Control Release. 2000;65(1):63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 73•.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan —a review. J Control Release. 2006;114(1):1–14. doi: 10.1016/j.jconrel.2006.04.017. Extensive review of intestinal delivery specific to natural polymer systems. [DOI] [PubMed] [Google Scholar]
- 74.Chen L, Tian Z, Du Y. Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomaterials. 2004;25(17):3725–32. doi: 10.1016/j.biomaterials.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 75.Chen S-C, Wu Y-C, Mi F-L, et al. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Control Release. 2004;96(2):285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Kulkarni AR, Soppimath KS, Aminabhavi TM, et al. In-vitro release kinetics of cefadroxil-loaded sodium alginate interpenetrating network beads. Eur J Pharm Biopharm. 2001;51(2):127–33. doi: 10.1016/s0939-6411(00)00150-8. [DOI] [PubMed] [Google Scholar]
- 77.Edelmana ER, Nathan A, Katada M, et al. Perivascular graft heparin delivery using biodegradable polymer wraps. Biomaterials. 2000;21(22):2279–86. doi: 10.1016/s0142-9612(00)00154-x. [DOI] [PubMed] [Google Scholar]
- 78.Rasmussen MR, Snabe T, Pedersen LH. Numerical modelling of insulin and amyloglucosidase release from swelling Ca–alginate beads. J Control Release. 2003;91(3):395–405. doi: 10.1016/s0168-3659(03)00262-1. [DOI] [PubMed] [Google Scholar]
- 79.Lee B-J, Min G-H. Oral controlled release of melatonin using polymer-reinforced and coated alginate beads. Int J Pharm. 1996;144(1):37–46. [Google Scholar]
- 80.Kim B, Bowersock T, Griebel P, et al. Mucosal immune responses following oral immunization with rotavirus antigens encapsulated in alginate microspheres. J Control Release. 2002;85(1):191–202. doi: 10.1016/s0168-3659(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 81.Romalde JL, Luzardo-Alvárez A, Ravelo C, et al. Oral immunization using alginate microparticles as a useful strategy for booster vaccination against fish lactoccocosis. Aquaculture. 2004;236(1):119–29. [Google Scholar]
- 82.Hurteaux R, Edwards-Lévy F, Laurent-Maquin D, et al. Coating alginate microspheres with a serum albumin-alginate membrane: application to the encapsulation of a peptide. Eur J Pharm Sci. 2005;24(2–3):187–97. doi: 10.1016/j.ejps.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Hébrard G, Hoffart V, Beyssac E, et al. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J Microencapsul. 2010;27(4):292–302. doi: 10.3109/02652040903134529. [DOI] [PubMed] [Google Scholar]
- 84.Pitarresi G, Craparo EF, Palumbo FS, et al. Composite nanoparticles based on hyaluronic acid chemically cross-linked with alpha,beta-polyaspartylhydrazide. Biomacromolecules. 2007;8(6):1890–8. doi: 10.1021/bm070224a. [DOI] [PubMed] [Google Scholar]
- 85.Pitarresi G, Pierro P, Giammona G, et al. Drug release from alpha,beta-poly (N-2-hydroxyethyl)-dl-aspartamide-based microparticles. Biomaterials. 2004;25(18):4333–43. doi: 10.1016/j.biomaterials.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Fiorica C, Pitarresi G, Palumbo FS, et al. A new hyaluronic acid pH sensitive derivative obtained by ATRP for potential oral administration of proteins. Int J Pharm. 2013;457(1):150–7. doi: 10.1016/j.ijpharm.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Han LN, Zhao YF, Yin LF, et al. Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS PharmSciTech. 2012;13(3):836–45. doi: 10.1208/s12249-012-9807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shepherd R, Reader S, Falshaw A. Chitosan functional properties. Glycoconj J. 1997;14(4):535–42. doi: 10.1023/a:1018524207224. [DOI] [PubMed] [Google Scholar]
- 89.Muzzarelli R, Baldassarre V, Conti F, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988;9(3):247–52. doi: 10.1016/0142-9612(88)90092-0. [DOI] [PubMed] [Google Scholar]
- 90.Yao KD, Peng T, Feng HB, et al. Swelling kinetics and release characteristic of crosslinked chitosan: polyether polymer network (semi-IPN) hydrogels. J Polymer Sci A. 1994;32(7):1213–23. [Google Scholar]
- 91.Mi F-L, Shyu S-S, Lee S-T, et al. Kinetic study of chitosantripolyphosphate complex reaction and acid-resistive properties of the chitosantripolyphosphate gel beads prepared by in-liquid curing method. J Polymer Sci B. 1999;37(14):1551–64. [Google Scholar]
- 92.Khalid MN, Ho L, Agnely F, et al. Swelling properties and mechanical characterization of a semi-interpenetrating chitosan/polyethylene oxide network: comparison with a chitosan reference gel. STP Pharma Sci. 2002;9(4):359–64. [Google Scholar]
- 93.Patel VR, Amiji MM. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res. 1996;13(4):588–93. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 94.Aly AS. Self-dissolving chitosan, I. Preparation, characterization and evaluation for drug delivery system. Die Angewandte Makromolekulare Chemie. 1998;259(1):13–18. [Google Scholar]
- 95.Yamada K, Chen T, Kumar G, et al. Chitosan Based Water-Resistant Adhesive. Analogy to Mussel Glue Biomacromolecules. 2000;1(2):252–8. doi: 10.1021/bm0003009. [DOI] [PubMed] [Google Scholar]
- 96.Guggi D, Krauland AH, Bernkop-Schnürch A. Systemic peptide delivery via the stomach: in vivo evaluation of an oral dosage form for salmon calcitonin. J Control Release. 2003;92(1):125–35. doi: 10.1016/s0168-3659(03)00299-2. [DOI] [PubMed] [Google Scholar]
- 97.Sandri G, Rossi S, Bonferoni MC, et al. Buccal penetration enhancement properties of N-trimethyl chitosan: influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm. 2005;297(1):146–55. doi: 10.1016/j.ijpharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Du Y, Huang R, et al. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials. 2003;24(27):5015–22. doi: 10.1016/s0142-9612(03)00408-3. [DOI] [PubMed] [Google Scholar]
- 99.Huguet ML, Groboillot A, Neufeld RJ, et al. Hemolglobin encapsulation in chitosan/calcium alginate beads. J Appl Polym Sci. 1994;51(8):1427–32. [Google Scholar]
- 100.You J, Zhang R, Xiong C, et al. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors. Cancer Res. 2012;72(18):4777–86. doi: 10.1158/0008-5472.CAN-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L, Guo R, Yang M, et al. Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Adv Mater. 2007;19(19):2988–92. [Google Scholar]
- 102.Jin Y, Song L, Su Y, et al. Oxime linkage: a robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules. 2011;12(10):3460–8. doi: 10.1021/bm200956u. [DOI] [PubMed] [Google Scholar]
- 103.Du Y, Chen W, Zheng M, et al. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials. 2012;33(29):7291–9. doi: 10.1016/j.biomaterials.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 104.Ahmed M, Narain R. Intracellular delivery of DNA and enzyme in active form using degradable carbohydrate-based nanogels. Mol Pharm. 2012;9(11):3160–70. doi: 10.1021/mp300255p. [DOI] [PubMed] [Google Scholar]
- 105.Lee ES, Kim D, Youn YS, et al. A Virus-Mimetic Nanogel Vehicle. Angew Chem. 2008;120(13):2452–5. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou T, Xiao C, Fan J, et al. A nanogel of on-site tunable pH-response for efficient anticancer drug delivery. Acta Biomater. 2013;9(1):4546–57. doi: 10.1016/j.actbio.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 107.Chang Kang H, Bae YH. Co-delivery of small interfering RNA and plasmid DNA using a polymeric vector incorporating endosomolytic oligomeric sulfonamide. Biomaterials. 2011;32(21):4914–24. doi: 10.1016/j.biomaterials.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thambi T, Yoon HY, Kim K, et al. Bioreducible block copolymers based on poly(ethylene glycol) and poly(γ-benzyl L-glutamate) for intracellular delivery of camptothecin. Bioconjug Chem. 2011;22(10):1924–31. doi: 10.1021/bc2000963. [DOI] [PubMed] [Google Scholar]
- 109.Rowland IR. Factors affecting metabolic activity of the intestinal microflora. Drug Metab Rev. 1988;19(3–4):243–61. doi: 10.3109/03602538808994135. [DOI] [PubMed] [Google Scholar]
- 110•.Hovgaard L, Brøndsted H. Dextran hydrogels for colon-specific drug delivery. J Control Release. 1995;36(1):159–66. Demonstration of exploiting microbial enzymes for site-specific drug delivery. [Google Scholar]
- 111.Basan H, Gumusderelioglu M, Orbey MT. Release characteristics of salmon calcitonin from dextran hydrogels for colon-specific delivery. Eur J Pharm Biopharm. 2007;65(1):39–46. doi: 10.1016/j.ejpb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 112.Yeh P-Y, Kopečkova P, Kopeček J. Degradability of hydrogels containing azoaromatic crosslinks. Macromol Chem Phys. 1995;196(7):2183–202. [Google Scholar]
- 113.Ghandehari H, Kopečková P, Yeh P-Y, et al. Biodegradable and pH sensitive hydrogels: synthesis by a polymer-polymer reaction. Macromol Chem Phys. 1996;197(3):965–80. [Google Scholar]
- 114.Akala EO, Kopečková P, Kopeček J. Novel pH-sensitive hydrogels with adjustable swelling kinetics. Biomaterials. 1998;19(11–12):1037–47. doi: 10.1016/s0142-9612(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 115.Glangchai LC, Caldorera-Moore M, Shi L, et al. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Control Release. 2008;125(3):263–72. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 116.Chellat F, Tabrizian M, Dumitriu S, et al. Study of biodegradation behavior of chitosan-xanthan microspheres in simulated physiological media. J Biomed Mater Res. 2000;53(5):592–9. doi: 10.1002/1097-4636(200009)53:5<592::aid-jbm20>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 117.He H, Cao X, Lee LJ. Design of a novel hydrogel-based intelligent system for controlled drug release. J Control Release. 2004;95(3):391–402. doi: 10.1016/j.jconrel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 118.Chowdary KPR, Rao YS. Mucoadhesive microspheres for controlled drug delivery. Biolo Pharm Bull. 2004;27(11):1717–24. doi: 10.1248/bpb.27.1717. [DOI] [PubMed] [Google Scholar]
- 119.Barthe L, Bessouet M, Woodley JF, et al. The improved everted gut sac: a simple method to study intestinal P-glycoprotein. Int J Pharm. 1998;173(1):255–8. [Google Scholar]
- 120.Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted gut sac model as a tool in pharmaceutical research: limitations and applications. J Pharm Pharmacol. 2012;64(3):326–36. doi: 10.1111/j.2042-7158.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- 121.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20(3):142–9. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 122.Mesiha M, Plakogiannis F, Vejosoth S. Enhanced oral absorption of insulin from desolvated fatty acid-sodium glycocholate emulsions. Int J Pharm. 1994;111(3):213–16. [Google Scholar]
- 123.Kotzé AF, Thanou MM, Luebetaen HL, et al. Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells (Caco-2) J Pharm Sci. 1999;88(2):253–7. doi: 10.1021/js980233c. [DOI] [PubMed] [Google Scholar]
- 124.Lueßen HL, Rentel CO, Kotzé AF, et al. Mucoadhesive polymers in peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitro. J Control Release. 1997;45(1):15–23. [Google Scholar]
- 125.Fasano A, Ghandehari H, Salama NN, et al. Tight junction modulation and its relationship to drug delivery. Adv Drug Deliv Rev. 2006;58(1):15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 126.Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89(4):429–42. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 127.Camenisch G, Alsenz J, van de Waterbeemd H, et al. Estimation of permeability by passive diffusion through Caco-2 cell monolayers using the drugs’ lipophilicity and molecular weight. Eur J Pharm Sci. 1998;6(4):313–19. [PubMed] [Google Scholar]
- 128.Giacomini KM, Huang S-M, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13(7):963–77. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- 130.Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin–transferrin conjugates. Biomaterials. 2006;27(20):3846–54. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 131.Kadiyala I, Loo Y, Roy K, et al. Transport of chitosan–DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur J Pharm Sci. 2010;39(1):103–9. doi: 10.1016/j.ejps.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport1PII of original article: S0169-409X(96)00415-2. The article was originally published in Advanced Drug Delivery Reviews 22 (1996) 67–84. 1. Adv Drug Del Rev. 2001;46(1):27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 133.Hilgers AR, Conradi RA, Burton PS. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7(9):902–10. doi: 10.1023/a:1015937605100. [DOI] [PubMed] [Google Scholar]
- 134.D’Souza VM, Shertzer HG, Menon AG, et al. High glucose concentration in isotonic media alters caco-2 cell permeability. AAPS PharmSci. 2003;5(3):E24. doi: 10.1208/ps050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okada T, Narai A, Matsunaga S, et al. Assessment of the marine toxins by monitoring the integrity of human intestinal Caco-2 cell monolayers. Toxicol In Vitro. 2000;14(3):219–26. doi: 10.1016/s0887-2333(00)00014-x. [DOI] [PubMed] [Google Scholar]
- 136.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–5. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 137.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man–fact or myth. Pharm Res. 1997;14(6):763–6. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 138.Yin L, Ding J, He C, et al. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30(29):5691–700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 139.Wikman-Larhed A, Artursson P. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur J Pharm Sci. 1995;3(3):171–83. [Google Scholar]
- 140.Behrens I, Stenberg P, Artursson P, et al. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX Cells. Pharm Res. 2001;18(8):1138–45. doi: 10.1023/a:1010974909998. [DOI] [PubMed] [Google Scholar]
- 141.Walter E, Janich S, Roessler BJ, et al. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: in vitro-in vivo correlation with permeability data from rats and humans. J Pharm Sci. 1996;85(10):1070–6. doi: 10.1021/js960110x. [DOI] [PubMed] [Google Scholar]
- 142••.Kim HJ, Huh D, Hamilton G, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–74. doi: 10.1039/c2lc40074j. A new technique to evaluate systems for oral drug delivery that more accurately represents the physiological environment and decreases cell culture time. [DOI] [PubMed] [Google Scholar]
- 143.Morishita M, Goto T, Peppas NA, et al. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J Control Release. 2004;97(1):115–24. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 144.Nakamura K, Murray RJ, Joseph JI, et al. Oral insulin delivery using P (MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release. 2004;95(3):589–99. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 145.McClean S, Prosser E, Meehan E, et al. Binding and uptake of biodegradable poly-dl-lactide micro- and nanoparticles in intestinal epithelia. Eur J Pharm Sci. 1998;6(2):153–63. doi: 10.1016/s0928-0987(97)10007-0. [DOI] [PubMed] [Google Scholar]
- 146.Morishita M, Goto T, Nakamura K, et al. Novel oral insulin delivery systems based on complexation polymer hydrogels: single and multiple administration studies in type 1 and 2 diabetic rats. J Control Release. 2006;110(3):587–94. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feijen J, Hennink WE, Sam AP, et al. Gene transfer to hemophilia a mice via oral delivery of FVIII–chitosan nanoparticles. J Control Release. 2008;132(3):252–9. doi: 10.1016/j.jconrel.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bowersock TL, Hogenesch H, Suckow M, et al. Oral vaccination with alginate microsphere systems. J Control Release. 1996;39(2):209–20. [Google Scholar]
- 149.Chen M-C, Wong H-S, Lin K-J, et al. The characteristics, biodistribution and bioavailability of a chitosan-based nanoparticulate system for the oral delivery of heparin. Biomaterials. 2009;30(34):6629–37. doi: 10.1016/j.biomaterials.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 150.Kamei N, Morishita M, Kanayama Y, et al. Molecular imaging analysis of intestinal insulin absorption boosted by cell-penetrating peptides by using positron emission tomography. J Control Release. 2010;146(1):16–22. doi: 10.1016/j.jconrel.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Bae YH, Grainger DW, Hennink WE, et al. Noninvasive imaging oral absorption of insulin delivered by nanoparticles and its stimulated glucose utilization in controlling postprandial hyperglycemia during OGTT in diabetic rats. J Control Release. 2013;172(2):513–22. doi: 10.1016/j.jconrel.2013.05.006. [DOI] [PubMed] [Google Scholar]