Abstract
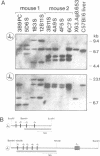
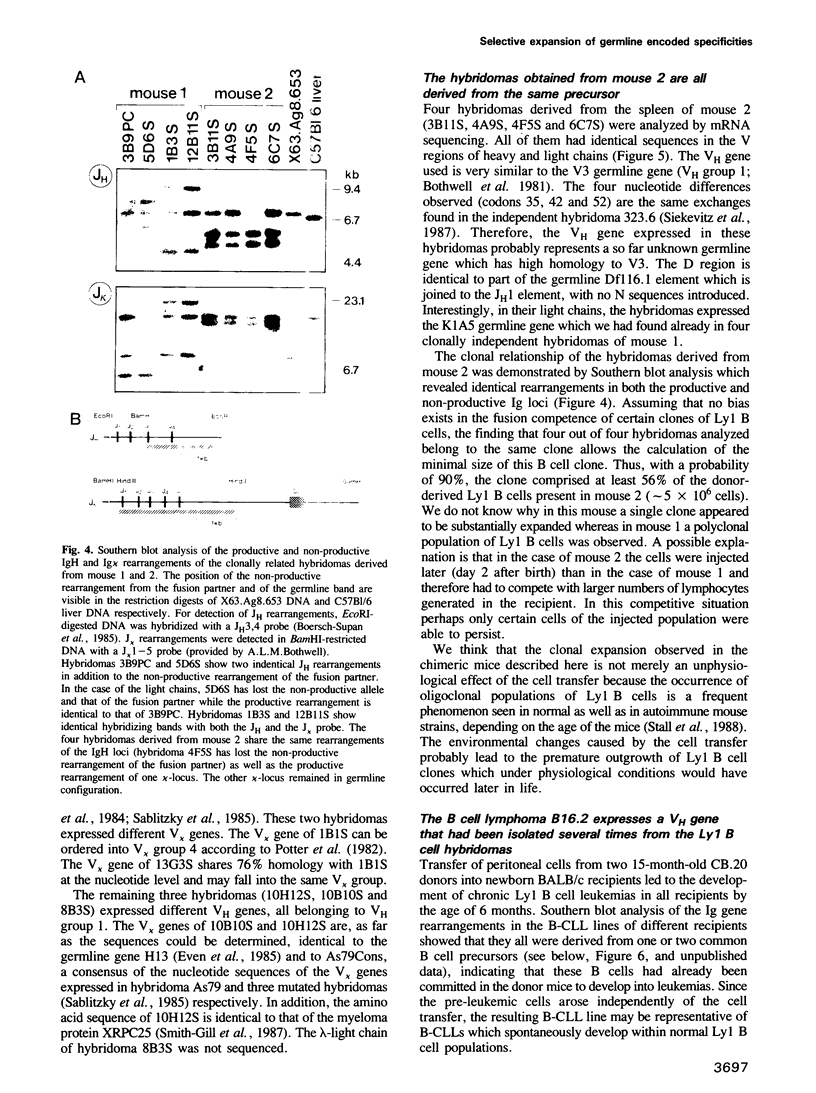
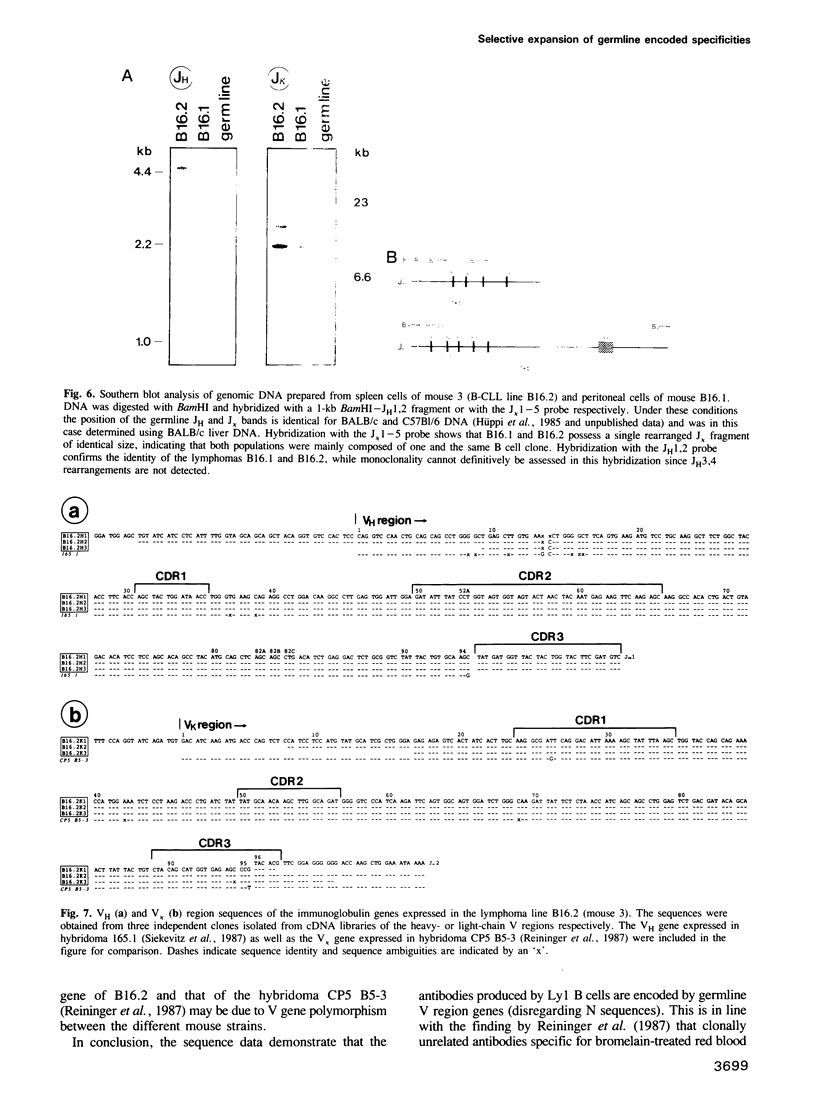
Antibody V gene expression was studied in a subpopulation of murine B cells (Ly1 B) which was enriched by cell transfer and had earlier been shown to persist in the immune system over long periods of time. Among 17 hybridomas derived from Ly1 B cells of two different mice, eight were progeny of only three different B cell precursors which apparently had expanded to clones of large size, in the absence of detectable somatic mutation of their antibody V regions. Furthermore, several clonally independent cells expressed identical, unmutated V genes. These data define a novel pathway of B cell development in which cells expressing a selected set of germline antibodies are continuously propagated in the organism. A Ly1 B cell leukemia derived from a similar transfer experiment expressed a VH gene that had been isolated in three independent Ly1 B cell hybridomas, suggesting that the leukemic cells had been equally selected in this pathway.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Berek C., Jarvis J. M., Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol. 1987 Aug;17(8):1121–1129. doi: 10.1002/eji.1830170808. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988 Oct;105:5–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Bonhomme F., Krawinkel U. Evolution of pseudogenes in the immunoglobulin VH-gene family of the mouse. Immunogenetics. 1987;26(4-5):237–248. doi: 10.1007/BF00346518. [DOI] [PubMed] [Google Scholar]
- Boersch-Supan M. E., Agarwal S., White-Scharf M. E., Imanishi-Kari T. Heavy chain variable region. Multiple gene segments encode anti-4-(hydroxy-3-nitro-phenyl)acetyl idiotypic antibodies. J Exp Med. 1985 Jun 1;161(6):1272–1292. doi: 10.1084/jem.161.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Boumsell L., Coppin H., Pham D., Raynal B., Lemerle J., Dausset J., Bernard A. An antigen shared by a human T cell subset and B cell chronic lymphocytic leukemic cells. Distribution on normal and malignant lymphoid cells. J Exp Med. 1980 Jul 1;152(1):229–234. doi: 10.1084/jem.152.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corbet S., Milili M., Fougereau M., Schiff C. Two V kappa germ-line genes related to the GAT idiotypic network (Ab1 and Ab3/Ab1') account for the major subfamilies of the mouse V kappa-1 variability subgroup. J Immunol. 1987 Feb 1;138(3):932–939. [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Davidson W. F., Fredrickson T. N., Rudikoff E. K., Coffman R. L., Hartley J. W., Morse H. C., 3rd A unique series of lymphomas related to the Ly-1+ lineage of B lymphocyte differentiation. J Immunol. 1984 Aug;133(2):744–753. [PubMed] [Google Scholar]
- Dildrop R., Bovens J., Siekevitz M., Beyreuther K., Rajewsky K. A V region determinant (idiotope) expressed at high frequency in B lymphocytes is encoded by a large set of antibody structural genes. EMBO J. 1984 Mar;3(3):517–523. doi: 10.1002/j.1460-2075.1984.tb01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Krawinkel U., Winter E., Rajewsky K. VH-gene expression in murine lipopolysaccharide blasts distributes over the nine known VH-gene groups and may be random. Eur J Immunol. 1985 Nov;15(11):1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- Even J., Griffiths G. M., Berek C., Milstein C. Light chain germ-line genes and the immune response to 2-phenyloxazolone. EMBO J. 1985 Dec 16;4(13A):3439–3445. doi: 10.1002/j.1460-2075.1985.tb04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Rocha B., Coutinho A. A. Lymphocyte population kinetics in the mouse. Immunol Rev. 1986 Jun;91:5–37. doi: 10.1111/j.1600-065x.1986.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Gadol N., Ault K. A. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986 Oct;93:23–34. doi: 10.1111/j.1600-065x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Grunow R., Jahn S., Porstmann T., Kiessig S. S., Steinkellner H., Steindl F., Mattanovich D., Gürtler L., Deinhardt F., Katinger H. The high efficiency, human B cell immortalizing heteromyeloma CB-F7. Production of human monoclonal antibodies to human immunodeficiency virus. J Immunol Methods. 1988 Feb 10;106(2):257–265. doi: 10.1016/0022-1759(88)90206-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Herzenberg L. A., Morse H. C., 3rd, Davidson W. F., Herzenberg L. A. Ly-1 as a differentiation antigen on normal and neoplastic B cells. Curr Top Microbiol Immunol. 1984;113:231–236. doi: 10.1007/978-3-642-69860-6_39. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Huppi K., Jouvin-Marche E., Scott C., Potter M., Weigert M. Genetic polymorphism at the kappa chain locus in mice: comparisons of restriction enzyme hybridization fragments of variable and constant region genes. Immunogenetics. 1985;21(5):445–457. doi: 10.1007/BF00430928. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Mäkelä O. Functional analogues of the VKOx1 gene in different strains of mice: evolutionary conservation but diversity based on V-J joining. J Immunol. 1987 Mar 1;138(5):1613–1617. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Chen P. P., Carson D. A. Autoantibody-associated kappa light chain variable region gene expressed in chronic lymphocytic leukemia with little or no somatic mutation. Implications for etiology and immunotherapy. J Exp Med. 1988 Mar 1;167(3):840–852. doi: 10.1084/jem.167.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R., Noonan D. J., Levy D. E., Wilson M. C., Møller N. P., Dixon F. J., Theofilopoulos A. N. Genetic elements used for a murine lupus anti-DNA autoantibody are closely related to those for antibodies to exogenous antigens. J Exp Med. 1985 Apr 1;161(4):805–815. doi: 10.1084/jem.161.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S., Levy S., Levy R. Retention of an idiotypic determinant in a human B-cell lymphoma undergoing immunoglobulin variable-region mutation. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5053–5057. doi: 10.1073/pnas.84.14.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle G., Kolb C., Kappen C., Schüppel R., Weiler E., Krawinkel U. A map of VH genes located next to the DH region in the Igh locus of two congenic Igh-recombinant mouse strains. Eur J Immunol. 1988 Aug;18(8):1275–1281. doi: 10.1002/eji.1830180819. [DOI] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S. A rapid method for cloning and sequencing variable-region genes of expressed immunoglobulins. Gene. 1987;54(2-3):167–173. doi: 10.1016/0378-1119(87)90484-7. [DOI] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Livant D., Blatt C., Hood L. One heavy chain variable region gene segment subfamily in the BALB/c mouse contains 500-1000 or more members. Cell. 1986 Nov 7;47(3):461–470. doi: 10.1016/0092-8674(86)90603-3. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Tamura G., Weissman I. L. Receptor mediated leukemogenesis: murine leukemia virus interacts with BCL1 lymphoma cell surface IgM. J Mol Cell Immunol. 1987;3(4):227–242. [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Pattern of sequence variation among kappa chains with limited sequence differences. Biochemistry. 1973 Feb;12(4):760–771. doi: 10.1021/bi00728a028. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Sasaki Y., Kina T., Amagai T., Katsura Y. A monoclonal antibody against Igh6-4 determinant. Immunogenetics. 1986;23(2):137–139. doi: 10.1007/BF00377976. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Osmond D. G. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986 Oct;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Painter C. J., Monestier M., Chew A., Bona-Dimitriu A., Kasturi K., Bailey C., Scott V. E., Sidman C. L., Bona C. A. Specificities and V genes encoding monoclonal autoantibodies from viable motheaten mice. J Exp Med. 1988 Mar 1;167(3):1137–1153. doi: 10.1084/jem.167.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Peters D. V. A simple enzyme-linked immunosorbent assay for antibodies to native DNA. J Immunol Methods. 1981;41(2):187–200. doi: 10.1016/0022-1759(81)90242-8. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Potter M., Newell J. B., Rudikoff S., Haber E. Classification of mouse VK groups based on the partial amino acid sequence to the first invariant tryptophan: impact of 14 new sequences from IgG myeloma proteins. Mol Immunol. 1982 Dec;19(12):1619–1630. doi: 10.1016/0161-5890(82)90273-5. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Reininger L., Ollier P., Poncet P., Kaushik A., Jaton J. C. Novel V genes encode virtually identical variable regions of six murine monoclonal anti-bromelain-treated red blood cell autoantibodies. J Immunol. 1987 Jan 1;138(1):316–323. [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Sablitzky F., Rajewsky K. Molecular basis of an isogeneic anti-idiotypic response. EMBO J. 1984 Dec 1;3(12):3005–3012. doi: 10.1002/j.1460-2075.1984.tb02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Wildner G., Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 1985 Feb;4(2):345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schiff C., Corbet S., Milili M., Fougereau M. Interstrain conservation of the murine GAT-specific antibody V kappa repertoire as analyzed at the germline gene level. EMBO J. 1983;2(10):1771–1776. doi: 10.1002/j.1460-2075.1983.tb01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Nemazee D. A., Sato V. L., Van Snick J., Carson D. A., Weigert M. G. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986 Aug 1;164(2):407–427. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Shultz L. D., Hardy R. R., Hayakawa K., Herzenberg L. A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986 Jun 13;232(4756):1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Hamel P. A., Lovoie T. B., Dorrington K. J. Contributions of immunoglobulin heavy and light chains to antibody specificity for lysozyme and two haptens. J Immunol. 1987 Dec 15;139(12):4135–4144. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stohrer R., Kearney J. Ontogeny of B cell precursors responding to alpha 1- greater than 3 dextran in BALB/c mice. J Immunol. 1984 Nov;133(5):2323–2326. [PubMed] [Google Scholar]
- Tarlinton D., Stall A. M., Herzenberg L. A. Repetitive usage of immunoglobulin VH and D gene segments in CD5+ Ly-1 B clones of (NZB x NZW)F1 mice. EMBO J. 1988 Dec 1;7(12):3705–3710. doi: 10.1002/j.1460-2075.1988.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol Rev. 1986 Dec;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Vakil M., Kearney J. F. Functional characterization of monoclonal auto-anti-idiotype antibodies isolated from the early B cell repertoire of BALB/c mice. Eur J Immunol. 1986 Sep;16(9):1151–1158. doi: 10.1002/eji.1830160920. [DOI] [PubMed] [Google Scholar]
- Vakil M., Sauter H., Paige C., Kearney J. F. In vivo suppression of perinatal multispecific B cells results in a distortion of the adult B cell repertoire. Eur J Immunol. 1986 Sep;16(9):1159–1165. doi: 10.1002/eji.1830160921. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]