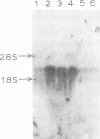
Abstract
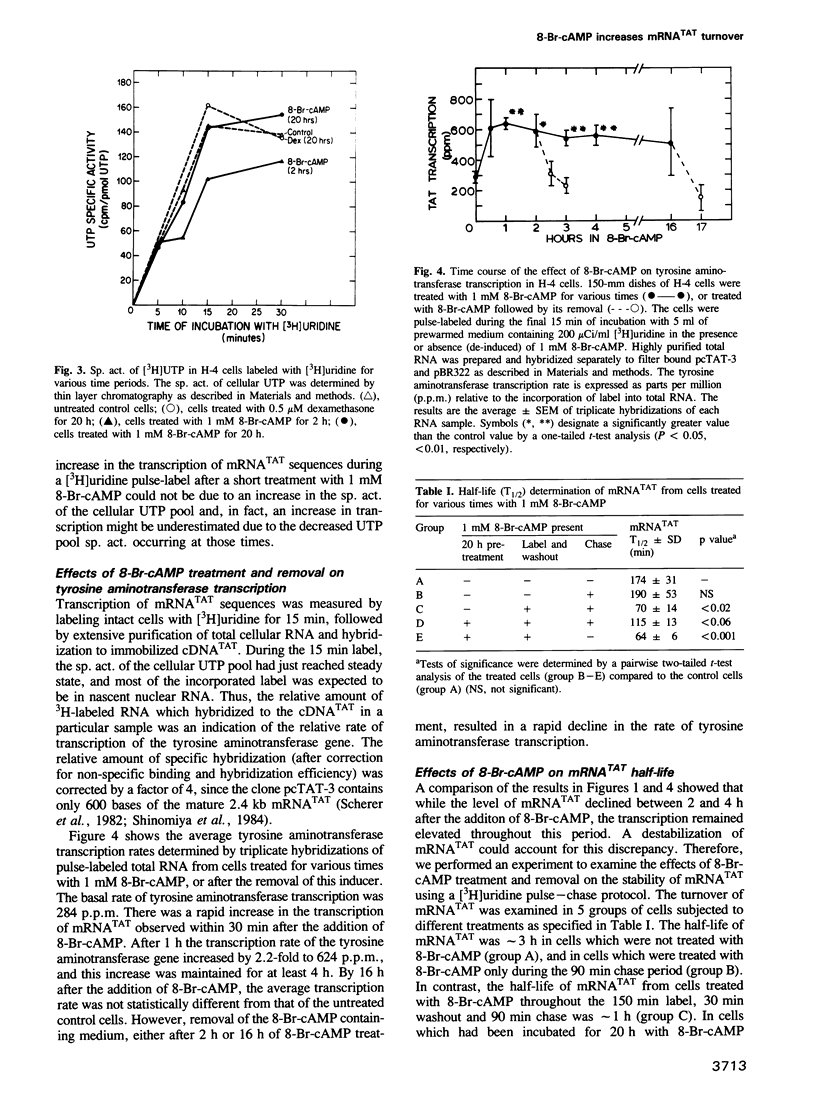
Treatment of H-4 rat hepatoma cells with 8-bromo-cyclic AMP (8-Br-cAMP) resulted in a transient induction of the gluconeogenic enzyme tyrosine aminotransferase. Synthesis of tyrosine aminotransferase and the level of its corresponding mRNA peaked 2 h after the addition of the cyclic nucleotide and declined thereafter. Tyrosine aminotransferase synthesis and mRNA failed to respond to the readdition of fresh 8-Br-cAMP, a process which we defined as desensitization. Removal of 8-Br-cAMP resulted in a decrease in tyrosine aminotransferase synthesis and mRNA, a process defined as de-induction. The relative transcription rate of the tyrosine aminotransferase gene and the turnover of its mRNA were determined by labeling intact cells with [3H]uridine. 8-Br-cAMP led to an increase in the rate of tyrosine aminotransferase transcription which was sustained for at least 4 h. The transcription rate declined upon de-induction. In addition, 8-Br-cAMP increased the turnover rate of tyrosine aminotransferase mRNA, but only after a 1.5-3 h time lag. This increased degradation rate persisted for at least 1.5 h after the removal of 8-Br-cAMP. These two contrasting and temporally distinct processes could account for the observed changes in tyrosine aminotransferase mRNA levels in response to 8-Br-cAMP treatment and removal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Chung S., Landfear S. M., Blumberg D. D., Cohen N. S., Lodish H. F. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. doi: 10.1016/0092-8674(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Culpepper J. A., Liu A. Y. Pretranslational control of tyrosine aminotransferase synthesis by 8-bromo-cyclic AMP in H-4 rat hepatoma cells. J Biol Chem. 1983 Nov 25;258(22):13812–13819. [PubMed] [Google Scholar]
- Deutsch P. J., Jameson J. L., Habener J. F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987 Sep 5;262(25):12169–12174. [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyházi E., Pigon A., Ossoinak A., Holst M., Tayip U. Phosphorylation of some chromosomal nonhistone proteins in active genes is blocked by the transcription inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB). J Cell Biol. 1984 Mar;98(3):954–962. doi: 10.1083/jcb.98.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest M. J. Regulation of tyrosine aminotransferase messenger ribonucleic acid in rat liver. effect of cycloheximide on messenger ribonucleic acid turnover. Biochemistry. 1982 Dec 21;21(26):6761–6767. doi: 10.1021/bi00269a022. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Kelley D. C., Miles M. F., Milkowski D. M. Cyclic AMP regulation of lactate dehydrogenase. Isoproterenol and N6,O2-dibutyryl cyclic amp increase the rate of transcription and change the stability of lactate dehydrogenase a subunit messenger RNA in rat C6 glioma cells. J Biol Chem. 1983 Apr 25;258(8):5312–5318. [PubMed] [Google Scholar]
- Kenney F., Lee K. L., Reel J. R., Hoel D. G. Regulation of tyrosine alpha-ketoglutarate transaminase in rat liver. IX. Studies of the mechanisms of hormonal inductions in cultured hepatoma cells. J Biol Chem. 1970 Nov 10;245(21):5806–5812. [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Calie P., Wicks W. D. Differences in rates of tyrosine aminotransferase deinduction with cyclic AMP and glucocorticoids. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5778–5782. doi: 10.1073/pnas.79.19.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. T., Pitot H. C. The regulation of ornithine aminotransferase synthesis by glucagon in the rat. Arch Biochem Biophys. 1976 May;174(1):262–272. doi: 10.1016/0003-9861(76)90345-3. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Miles M. F., Hung P., Jungmann R. A. Cyclic AMP regulation of lactate dehydrogenase. Quantitation of lactate dehydrogenase M-subunit messenger RNA in isoproterenol-and N6,O2'-dibutyryl cyclic AMP-stimulated rat C6 glioma cells by hybridization analysis using a cloned cDNA probe. J Biol Chem. 1981 Dec 10;256(23):12545–12552. [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M. M., Merrill M. J., Pitot H. C. Translational and pretranslational control of ornithine aminotransferase synthesis in rat liver. J Biol Chem. 1983 May 25;258(10):6109–6114. [PubMed] [Google Scholar]
- Murdoch G. H., Rosenfeld M. G. Eukaryotic transcriptional regulation and chromatin-associated protein phosphorylation by cyclic AMP. Science. 1982 Dec 24;218(4579):1315–1317. doi: 10.1126/science.6293056. [DOI] [PubMed] [Google Scholar]
- Murphy W., Attardi G. Stability of cytoplasmic messenger RNA in HeLa cels. Proc Natl Acad Sci U S A. 1973 Jan;70(1):115–119. doi: 10.1073/pnas.70.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Blume J. E., Nielsen D. A. Regulation of messenger RNA stability in eukaryotic cells. Bioessays. 1987 May;6(5):221–226. doi: 10.1002/bies.950060507. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Scherer G., Schmid W., Zentgraf H., Schütz G. Isolation and characterization of the rat tyrosine aminotransferase gene. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1346–1350. doi: 10.1073/pnas.81.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Simon L. N., Shuman D. A., Robins R. K. The chemistry and biological properties of nucleotides related to nucleoside 3',5'-cyclic phosphates. Adv Cyclic Nucleotide Res. 1973;3:225–353. [PubMed] [Google Scholar]
- Smith J. D., Liu A. Y. The induction, desensitization and de-induction of tyrosine aminotransferase by 8-bromo-cyclic AMP in rat hepatoma cells. Biochem J. 1988 Apr 1;251(1):261–267. doi: 10.1042/bj2510261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., Voorma H. O., Van Wijk R. Further evidence for translational regulation of tyrosine aminotransferase synthesis by dibutyryl cyclic AMP in Reuber H35 hepatoma cells. Biochim Biophys Acta. 1981 Aug 27;655(1):107–112. doi: 10.1016/0005-2787(81)90073-3. [DOI] [PubMed] [Google Scholar]
- Snoek G. T., van de Poll K. W., Voorma H. O., van Wijk R. Studies on the posttranscriptional site of cAMP action in the regulation of the synthesis of tyrosine aminotransferase. Eur J Biochem. 1981;114(1):27–31. doi: 10.1111/j.1432-1033.1981.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Spielholz C., Schlichter D., Wicks W. D. Cyclic adenonosine monophosphate does not affect the stability of the messenger ribonucleic acid for tyrosine aminotransferase in cultured hepatoma cells. Mol Endocrinol. 1988 Apr;2(4):344–349. doi: 10.1210/mend-2-4-344. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Thompson E. B., Hayashi S., Gelehrter T., Granner D., Peterkofsky B. Tyrosine transaminase induction in mammalian cells in tissue culture. Cold Spring Harb Symp Quant Biol. 1966;31:349–360. doi: 10.1101/sqb.1966.031.01.045. [DOI] [PubMed] [Google Scholar]
- Wicks W. D., McKibbin J. B. Evidence for translational regulation of specific enzyme synthesis by N 6 , O 2' -dibutyryl cyclic AMP in hepatoma cell cultures. Biochem Biophys Res Commun. 1972 Jul 11;48(1):205–211. doi: 10.1016/0006-291x(72)90364-6. [DOI] [PubMed] [Google Scholar]
- Zandomeni R., Zandomeni M. C., Shugar D., Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986 Mar 5;261(7):3414–3419. [PubMed] [Google Scholar]