Abstract
The diagnosis and staging of patients with lung cancer in recent decades has increasingly relied on minimally invasive tissue sampling techniques, such as endobronchial ultrasound (EBUS) or endoscopic ultrasound (EUS) needle aspiration, transbronchial biopsy, and transthoracic image guided core needle biopsy. These modalities have been shown to have low complication rates, and provide adequate cellular material for pathologic diagnosis and necessary ancillary molecular testing. As an important component to a multidisciplinary team approach in the care of patients with lung cancer, these minimally invasive modalities have proven invaluable for the rapid and safe acquisition of tissue used for the diagnosis, staging, and molecular testing of tumors to identify the best evidence-based treatment plan. The continuous evolution of the field of lung cancer staging and treatment has translated into improvements in survival and quality of life for patients. Although differences in clinical practice between academic and community hospital settings still exist, improvements in physician education and training as well as adoption of technological advancements should help narrow this gap going forward.
Keywords: Lung cancer, staging, molecular testing, minimally invasive, endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA), interventional pulmonology
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. The most frequently encountered primary lung cancers include epithelial-derived non-small-cell lung cancer (NSCLC), with adenocarcinoma and squamous cell carcinoma as the main histologic subtypes; and neuroendocrine carcinomas, with small cell lung cancer (SCLC) as the major high-grade neuroendocrine carcinoma. Most NSCLCs are diagnosed at advanced stages, and historically (up to the early 2000s), palliative therapeutic decisions were based solely on the differentiation between NSCLC and SCLC. Hence, the main diagnostic modalities and focus on tissue acquisition were geared towards obtaining small samples for simple histopathological characterization that would be added to non-invasive imaging studies to complete tumor, node, metastasis (TNM) staging. The paradigm of NSCLC histology not otherwise specified (NOS) with advanced TNM staging drove the development of anti-cancer therapies for NSCLCs in the 1980s, 1990s, and early 2000s; with the evidence-based introduction of platinum-doublets as the main palliative modality for stage IV NSCLC (1).
A need to better define NSCLC subtypes occurred in the early 2000s with the introduction of novel cytotoxic chemotherapies (pemetrexed) and biological agents (bevacizumab) that had enhanced efficacy or worsened toxicity, respectively, based on histology (2,3). To this end, a diagnosis of NSCLC NOS was no longer sufficient, and the more widespread use of both histochemical and immunohistochemical ancillary studies helped to more consistently distinguish adenocarcinoma from squamous cell carcinoma in small biopsy/cytology specimens. The 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) lung adenocarcinoma classification was developed by an international core panel of expert medical oncologists, pulmonologists, pathologists, and thoracic surgeons, to address minimum requirements in immunohistochemical testing markers to differentiate between adenocarcinoma and squamous cell in small samples that were previously classified as NSCLC NOS (4). This shift in tumor acquisition goals and requirements, continues to reverberate in clinical lung cancer care and drug development, with, for example, the initial approval by the US Food and Drug Administration (FDA) of the immune-checkpoint, anti-programmed death-ligand 1 (PD-1) inhibitor, nivolumab, for advanced squamous cell lung cancer (5).
The need for adequate tissue for the diagnosis and management of NSCLC has increased substantially over the last decade, as new anti-cancer therapies have begun to explore vulnerabilities in the genomic underpinnings of cancer. Cancer is a heterogeneous group of diseases that lead to invasion and metastasis, induction angiogenesis, replicative immortality, resistance to cell death, reprogramming of energy metabolism, evasion of immune surveillance, circumvention of growth suppressors, and sustained proliferative signaling (6). The latter is especially prevalent in subgroups of NSCLC, since sustained proliferative signaling is usually derived from genomic mutations in key oncogenes that encode for activated tyrosine kinases.
Three main genomic events lead to the direct activation of tyrosine kinases in NSCLC: overexpression or amplification (due to increased copy numbers of a certain oncogene), mutation (due to point mutations or insertions/deletions), and rearrangement with partner genes (by preserving or activating the kinase domain of oncogenes). The most prevalent oncogenes that are amplified, mutated or rearranged in NSCLCs are listed in Table 1 (7-9).
Table 1. Known driver mutations in NSCLC with associated targeted therapeutics.
| Molecular target/driver oncogene | Prevalence (%) | US FDA-approved TKIs in 2015 | US FDA-breakthrough designation TKIs in 2015 | Off label use of TKIs with significant level of evidence (NCCN category 2A) | Off label use of TKIs with lesser levels of evidence |
|---|---|---|---|---|---|
| Adenocarcinoma | |||||
| KRAS mutations | 25-30 | None | None | None | None |
| EGFR mutations | 15-20 | Erlotinib, afatinib | AZD9291, rociletinib | N/A | N/A |
| ALK rearrangements | 3-7 | Crizotinib, ceritinib | Alectinib | N/A | N/A |
| ROS1 rearrangements | 2-4 | None | Crizotinib | Crizotinib | Cabozantinib |
| MET exon 14 skipping mutation | 2-4 | None | None | None | Crizotinib |
| ERBB2 mutations | 1-3 | None | None | None | Afatinib |
| BRAF mutations (V600E) | 1-3 | None | Dafrafenib, dafrafenib + trametinib | Dafrafenib, vemurafenib | N/A |
| RET rearrangements | 1-2 | None | None | None | Cabozantinib |
| MET amplification | 1-2 | None | None | Crizotinib | N/A |
| MAP2K1 mutations | 1 | None | None | None | None |
| NTRK1 rearrangements | <1 | None | None | None | None |
| FGFR2/3/4 rearrangements | <1 | None | None | None | None |
| Squamous cell carcinoma | |||||
| FGFR1 amplifications | 15-20 | None | None | None | None |
| FGFR2/3/4 mutations/rearrangements | 5-10 | None | None | None | None |
| PI3KCA mutations | 5-10 | None | None | None | None |
| DDR2 mutations | 1-5 | None | None | None | Dasatinib |
NSCLC, non-small-cell lung cancer; FDA, Food and Drug Administration; TKIs, tyrosine kinase inhibitors; N/A, non-applicable.
Tyrosine kinase inhibitors (TKIs), small molecules that can block the function of kinases, have been developed as precision therapies in NSCLC. As of mid-2015, EGFR and ALK mutations are the most prevalent, clinically relevant driver oncogenes in NSCLC care. First generation reversible EGFR TKIs (gefitinib and erlotinib) and second generation irreversible EGFR TKIs (afatinib) have been shown in multiple randomized phase III trials to be superior to standard platinum-doublet chemotherapies in the first line treatment of advanced EGFR mutant lung adenocarcinomas and are FDA approved for use in this setting (10-13). In addition, novel third generation covalent EGFR TKIs that are more specific to the most common first/second generation TKI resistance mutation (EGFR-T790M) are active and have FDA ‘breakthrough’ review designation.
ALK mutations in lung adenocarcinomas occur through gene rearrangements (the most common partner is EML4) that lead to constitutive activation of the tyrosine kinase domain of ALK. The multitargeted ALK/MET/ROS1 TKI crizotinib led to significant responses in phase I and II trials of ALK rearranged lung adenocarcinoma, and phase III randomized trials in the second line (crizotinib versus docetaxel or pemetrexed) and first line (crizotinib versus platinum-pemetrexed) setting have confirmed that crizotinib is more effective than chemotherapy for these tumors (14-17). The FDA label of crizotinib requires tumor identification of ALK rearrangement status. In addition, the second generation ALK TKI ceritinib is FDA approved for the therapy of crizotinib-resistant ALK rearranged lung adenocarcinoma and the related compound alectinib has a FDA breakthrough designation (18,19). Other TKIs have differing levels of evidence for off-label use in lung adenocarcinomas with other genotypes (Table 1).
To standardize the use of tissue for the ever-changing needs of molecular diagnostics in lung cancer, in 2013, IASLC, Association for Molecular Pathology (AMP), and College of American Pathologists (CAP) published minimum molecular testing guidelines for selection of lung cancer patients for EGFR and ALK TKIs that are now widely used for day-to-day medical oncology care (20). The current guidelines prioritize use of rapid single gene assays for these two driver oncogenes. However, it is becoming evident that technological advances have reached a point where comprehensive molecular profiling using a variety of next generation sequencing (NGS) platforms is feasible in routine clinical practice; with a multitude of commercial or academic vendors providing Clinical Laboratory Improvement Amendments (CLIA)-certified NGS assays that use formalin-fixed paraffin-embedded (FFPE) specimens or cytology specimens to isolate DNA and/or RNA for analyses of a targeted panel of genes to select for the most readily targetable alterations (Table 1) (21,22).
Therefore, the need for sufficient, high-quality tissue material for diagnosis, staging, and treatment selection has grown significantly, concurrently with the expansion of minimally-invasive tissue acquisition methods. We will address current minimally invasive methods for tissue acquisition in the diagnosis and management of patients with lung cancer, their performance characteristics, and consider current gaps in patient care in different practice environments.
Minimally invasive techniques for tissue acquisition
Prompt and accurate diagnosis and staging of patients with lung cancer should be sought through an efficient process: one that minimizes the number of procedures before initiating treatment. Ideally, the preferred initial procedure would be able to simultaneously provide tissue for diagnosis, tumor classification, molecular testing, as well as provide staging information. However, this may or may not be possible depending on the individual patient and the need for sufficient and appropriate tissue for current and future cytological, immunohistochemical, and molecular studies. The available techniques are: mediastinoscopy, endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA), endoscopic ultrasound (EUS) with fine needle aspiration (FNA), traditional bronchoscopic TBNA and computed-tomography guided core needle biopsy (CT-CNB) or CT-FNA. The overall performance measures of these different techniques are summarized in Table 2.
Table 2. Non-invasive and minimally-invasive staging modalities for non-small cell lung carcinoma*.
| Procedure | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Number of studies | Number of specimens | Cancer prevalence (%) |
|---|---|---|---|---|---|---|---|
| CT | 55 | 81 | 58 | 83 | 43 | 7,368 | 30 |
| Integrated PET-CT | 62 | 90 | 63 | 90 | 19 | 2,014 | 22 |
| Mediastinoscopy^ | 81 | 100 | 100 | 91 | 35 | 10,648 | 34 |
| TBNA | 78 | 100 | 100 | 77 | 27 | 2,408 | 81 |
| EUS-FNA | 89 | 100 | 100 | 86 | 26 | 2,443 | 58 |
| EBUS-TBNA | 89 | 100 | 100 | 91 | 26 | 2,756 | 58 |
| EBUS-TBNA + EUS-FNA | 91 | 100 | 100 | 96 | 7 | 811 | 33 |
*, median data values, compiled from the most recent 3rd edition ACCP Guidelines for the Diagnosis and Management of Lung Cancer [Silvestri et al. (23)]. PPV, positive predictive value; NPV, negative predictive value; PET-CT, positron emission tomography-computed tomography; TBNA, transbronchial needle aspiration; EUS-FNA, endoscopic ultrasound guided fine needle aspiration; EBUS-TBNA, endobronchial ultrasound with transbronchial needle aspiration; ^, includes traditional mediastinoscopy and video-assisted mediastinoscopy.
Mediastinoscopy
Mediastinoscopy is a surgical procedure that allows for the exploration of the superior mediastinum from the sternal notch to the subcarinal space and sometimes can reach the main bronchi (Figure 1). It is done under general anesthesia, with the neck maximally extended and through a 2-3 cm collar incision at the sternal notch carried out through the platysma. The strap muscles are separated to expose the trachea and after incising the pretracheal fascia, the pretracheal plane is developed. Finger dissection is initially used as caudally as possible while palpating key structures such as the innominate artery and the aortic arch. This space is then used to advance the video-mediastinoscope. This process is continued by using suction/coagulation device sweeps to advance caudally. Before carrying out biopsies, the surgeon identifies the innominate artery, aortic arch, pulmonary artery and the azygos vein. Occasionally, the appearance of a lymph node and a vascular structure are similar, and a fine needle is used to gently penetrate the structure and identify if there is blood flow or not (24).
Figure 1.
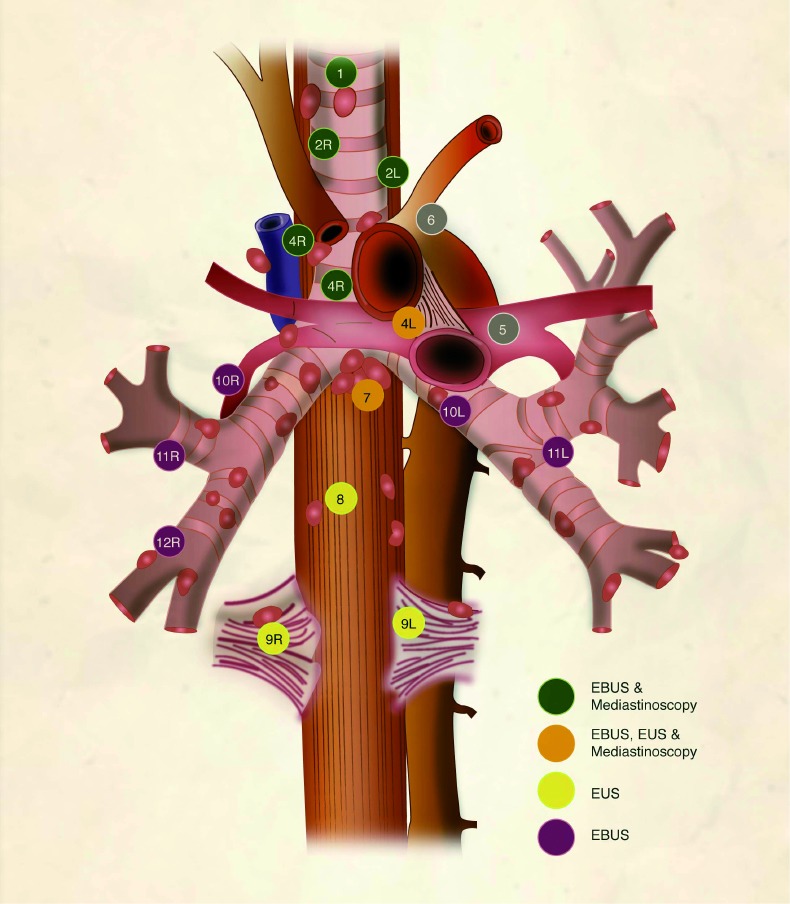
Lymph node map adapted from the 2009 IASLC lung cancer staging project. The lymph node stations are color coded to indicate the minimally-invasive staging techniques that can readily access each lymph node station. The close proximity to vascular structures highlights the importance of direct visualization or ultrasound guidance to avoid bleeding complications. EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; IASLC, International Association for the Study of Lung Cancer.
In a similar fashion to EBUS or EUS, exploration of the lymph nodes starts on the contralateral side of the tumor to rule out N3 disease and then proceeds in a systematic way. The subcarinal lymph nodes are usually sampled last because bronchial artery and perinodal bleeding can be more difficult to control. It is important to mention that by convention the specificity and positive predictive values of cervical mediastinoscopy are considered 100%, as entire lymph nodes are excised for histologic evaluation. However, positive results are not confirmed by other tests. The median sensitivity of conventional mediastinoscopy is reported to be 78% with a median negative predictive value of 91% (23). Video-mediastinoscopy has a median sensitivity of 89% with a negative predictive value of 92%. Although rare, complications occur in 3% of cases with serious bleeding in 0.4% occasionally requiring mediastinotomy (25,26). Mortality is under 0.5% (27,28).
There are two technical variations of mediastinoscopy intended for systematic removal of mediastinal lymph nodes: video-assisted mediastinoscopic lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA). These two procedures, also called “supermediastinoscopies”, are not widely used but their exceptional operating characteristics warrant a comment. Both are done through an incision similar to the one used for mediastinoscopy but with systematic removal of the lymph nodes. In VAMLA, the removal of subcarinal and right inferior paratracheal lymph nodes en block followed by the left inferior paratracheal lymph nodes is done through a 2-blade spreadable mediastinoscope (29).
In TEMLA, a sternal retractor elevates the sternum allowing for complete mediastinal lymphadenectomy from the supraclavicular to the paraesophageal lymph nodes. A thoracoscope is also used to remove the subaortic and para-aortic lymph nodes (30).
Although both are rarely used, the sensitivity of VAMLA was close to 100%, while TEMLA has shown to be superior to mediastinoscopy and EBUS (31,32).
Interestingly, some experts and authors of the prior research studies, conclude that VAMLA and TEMLA have no current role in the routine mediastinal staging of lung cancer. In part due to their invasiveness and high risk of complications when compared to equally accurate but less invasive options including EBUS and EUS (33). Furthermore, VAMLA and TEMLA are not mentioned (23) or recommended only within clinical trials (34) in the most recent guidelines for staging of lung cancer.
Endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA)
Endoscopic techniques have emerged as the procedure of choice for diagnosis and staging of lung cancer (23). These techniques have also been associated with lower morbidity and mortality, and have been suggested to be more cost effective than mediastinoscopy (35,36). Complications are very rare, with the rate of pneumothorax between 0.07% and 0.2% (37). The procedure is usually done in the outpatient setting by pulmonologists, interventional pulmonologists, or thoracic surgeons in a procedure suite or in the operating room. Anesthesia largely depends on local practices, but may involve moderate sedation or general anesthesia. A dedicated flexible bronchoscope with an ultrasound (5, 7.5, 10 and 12 MHz) at the distal end is inserted through the mouth, an endotracheal tube, or a laryngeal mask and advanced to the distal trachea where apposition of the ultrasound probe to the airway wall reveals adjacent structures in high detail. After identifying the lymph node station based on anatomic landmarks, a 21 or 22 gauge needle is advanced under direct visualization on ultrasound.
Although there is no consensus on the number of times each lymph node is punctured (passes), in our experience, three passes with 15 needle excursions per pass provides diagnostic material in over 95% of cases (38). After each pass, the needle is withdrawn and a small amount of material can be either placed on a slide for immediate preparation or the entire sample can be placed in a preservative solution for cytologic analysis and cellblock preparation. As shown in Figure 1, EBUS can access the following stations: 2R and 2L (upper paratracheal), 4R and 4L (lower paratracheal), 7 (subcarinal), 10R and 10L (hilar), 11R and 11L (interlobar), on occasion 12R and 12L (lobar) as well as paratracheal and parabronchial masses that occur close to the airway. At least one case series that encompasses multiple institutions described access to station 5 (subaortic) through a transpulmonary artery route (39).
Endoscopic ultrasound guided fine needle aspiration (EUS-FNA)
EUS is also a real-time ultrasound procedure guiding trans-esophageal needle aspiration. It allows posterior mediastinal sampling through the esophageal wall. The lymph nodes preferentially accessible to EUS are the inferior pulmonary ligament (level 9), paraesophageal (level 8), subcarinal (level 7), and left paratracheal (level 4L) (Figure 1). However, anterolateral paratracheal (levels 2R, 2L, and 4R) are difficult to sample with EUS. EUS also has a high safety profile, similar to EBUS (40,41). The main feature that sets apart EUS from other techniques is the access to locations outside of the mediastinum, such as the left lobe of the liver, a significant part of the right lobe of the liver, and the left adrenal gland (42). Given its relative strengths and weaknesses, it is best to think of EUS as a complement to EBUS for the diagnosis and staging of lung cancer patients. When used in combination, the yield is higher than with either technique used alone. Pooled analyses have shown sensitivity of 91% and specificity of 100% (23,43).
CT-guided biopsy
Computed tomography provides details on the anatomic location, shape, margins, attenuation of the primary lesion as well as the extent of invasion of the chest wall, presence of suspicious mediastinal, hilar, segmental lymph nodes, and proximity to surrounding structures (44). However, this radiologic evaluation is not entirely specific and should not be used as the single source of staging. The median sensitivity and specificity of CT for identification of mediastinal lymph node involvement were 55% and 81% respectively (23). Other studies have shown similar low sensitivity when pooled in meta-analysis demonstrating sensitivity of 51-64% for NSCLC (45,46). Whenever CT guidance is used to obtain tissue by core needle biopsy or fine needle aspiration, the pooled sensitivity and specificity are 90% and 97% respectively (47). However, the complications include a 15% risk of pneumothorax and 1% risk of major hemorrhage (48). The risk factors for major complications during trans-thoracic needle aspiration include emphysema, small lesion, greater depth of needle penetration, and multiple needle passes. For these reasons, it is not common to use trans-thoracic needle aspiration to sample mediastinal lymph nodes.
In summary, the different minimally invasive techniques are designed to help clinicians identify lung cancer patients who are likely to benefit from primary resection, neo-adjuvant chemotherapy and/or radiation, or palliative chemotherapy. However, recent studies suggest that the strategic combination of staging techniques (such as EBUS, followed, when negative, by mediastinoscopy) provides better outcomes and may be more cost-effective (49). A study by Farjah and colleagues reported severe underuse of multimodality staging; with the use of multimodality staging increasing over time from 1998 to 2005 resulting in an association between use of multimodality staging and improved survival, irrespective of the stage of disease (50).
If only imaging studies are used for staging, 15-40% of patients will be denied curative intent therapy (51). For these reasons, radiologic images that are concerning for lung cancer or metastatic disease should be confirmed with cytology or histopathology. Inadequate lymph node evaluation is unfortunately common and its consequences are hard to estimate, but likely translates into reduced lung cancer survival if nodal disease is not identified and treated (52-54).
Lymph node mapping
Regardless of how thoracic lymph nodes are sampled for staging purposes, it is important to use a common vocabulary when describing the location of these lymph node stations as well as to state what specific lymph node stations were sampled. The Japanese (Naruke) and US/European (Mountain and Dresler) lymph node maps were reconciled into a single universal map by the IASLC in 2009 (55). This provides a uniform, specific anatomic definition of the lymph node stations, and facilitates the identification of the exact location during surgery, radiologic interpretation and minimally-invasive biopsy techniques (see Rami-Porta et al. in this special issue). It is recommended that we abandon loose anatomic descriptions such as “lower paratracheal” or “parahilar” as these terms are not specific to a lymph node station and can easily be misinterpreted.
Definitions for mediastinal lymph node evaluation
Using standard definitions for the thoroughness of mediastinal nodal staging is as important as using a uniform mediastinal lymph node map (56). The following categories have been used for surgical staging, but they can easily be extrapolated to minimally invasive techniques such as EBUS TBNA. The extent of lymph node assessment can be broadly categorized into the following groups (57):
Random sampling: the sampling of lymph nodes by convenience or by preoperative or intraoperative findings. The most common situation is the sampling of a single enlarged lymph node. Unfortunately, this practice has been found to be very common in the mediastinoscopy literature (52).
Systematic sampling: the sampling of predetermined lymph node stations, such as 2L, 4L, 7, and 10L for a left sided lung tumor, and 2R, 4R, 7 and 10R for a right sided tumor.
Mediastinal lymph node dissection: the complete surgical removal of all identifiable mediastinal lymph node tissue based on anatomic landmarks.
Extended lymph node dissection: the removal of bilateral paratracheal and cervical lymph nodes by formal dissection.
Lobe-specific systematic node dissection: the removal of ipsilateral mediastinal lymph node tissue based on the location of the tumor.
Guidelines on tissue acquisition and processing for diagnosis, staging, and genotyping
The American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines, the European Society of Thoracic Surgeons (ESTS) guidelines, and Cancer Care Ontario (CCO) Program in Evidence-Based Care Practice Guidelines are in agreement on their recommendations for indications and techniques for invasive staging (23,34,58). It is important to emphasize that random sampling or sampling of a single enlarged lymph node is considered inadequate surgical staging. Some authors have extrapolated this to minimally invasive techniques and have advocated against random sampling (59). It is recommended that appropriate staging include stations 2R, 2L, 4R, 4L, and 7. However, TBNA of lymph nodes that are smaller than 5 mm is very difficult and likely will result in sub-optimal amount of tissue for diagnosis. Clinically suspicious lymph nodes, such as enlarged (≥1 cm short axis diameter) or FDG-avid nodes, should also be sampled. Guidelines, such as those published by ESTS, the United Kingdom’s National Institute for Health and Care Excellence, and CCO, recommend that appropriate lymph node assessment should be systematic and include a minimum of three mediastinal lymph node stations, one of which should be station 7 (subcarinal) (34,58,60).
Sample acquisition and processing differences: how does needle aspiration (cytology) differ from core biopsy (histology)?
It is important to have an appreciation for how small biopsies obtained by minimally invasive means are processed and evaluated by the pathologist/cytopathologist. In general, these small biopsy or cytology specimens must be sufficient to establish a diagnosis of malignancy, to make a reliable subclassification of disease (e.g., adenocarcinoma vs. squamous cell carcinoma) using immunochemical stains, and, increasingly, for molecular testing to identify targetable driver mutations. The amount of information to be gleaned from these small biopsy and cytologic specimens is great, and has increased dramatically over the past decade.
Minimally invasive biopsy specimens are small, with limited cellular material. Transbronchial/endobronchial biopsies and transthoracic core needle biopsies of lung lesions can provide some tissue architecture, helpful in delineating invasive carcinoma from in-situ/lepidic pattern of spread, though sampling limitations can be an issue for these specimens. Cytologic aspirates (EBUS-TBNA or EUS-FNA) oftentimes lack these architectural cues, though frequently larger tissue fragments that are almost biopsy-like can be aspirated and appreciated on direct smears or cell block preparations. Establishing a diagnosis of malignancy on cytologic specimens should rarely be a problem though, as the cytologic features of malignancy are generally easy to appreciate. In contrast to biopsy specimens, which are nearly always formalin-fixed and paraffin-embedded, cytologic specimens can be processed and evaluated in a number of ways, including by direct smears or touch-preparations of tissue biopsies (either air-dried or alcohol fixed), alcohol-fixed liquid based concentration methods (such as using cytospin, ThinPrep, or SurePath), as well as the creation of a tissue cell block. The latter captures the cellular material into a cell pellet that is formalin-fixed and paraffin-embedded, creating for all intents and purposes a tissue-biopsy-like specimen from which multiple serial slides can be cut from the paraffin block and used for immunohistochemical stains and molecular testing. In reality, the lines between small biopsy specimens and cytology specimens (especially with the creation of a good cell block) have become blurred, with both types of specimens capable of providing specific histopathologic diagnoses and serving as substrates for molecular testing.
In order to preserve cellular material for downstream molecular testing, the 2015 iteration of the WHO classification of lung tumors (61) and the 2011 IASLC/ATS/ERS classification of lung carcinomas on small biopsy/cytology specimens (62) recommends that a focused panel of immunostains be employed for the work-up of a suspected primary NSCLC when histology or cytomorphology alone is insufficient to distinguish adenocarcinoma from squamous cell carcinoma. Specifically, one lung adenocarcinoma marker (traditionally the transcription factor TTF-1) and one squamous cell marker (usually p63 or more recently p40—the N-terminal truncation isoform of p63 shown to be more specific for squamous cell carcinoma) (63). If these results are inconclusive, then second line lung adenocarcinoma markers (such as the aspartic proteinase Napsin-A) and squamous cell carcinoma markers (cytokeratin 5/6) can be employed. A mucicarmine histochemical stain can also be helpful to demonstrate glandular differentiation. Clinical and radiologic correlation are always helpful, to focus the immunohistochemical work-up of carcinoma metastatic to the lungs, especially when more lung-specific markers are negative.
Genotyping: yield of different techniques
The most current guidelines from the CAP, IASLC, and AMP call for testing all advanced stage lung adenocarcinomas (or mixed tumors with an adenocarcinoma component) for EGFR mutations, generally by PCR-based methods, and ALK gene rearrangements (via FISH assay or with screening immunohistochemistry) (20). Lung cancers are also commonly tested for KRAS mutations which are associated with resistance to tyrosine kinase inhibitors. In addition to these three main molecular targets, the list of less common driver mutations (Table 1) in lung adenocarcinoma is growing rapidly. With the growing number of actionable targets for lung cancer, relying on the current paradigm of one-off testing using these small biopsy or cytology specimens will inevitably deplete the cellular material despite the cytopathologist’s best efforts to maximize cell block cellularity and minimize material loss during the initial diagnostic work-up. Therefore, a shift towards multiplexed panels seems inevitable in future (21).
Many groups have published very good molecular testing success rates using small biopsy and cytology specimens. In general, the success rates for small biopsy specimens (including transthoracic core needle biopsies or transbronchial biopsies) are comparable to those for cytology cell block specimens. Recent studies comparing these modalities report a molecular testing success rate for small biopsy specimens of 55-100%, and a success rate for FNA or EBUS-TBNA cell block specimens of 46-95%, depending on the study parameters (64-67). In general there is a higher molecular testing failure rate from small biopsy or cytology specimens as compared to larger surgical resection specimens, inferred from the limiting tumor cellularity present in the former (68).
A recent publication from the Lung Cancer Mutation Consortium, a multi-institutional program investigating selected oncogene drivers in lung adenocarcinoma, revealed that in an 8-gene panel testing approach, 35% of cytology specimens and 26% of small biopsies were insufficient for molecular testing (compared to only 5% of surgical resection specimens). Importantly however, the authors comment that once a specimen was deemed adequate for molecular testing (i.e., has sufficient tumor cellularity), the specimen type (cytology/small biopsy/surgical resection) had no influence on subsequent molecular testing performance and (69) that minor differences between completion rates were not felt to be clinically significant. Therefore, cytology and small biopsy specimens have been proven to be excellent substrates for molecular testing, as long as enough tumor cells are obtained and the preceding pathologic work-up is efficient and minimized tumor cell loss.
Advanced bronchoscopy techniques in non-academic settings
EBUS-TBNA has become increasingly commonplace outside of academic medical centers. However, appropriate training for thorough and systematic mediastinal staging is still lagging (59). Electromagnetic navigation bronchoscopy (ENB), and other advanced diagnostic techniques have also become increasingly commonplace in the community setting. Each of these procedures has an associated learning curve, requiring the development of a systematic approach to proper procedural techniques for biopsies and tissue handling. Increasing interest has led to implementation of training in advanced bronchoscopy techniques in pulmonary/critical care fellowships, as well as dedicated interventional pulmonary fellowships.
For physicians who did not have exposure to these techniques during their formal training, the training options include taking a sabbatical year, participating in an intense 1-7 day course, or direct proctoring by experienced colleagues. Current ACCP guidelines for procedural training are based on minimum number of procedures and not necessarily on the cognitive and technical skills required (70). In the United States, the need for the procedures at community and regional hospitals has led to the implementation of bronchoscopy services, including EBUS, or the creation of referral channels to tertiary care centers (71). Ultimately, the success of community programs depends on adequate investment of human and technological capital, ideally within multidisciplinary teams of pulmonologists, thoracic surgeons, radiologists, cytopathologists, radiation oncologists, and medical oncologists, who should collaborate to apply evidence-based guidelines while continuously evaluating their performance using mutually accepted yield and quality metrics.
A number of authors have advocated the utility of rapid onsite examination (ROSE) for the evaluation of EBUS samples. Although immediate feedback for the bronchoscopist as well as appropriate specimen collection and triage can be helpful in certain circumstances, the current guidelines from the World Association for Bronchology and Interventional Pulmonology state that use of ROSE is not recommended for every case if the operator is experienced (72), and certainly should not limit the implementation of a much needed service for lung cancer patients. In this setting, EBUS-TBNA samples for driver oncogene mutation analysis has been successful in close to 95% of the cases, even with use of a commercial laboratory and no sample enrichment (64). Appropriate tissue handling and preparation with methanol based fixatives and paraffin-embedded cell blocks have been used successfully by our group and others (68,73).
Conclusions
The diagnosis and treatment of lung cancer has undergone multiple dramatic changes in the last decade. We have a better understanding of the molecular biology of lung cancer and driver mutations that can be targeted through the use of specific tyrosine-kinase inhibitors. Significant technological advances allow interventional pulmonologists and surgeons to obtain diagnostic material in a safe and minimally invasive manner. Ongoing refinements in diagnostic and ancillary molecular testing by pathologists and cytopathologists has allowed small biopsy and cytology specimens to be used to accurately diagnose and characterize lung cancer, helping direct appropriate therapeutic decisions. Moving forward, a pressing task for the health care community at large will be to narrow existing practice gaps between high-performing (often academic) and lower performing (often community-based) care delivery settings.
Acknowledgements
The authors would like to thank Diana Obregon for lending her artistic skills in creating the figures for this manuscript.
Footnotes
Conflicts of Interest: DB Costa has received consulting fees and honoraria from Pfizer and Boehringer Ingelheim (unrelated to the current work). E Folch has served as scientific consultant for Boston Scientific, education consultant for Olympus and is the principal investigator for an ongoing trial in navigation bronchoscopy funded by Medtronic. J Wright and PA VanderLaan have no conflicts of interest to declare.
References
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [DOI] [PubMed] [Google Scholar]
- 8.Gerber DE, Gandhi L, Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am Soc Clin Oncol Educ Book 2014:e353-65. [DOI] [PubMed] [Google Scholar]
- 9.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res 2014;47:929-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [DOI] [PubMed] [Google Scholar]
- 14.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [DOI] [PubMed] [Google Scholar]
- 17.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:2537-9. [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 2014;20:1479-84. [DOI] [PubMed] [Google Scholar]
- 23.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S. [DOI] [PubMed] [Google Scholar]
- 24.Weeden D, Tsang VT. Cardiothoracic surgery. In: Johnson CD, Cumming J, editers. Essential surgical technique. New York: Springer, 1997:197-232. [Google Scholar]
- 25.Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg 2003;238:180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park BJ, Flores R, Downey RJ, et al. Management of major hemorrhage during mediastinoscopy. J Thorac Cardiovasc Surg 2003;126:726-31. [DOI] [PubMed] [Google Scholar]
- 27.Kirschner PA. Cervical mediastinoscopy. Chest Surg Clin N Am 1996;6:1-20. [PubMed] [Google Scholar]
- 28.Urschel JD. Conservative management (packing) of hemorrhage complicating mediastinoscopy. Ann Thorac Cardiovasc Surg 2000;6:9-12. [PubMed] [Google Scholar]
- 29.Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [DOI] [PubMed] [Google Scholar]
- 30.Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90; discussion 390. [DOI] [PubMed] [Google Scholar]
- 31.Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuzdzal J, Zieliński M, Papla B, et al. The transcervical extended mediastinal lymphadenectomy versus cervical mediastinoscopy in non-small cell lung cancer staging. Eur J Cardiothorac Surg 2007;31:88-94. [DOI] [PubMed] [Google Scholar]
- 33.Kużdżał J, Warmus J, Grochowski Z. Optimal mediastinal staging in non-small cell lung cancer: what is the role of TEMLA and VAMLA? Lung Cancer 2014;86:1-4. [DOI] [PubMed] [Google Scholar]
- 34.De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [DOI] [PubMed] [Google Scholar]
- 35.Steinfort DP, Liew D, Conron M, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010;5:1564-70. [DOI] [PubMed] [Google Scholar]
- 36.Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [DOI] [PubMed] [Google Scholar]
- 37.Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderLaan PA, Wang HH, Majid A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): an overview and update for the cytopathologist. Cancer Cytopathol 2014;122:561-76. [DOI] [PubMed] [Google Scholar]
- 39.Folch E, Santacruz J, Machuzak M, et al. Safety and efficacy of EBUS-guided TBNA through the pulmonary artery: a preliminary report. Chest 2011;140:600A. [Google Scholar]
- 40.Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S. [DOI] [PubMed] [Google Scholar]
- 41.Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [DOI] [PubMed] [Google Scholar]
- 42.Chang KJ, Erickson RA, Nguyen P. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration of the left adrenal gland. Gastrointest Endosc 1996;44:568-72. [DOI] [PubMed] [Google Scholar]
- 43.Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [DOI] [PubMed] [Google Scholar]
- 44.Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [DOI] [PubMed] [Google Scholar]
- 45.Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology 1999;213:530-6. [DOI] [PubMed] [Google Scholar]
- 46.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [DOI] [PubMed] [Google Scholar]
- 47.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S. [DOI] [PubMed] [Google Scholar]
- 48.Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [DOI] [PubMed] [Google Scholar]
- 50.Farjah F, Flum DR, Ramsey SD, et al. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol 2009;4:355-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S. [DOI] [PubMed] [Google Scholar]
- 52.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [DOI] [PubMed] [Google Scholar]
- 53.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806. [DOI] [PubMed] [Google Scholar]
- 54.Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol 2012;7:390-6. [DOI] [PubMed] [Google Scholar]
- 55.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77. [DOI] [PubMed] [Google Scholar]
- 56.Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [DOI] [PubMed] [Google Scholar]
- 57.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [DOI] [PubMed] [Google Scholar]
- 58.Darling GE, Dickie AJ, Malthaner RA, et al. Invasive mediastinal staging of non-small-cell lung cancer: a clinical practice guideline. Curr Oncol 2011;18:e304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folch E, Majid A. Point: are >50 supervised procedures required to develop competency in performing endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal staging? Yes. Chest 2013;143:888-91; discussion 894-5. [DOI] [PubMed]
- 60.National Collaborating Centre for Cancer (Great Britain), National Institute for Health and Clinical Excellence (Great Britain). The diagnosis and treatment of lung cancer (update). NICE clinical guidelines no 121. Cardiff, UK: National Collaborating Centre for Cancer (UK); 2011:1. [Google Scholar]
- 61.Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: WHO, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012;25:405-15. [DOI] [PubMed] [Google Scholar]
- 64.Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013;8:1438-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider F, Smith MA, Lane MC, et al. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am J Clin Pathol 2015;143:193-200; quiz 306. [DOI] [PubMed] [Google Scholar]
- 66.Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol 2015;123:318-26. [DOI] [PubMed] [Google Scholar]
- 67.Wang S, Yu B, Ng CC, et al. The suitability of small biopsy and cytology specimens for EGFR and other mutation testing in non-small cell lung cancer. Transl Lung Cancer Res 2015;4:119-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer 2014;84:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [DOI] [PubMed] [Google Scholar]
- 71.Kinsey CM, Channick CL. Counterpoint: are >50 supervised procedures required to develop competency in performing endobronchial ultrasound-guided transbronchial needle aspiration for lung cancer staging? No. Chest 2013;143:891-3; discussion 893-4. [DOI] [PubMed]
- 72.van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds JP, Tubbs RR, Minca EC, et al. EGFR mutational genotyping of liquid based cytology samples obtained via fine needle aspiration (FNA) at endobronchial ultrasound of non-small cell lung cancer (NSCLC). Lung Cancer 2014;86:158-63. [DOI] [PubMed] [Google Scholar]


