Abstract
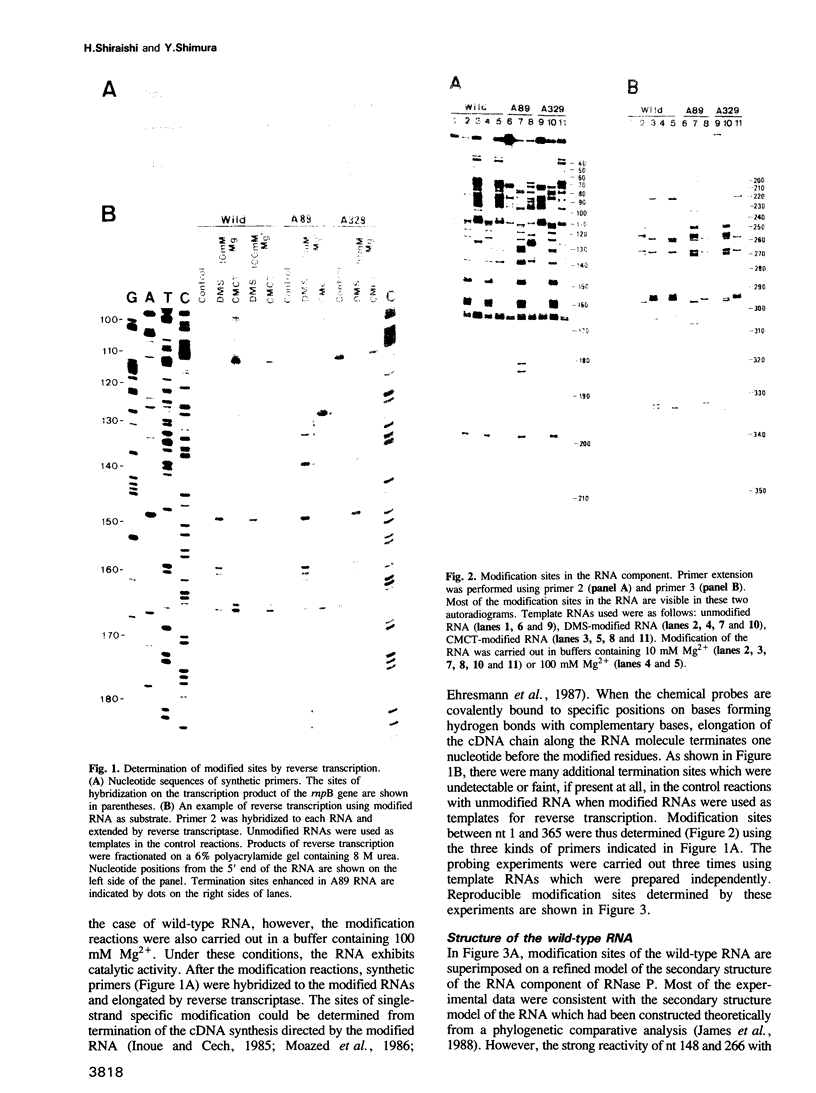
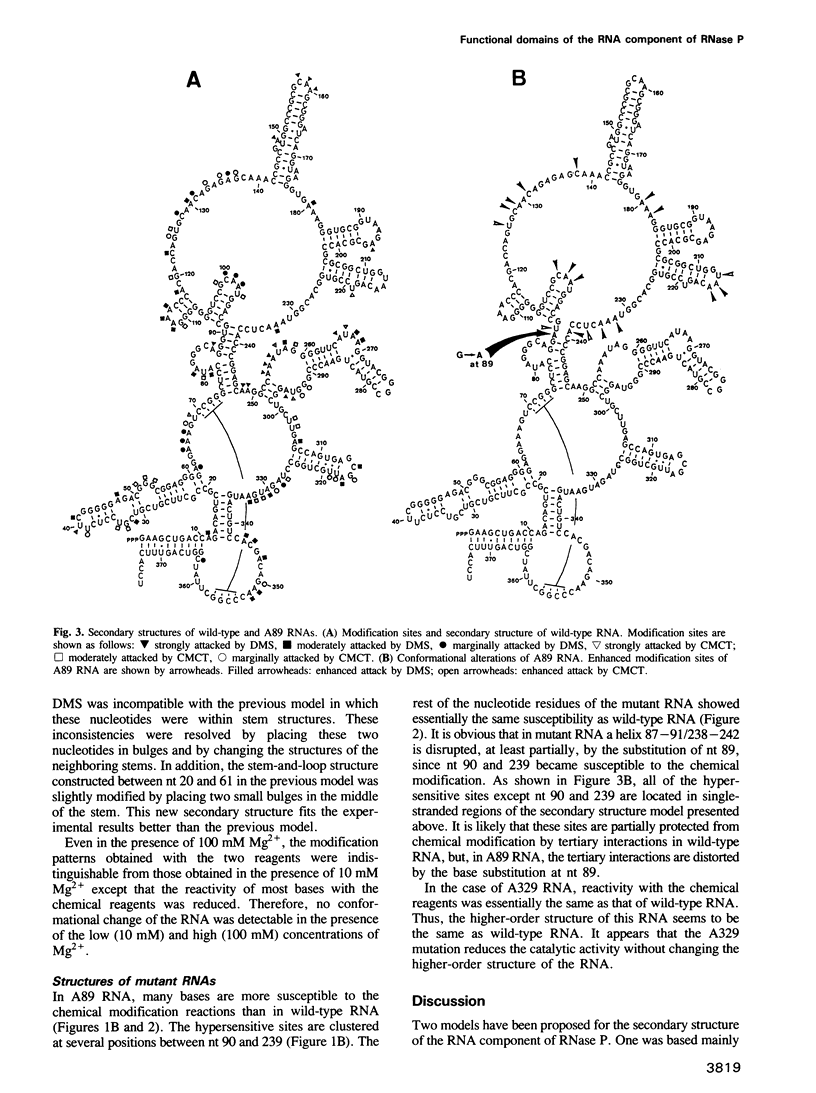
The higher-order structure of the RNA component of ribonuclease P from Escherichia coli was analyzed using chemical probes. The secondary structure model which had been constructed from the comparative sequence analysis of the RNA was refined using the experimental data. In a mutant RNA (A89 RNA), which contains a G----A substitution at nucleotide 89, we detected a number of conformational alterations clustered between nucleotides 90 and 239. In view of the fact that A89 RNA is as catalytically active as wild-type RNA, but defective in association with the protein component, it is clear that the catalytic function of the RNA component resides on the structure which is not disrupted by the A89 mutation and that the structures altered by the mutation represent the region(s) interacting with the protein component. Another mutant (A329 RNA), which has a G----A substitution at nucleotide 329 and is defective in catalytic function, showed no detectable change in higher-order structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Smith J. D. Tyrosine tRNA precursor molecule polynucleotide sequence. Nat New Biol. 1971 Sep 8;233(36):35–39. doi: 10.1038/newbio233035a0. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. M1 RNA with large terminal deletions retains its catalytic activity. Cell. 1986 Apr 25;45(2):177–183. doi: 10.1016/0092-8674(86)90381-8. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. Structure in solution of M1 RNA, the catalytic subunit of ribonuclease P from Escherichia coli. Biochemistry. 1984 Dec 18;23(26):6327–6334. doi: 10.1021/bi00321a006. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B. D., Olsen G. J., Liu J. S., Pace N. R. The secondary structure of ribonuclease P RNA, the catalytic element of a ribonucleoprotein enzyme. Cell. 1988 Jan 15;52(1):19–26. doi: 10.1016/0092-8674(88)90527-2. [DOI] [PubMed] [Google Scholar]
- Lawrence N. P., Richman A., Amini R., Altman S. Heterologous enzyme function in Escherichia coli and the selection of genes encoding the catalytic RNA subunit of RNase P. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6825–6829. doi: 10.1073/pnas.84.19.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell. 1983 Oct;34(3):911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R. Structure-independent nucleotide sequence analysis. Proc Natl Acad Sci U S A. 1979 May;76(5):2232–2235. doi: 10.1073/pnas.76.5.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986 Dec 26;47(6):985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Reed R. E., Baer M. F., Guerrier-Takada C., Donis-Keller H., Altman S. Nucleotide sequence of the gene encoding the RNA subunit (M1 RNA) of ribonuclease P from Escherichia coli. Cell. 1982 Sep;30(2):627–636. doi: 10.1016/0092-8674(82)90259-8. [DOI] [PubMed] [Google Scholar]
- Reich C., Gardiner K. J., Olsen G. J., Pace B., Marsh T. L., Pace N. R. The RNA component of the Bacillus subtilis RNase P. Sequence, activity, and partial secondary structure. J Biol Chem. 1986 Jun 15;261(17):7888–7893. [PubMed] [Google Scholar]
- Robertson H. D., Altman S., Smith J. D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid presursor. J Biol Chem. 1972 Aug 25;247(16):5243–5251. [PubMed] [Google Scholar]
- Sakamoto H., Kimura N., Nagawa F., Shimura Y. Nucleotide sequence and stability of the RNA component of RNase P from a temperature-sensitive mutant of E. coli. Nucleic Acids Res. 1983 Dec 10;11(23):8237–8251. doi: 10.1093/nar/11.23.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Kimura N., Shimura Y. Processing of transcription products of the gene encoding the RNA component of RNase P. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6187–6191. doi: 10.1073/pnas.80.20.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Yamada S., Ikemura T., Shimura Y., Ozeki H. Temperature sensitive mutants of Escherichia coli for tRNA synthesis. Nucleic Acids Res. 1974 Mar;1(3):355–371. doi: 10.1093/nar/1.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H., Shimura Y. A rapid and efficient method for targeted random mutagenesis. Gene. 1988 Apr 29;64(2):313–319. doi: 10.1016/0378-1119(88)90346-0. [DOI] [PubMed] [Google Scholar]
- Shiraishi H., Shimura Y. Mutations affecting two distinct functions of the RNA component of RNase P. EMBO J. 1986 Dec 20;5(13):3673–3679. doi: 10.1002/j.1460-2075.1986.tb04698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]