Abstract
Objectives:
To determine risk factors associated with postoperative hypoxemia after surgery for acute type A aortic dissection.
Methods:
We retrospectively analyzed the clinical data of 192 patients with acute type A aortic dissection who underwent surgery in Qingdao Municipal Hospital, Medical College of Qingdao University, Qingdao, China between January 2007 and December 2013. Patients were divided into hypoxemia group (n=55) [arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤200 mm Hg] and non-hypoxemia group (n=137) [PaO2/FiO2 >200 mm Hg]. Perioperative clinical data were analyzed and compared between the 2 groups.
Results:
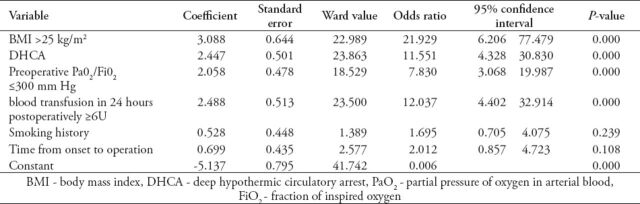
The incidence of postoperative hypoxemia after surgery for acute aortic dissection was 28.6% (55/192). Perioperative death occurred in 13 patients (6.8%). Multivariate regression identified body mass index (BMI) >25 kg/m2 (OR=21.929, p=0.000), deep hypothermic circulatory arrest (DHCA) (OR=11.551, p=0.000), preoperative PaO2/FiO2 ≤300 mm Hg (OR=7.830, p=0.000) and blood transfusion >6U in 24 hours postoperatively (OR=12.037, p=0.000) as independent predictors of postoperative hypoxemia for patients undergoing Stanford A aortic dissection surgery.
Conclusion:
Our study demonstrated that BMI >25 kg/m2, DHCA, preoperative PaO2/FiO2 ≤300 mm Hg, and blood transfusion in 24 hours postoperatively >6U were independent risk factors of the hypoxemia after acute type A aortic dissection aneurysm surgery.
Acute type A aortic dissection is a serious life-threatening cardiovascular disease that requires positive surgical treatment. Although the preoperative recognition, perioperative management, and surgical techniques have been significantly improved, and the operations for acute type A aortic dissections are still associated with high mortality.1,2 In-hospital mortality after surgery for Stanford type A acute aortic dissection range from 9-30% in previous study.3 The international registry of acute aortic dissection experience indicates that the overall in-hospital mortality is 31.4% in hemodynamically instable patients, and 16.7% in hemodynamically stable patients.4 On analysis of the German registry for acute aortic dissection type A in 658 patients with type I Debakey aortic dissection has an overall 30-day mortality of 20.2%.5 Hypoxemia is a common postoperative complication for acute aortic dissection, especially for Stanford type A aortic dissection. As consequence, the duration of mechanical ventilation and ICU stay prolonged, and perioperative mortality increased accordingly. However, the association between acute type A aortic dissection and hypoxemia is still not fully investigated. Therefore, it is essential to investigate the risk factors of hypoxemia after surgery for acute type A aortic dissection to improve the surgical treatment effect and reduce the perioperative mortality by early intervention and treatment of hypoxemia.
Methods
The study took place in Qingdao Municipal Hospital, Medical College of Qingdao University, Qingdao, China between January 2007 and December 2013. One hundred and ninety-two patients with acute type A aortic dissection underwent surgical procedure in our hospital. We enrolled 192 consecutive patients with acute type A aortic dissection diagnosed with enhanced computed tomography in this study. Of the 192 patients, 55 cases suffered from postoperative hypoxemia. This study was conducted in accordance with the principles of the Helsinki Declaration and was approved by the Ethics Committee of Qingdao Municipal Hospital, Medical College of Qingdao University, Qingdao, China. There were 152 males and 40 females with an average age of 56.1 years (range 21-79 years). All patients underwent surgical treatment within 7 days after the onset of symptoms, and 136 patients underwent operation within 48 hours. Postoperative hypoxemia was defined as an arterial partial oxygen (mm Hg)/inspired oxygen fraction (%) (partial pressure of oxygen in arterial blood [PaO2]/fraction of inspired oxygen [FiO2]) ratio of 200 or lower, while for non-hypoxemia the above ratio was greater than 200. All the data were evaluated approximately 6 hours after arrival in the intensive care unit (ICU). According to the occurrence of postoperative hypoxemia, 192 patients were divided into 2 groups: hypoxemia group (n=55) and non-hypoxemia group (n=137). Preoperative clinical materials of these 2 groups are listed in Table 1. All the patients with acute type A aortic dissection underwent surgical treatment by prosthetic graft replacement of ascending aorta or aortic arch. Operations were performed through a standard longitudinal median sternotomy. Cardiopulmonary bypass (CPB) was established by cannulation of right atrium or separately the superior and inferior vena cava. The right or left femoral artery was optionally the site of cannulation. Myocardial protection was provided by intermittent, antegrade, cold blood cardioplegic solution via coronary ostia. If an intimal tear was localized to the ascending aorta, the distal anastomosis was constructed proximal to the innominate artery. If the intimal tear originated in or extended into the arch, deep hypothermic circulatory arrest (DHCA) was instituted to extend the aortic replacement to include the intimal tear. Of the 192 patients, 61 cases were performed with moderate hypothermic CPB. Cerebral protection was performed with DHCA and selective antegrade cerebral perfusion in 131. When the proximal aortic root procedure was finished, the rectal temperature was approximately 18°C. The circulation was arrested, cross clamping was removed, and open distal repair was performed. Antegrade selective cerebral perfusion was applied during circulatory arrest, and the patients’ heads were wrapped in ice bags. During the operation, when the intimal tear was located only in the ascending aorta, we simply replaced it. In aortic root replacement, Bentall procedure were performed for patients with dilation of aortic root. Partial or total arch replacement was performed when the entry site was present or extended into the aortic arch. All patients underwent corrective surgery, ascending aorta/hemiarch replacement in 84, Bentall procedure in 65, David procedure in 11, total arch replacement in 28, and aortoplasty in 4; at the same time, 20 patients underwent coronary artery bypass grafting, and 62 patients underwent descending aorta stent implantation. We appropriately extended the ventilator-assisted breathing time until oxygenation improvement in patients with hypoxemia. Some patients were given noninvasive ventilator support after removing endotracheal intubation, positive end-expiratory pressure was 5×10 cmH2O during the mechanical ventilation. The criteria to remove the extubation ventilator are the following: patients with clear consciousness can reply and cooperate with the treatment; PaO2 ≥80 mm Hg, FiO2 ≤0.4; breathing rate <30 times/min; and hemodynamics was stable. We evaluated and compared the frequencies of the following variables in the hypoxemia group and the non-hypoxemia group: gender, age, complicated with diabetes mellitus, New York Heart Association functional class, European system for cardiac operative risk evaluation (EuroSCORE) scoring; abnormal renal function (serum creatinine >1.5 mg/dl); body mass index (BMI) >25 kg/m2; history of chronic obstructive pulmonary disease (COPD); smoking history; interval between the onset of the symptoms and surgery; PaO2/FiO2 ratio after the induction of anesthesia, but before surgery the preoperative PaO2/FiO2 ratio must be 300 or lower.
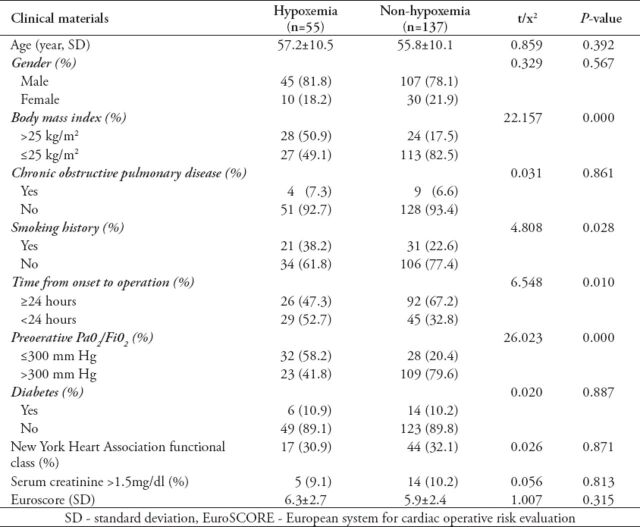
Table 1.
Univariate analysis of the preoperative characteristics of patients undergoing surgery for acute aortic dissection.
Statistical analysis
Statistical analysis was carried out using SPSS version 16.0 statistical software (SPSS Inc, Chicago, IL, USA). Continuous variables were expressed as means ± standard deviation and analyzed with Student’s t-text or t’-test. The categorical variables were expressed as percentages, and analyzed with the Chi-squared test. To identify independent risk factors for hypoxemia after surgery for acute aortic dissection, variables that were statistically significant in the univariate analysis were put into a multivariate logistic regression model. Using the method of maximum likelihood to identify independent risk factors. Odds ratios (OR) were calculated with 95% confidence intervals (CI). A p value of less than 0.05 was considered statistically significant.
Results
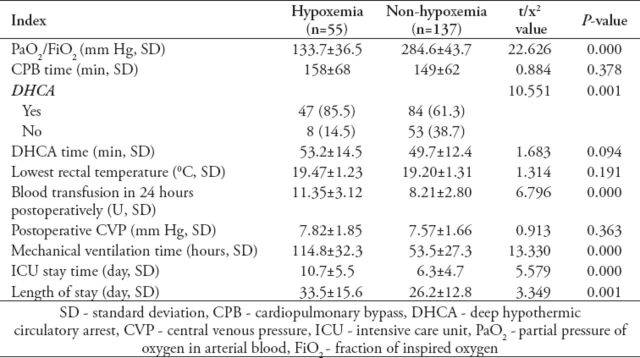
Thirteen patients died postoperatively, accounting for an in-hospital mortality of 6.8% (13/192). Of them, 8 cases (14.5%, 8/55) in the hypoxemia group, and 5 cases (3.6%, 5/137) in the non-hypoxemia group (p<0.05). Causes of hospital mortality included stroke in 4, uncontrolled bleeding in 5, low cardiac output in one, multiple organ failure in 2, and arrhythmia secondary to late cardiac tamponade in one patient. The incidence of postoperative hypoxemia was 28.6% (55/192). Among the preoperative characteristics, the ratio of BMI >25 kg/m2, smoking history ratio, the ratio of time from onset to operation <24 hours, and the ratio of preoperative PaO2/FiO2 ≤300 mm Hg were significantly greater in the hypoxemia group than in the non-hypoxemia group (p<0.05) (Table 1). Among the intraoperative and postoperative characteristics, the DHCA ratio, blood transfusion in 24 hours postoperatively, mechanical ventilation time, length of ICU stay, and hospital stay in the hypoxemia group were significantly higher or longer than those in the non-hypoxemia group (p<0.05) (Table 2). Multivariate regression analysis showed that BMI >25 kg/m2, DHCA, preoperative PaO2/FiO2 ≤300 mm Hg, and blood transfusion >6U in 24 hours postoperatively were significantly independent predictors for hypoxemia in patients undergoing Stanford A aortic dissection surgery (Table 3).
Table 2.
Univariate analysis of the intraoperative and postoperative characteristics of patients undergoing surgery for acute aortic dissection.
Table 3.
Multivariate analysis for hypoxemia after open surgery for acute aortic dissection.
Discussion
Perioperative hypoxemia is a common complication after heart surgeries, especially for acute aortic dissection patients. It can result in increased duration of mechanical ventilation and prolonged stay in the ICU, nosocomial infection increased accordingly, and consequently in costs of hospitalization. Therefore, it is particularly important for us to have an intensive study on the risk factors for hypoxemia after surgery for acute aortic dissection in order to improve the clinical outcomes. There are many studies6-8 on postoperative hypoxemia after coronary artery bypass grafting and valve replacement. From these studies we can observed that COPD, obesity, smoking history, hemodynamic instability, emergency operation, complex cardiac surgery, and postoperative lung infections are risk factors of postoperative hypoxemia.6-8 However, there are few studies10 on hypoxemia after surgery for acute type A aortic dissection. The American European Consensus Conference (AECC) acute lung injury (ALI) and acute respiratory disease syngrome (ARDS) criteria are used most commonly to diagnose ALI and ARDS in adults and children.9 We used AECC criteria for diagnosis of ALI/ARDS. Postoperative hypoxemia was defined as an PaO2/FiO2 <200 mm Hg, and was evaluated at 6 hours after arrival in the ICU and non-hypoxemia was defined as PaO2/FiO2 ≥200 mm Hg.10 Furthermore, hypoxemia in the hypoxemia group patients usually was not related to cardiogenic pulmonary edema; the imaging changes such as pulmonary infiltrates do not exist immediately after surgery.
In our study, multivariate analysis showed BMI of >25 kg/m2, DHCA, preoperative PaO2/FiO2 ≤300 mm Hg, and blood transfusion in 24 hours postoperatively >6U were independent risk factors of hypoxemia after acute type A aortic dissection aneurysm surgery.
The decrease of lung compliance is particularly obvious in obese patients. This may be related to the following factors: fatty infiltration of the chest wall, increased lung blood flow, and excess adipose tissue compress chest. Therefore, work of breathing is obviously increased in obese population. In addition, respiratory resistance has been shown to be increased in obese subjects. Studies have shown that occurrence of ALI/ARDS are related with the imbalance of anti-inflammatory and pro-inflammatory cytokines, oxidants and anti-oxidants, and coagulation factors. Most of the obese patients have chronic excessive inflammation and oxidative stress.11,12 Abnormal cytokine products and acute phase reactants increased significantly in obese patients, and proinflammatory signaling pathways had obvious upregulation. Furthermore, induction of proinflammatory cytokines and mediators increased accordingly for the stimulation of extra weight gain.13,14 Obesity can increase the oxidative stress and reactive oxygen products, which can cause direct damage to the cellular membranes, monocytes cellular adhesion, and release of chemotactic factors and vasoactive substances.
Lung injury is still one of the main complications after extracorporeal circulation operation. The incidence of pulmonary dysfunction is higher after extracorporeal circulation operation. Severe cases may appear acute respiratory distress syndrome and death.15 Currently, it was considered that systemic inflammatory response caused by the extracorporeal circulation and lung ischemia-reperfusion injury can lead to lung injury after extracorporeal circulation cardiac surgery.16-18 Currently, the recognized possible mechanisms are: 1) ischemia-reperfusion injury results in reactive oxygen species and inflammatory molecules (especially cytokines) releasing into the circulation,19 2) activation of white blood cells, platelets, complements and the blood coagulation system, and the releases of other inflammatory cytokines, which may be secondary to the contact of blood with extracorporeal circulation pipe,20 and 3) relative insufficient visceral blood perfusion in intraoperative and postoperative can cause intestinal ischemia and increased capillary permeability, and this may lead to intestinal flora ectopicly entering into circulation. The pathophysiological process of DHCA is similar to the lung transplantation after cryopreserved process or the ischemia and reperfusion process of the lung. Pulmonary vascular endothelial cell hypoxia can lead to mitochondrial swelling and edema. A large number of oxygen free radicals, calcium overload, and energy metabolic disorders will lead to more severe ischemia-reperfusion injury during blood reperfusion. In this group, DHCA is the independent risk factors for postoperative hypoxemia in aortic dissection aneurysm patients.
Previous study21 has shown that non-strict control of postoperative blood transfusion is not good for health, even harmful. Blood transfusion can cause all kinds of micro embolus formation that could directly affect the lung function. A large number of cellular debris and foreign proteins entering into the body contained in banked blood also can induce the same result. Banked blood has several shortcomings: the ability to carry oxygen of red blood cells in banked blood is poor, and the inflammatory mediators can damage the lung function and prolong the ventilation time,22 banked blood has the function of immune suppression, so that, the risk of nosocomial infections increased. It was reported that intraoperative transfusion of packed red blood cells (or even 1-2 units) could significantly increase the hospital mortality and complications such as pneumonia in patients undergoing general surgery.23 Our study showed that blood transfusion >6U in 24 hours postoperatively was the independent risk factor for postoperative hypoxemia in patients with aortic dissection aneurysm. Using blood conservation measures to reduce transfusions can reduce the incidence of complications in cardiac surgery.24 Therefore, it is necessary for reducing the incidence of hypoxemia to have quick and effective bleeding control, replenish coagulation factors, and reduce the amount of blood transfusion.25,26
Our data demonstrates that preoperative PaO2/FiO2 ≤300 mm Hg is the independent risk factor of postoperative hypoxemia in patients with aortic dissection. The PaO2/FiO2 may decline after aortic dissection in patients with aortic dissection aneurysm who have no basic lung diseases and smoking history. A study shows that PaO2/FiO2 decline may be associated with the occurrence of systemic inflammatory response in patients with aortic dissection.27 In the course of systemic inflammatory reaction, the release of pro-inflammatory cytokines causes lung neutrophil accumulation and activation. Activated neutrophils can release some toxin media, which may damage pulmonary capillary endothelial cells and increase capillary permeability. The changes lead to fluid accumulation in alveolar and respiratory failure.28
In our heart center, we provide the patients a large dose of methylperdnisolone during CPB in the operation, and in 3 days after surgery, a small dose of methylperdnisolone are regularly used in these patients, to decrease the inflammation and lung injury. We also run the operation and control bleeding as fast as possible to reduce the CPB or DHCA time, and excessive transfusion. Routine use of intraoperative ultrafiltration will reduce fluid loading, clear inflammatory mediators, and reduce tissue edema.
Study limitations
Similar with other retrospective studies, the present study also has several limitations. First, the patient population collected for investigation was relatively small and only in a single institution. Second, factors such as experience of the individual surgeon, and institutional philosophy influence the decision for method of treatment cannot be taken into account in this analysis. Third, long-term results are presently not obtained by the registry data. Finally, since this is a retrospective study, the potential information bias could not be completely excluded.
In conclusion, our study demonstrated that BMI >25 kg/m2, DHCA, preoperative PaO2/FiO2 ≤300 mm Hg, and blood transfusion in 24 hours postoperatively >6U were independent risk factors of hypoxemia after acute type A aortic dissection aneurysm surgery. We should fully understand the medical history (history of smoking, lung disease history, and so forth) and preoperative blood gas analysis of the patient with aortic dissection, and this has guiding significance to predict the postoperative hypoxemia. We ought to take appropriate preventive measures to minimize the incidence of hypoxemia for patients at high risk of postoperative hypoxemia. Thus, this can shorten the length of stay in the ICU, improve the quality of life, and reducing hospital costs.
Footnotes
New Peer Reviewers
Join our team of expert peer reviewers for Saudi Medical Journal by registering through the website at http://www.smj.org.sa/_Authors/ and select “register now” or sending an enquiry and summarized CV to info@smj.org.sa. Note that SMJ reviewers, whose reviews are returned on time and are judged satisfactory by the Editors, may receive 1 CME credit per review, with a maximum of 5 credit per year, from the Saudi Council for Health Specialties.
References
- 1.Olsson C, Hillebrant CG, Liska J, Lockowandt U, Eriksson P, Franco-Cereceda A. Mortality and reoperations in survivors operated on for acute type A aortic dissection and implications for catheter-based or hybrid interventions. J Vasc Surg. 2013;58:333–339.e1. doi: 10.1016/j.jvs.2012.12.078. [DOI] [PubMed] [Google Scholar]
- 2.Charlton-Ouw KM, Azizzadeh A, Sandhu HK, Sawal A, Leake SS, Miller CC, 3rd, et al. Management of common carotid artery dissection due to extension from acute type A (DeBakey I) aortic dissection. J Vasc Surg. 2013;58:910–916. doi: 10.1016/j.jvs.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 3.Hata M, Suzuki M, Sezai A, Niino T, Yoshitake I, Unosawa S, et al. Outcome of less invasive proximal arch replacement with moderate hypothermic circulatory arrest followed by aggressive rapid re-warming in emergency surgery for type A acute aortic dissection. Circ J. 2009;73:69–72. doi: 10.1253/circj.cj-08-0499. [DOI] [PubMed] [Google Scholar]
- 4.Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD) Eur J Vasc Endovasc Surg. 2009;37:149–159. doi: 10.1016/j.ejvs.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Easo J, Weigang E, Hölzl PP, Horst M, Hoffmann I, Blettner M, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg. 2012;144:617–623. doi: 10.1016/j.jtcvs.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Delgado M, Navarrete I, Garcia-Palma MJ, Colmenero M. Postoperative respiratory failure after cardiac surgery: use of noninvasive ventilation. J Cardiothorac Vasc Anesth. 2012;26:443–447. doi: 10.1053/j.jvca.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Santos NP, Mitsunaga RM, Borges DL, Costa Mde A, Baldez TE, Lima IM, et al. Factors associated to hypoxemia in patients undergoing coronary artery bypass grafting. Rev Bras Cir Cardiovasc. 2013;28:364–370. doi: 10.5935/1678-9741.20130056. [DOI] [PubMed] [Google Scholar]
- 8.Kilger E, Möhnle P, Nassau K, Beiras-Fernandez A, Lamm P, Frey L, et al. Noninvasive mechanical ventilation in patients with acute respiratory failure after cardiac surgery. Heart Surg Forum. 2010;13:E91–E95. doi: 10.1532/HSF98.20091116. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T, Kawazoe K, Izumoto H, Kataoka T, Niinuma H, Shirahashi N. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today. 2006;36:680–685. doi: 10.1007/s00595-006-3226-5. [DOI] [PubMed] [Google Scholar]
- 11.Kordonowy LL, Burg E, Lenox CC, Gauthier LM, Petty JM, Antkowiak M, et al. Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am J Respir Cell Mol Biol. 2012;47:120–127. doi: 10.1165/rcmb.2011-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazloom Z, Hejazi N, Dabbaghmanesh MH. Effects of obesity on inflammation and lipid profile of obese women. Saudi Med J. 2009;30:1357–1358. [PubMed] [Google Scholar]
- 13.Varol C, Zvibel I, Spektor L, Mantelmacher FD, Vugman M, Thurm T, et al. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J Immunol. 2014;193:4002–4009. doi: 10.4049/jimmunol.1401149. [DOI] [PubMed] [Google Scholar]
- 14.Balentine CJ, Marshall C, Robinson C, Wilks J, Anaya D, Albo D, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res. 2010;164:18–22. doi: 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 15.Apostolakis EE, Koletsis EN, Baikoussis NG, Siminelakis SN, Papadopoulos GS. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg. 2010;5:1. doi: 10.1186/1749-8090-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao YK, Wu YC, Yang KJ, Chiang LL, Liu HP, Lin PJ, et al. Pulmonary perfusion with L-arginine ameliorates post-cardiopulmonary bypass lung injury in a rabbit model. J Surg Res. 2011;167:e77–e83. doi: 10.1016/j.jss.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Dong LY, Zheng JH, Qiu XX, Yu M, Ye YZ, Shi S, et al. Ischemic preconditioning reduces deep hypothermic circulatory arrest cardiopulmonary bypass induced lung injury. Eur Rev Med Pharmacol Sci. 2013;17:1789–1799. [PubMed] [Google Scholar]
- 18.Yamazaki S, Inamori S, Nakatani T, Suga M. Activated protein C attenuates cardiopulmonary bypass-induced acute lung injury through the regulation of neutrophil activation. J Thorac Cardiovasc Surg. 2011;141:1246–1252. doi: 10.1016/j.jtcvs.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Merry HE, Phelan P, Doak MR, Zhao M, Hwang B, Mulligan MS. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg. 2015;99:1193–1199. doi: 10.1016/j.athoracsur.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 20.Kagawa H, Morita K, Nagahori R, Shinohara G, Kinouchi K, Hashimoto K. Prevention of ischemia/reperfusion-induced pulmonary dysfunction after cardiopulmonary bypass with terminal leukocyte-depleted lung reperfusion. J Thorac Cardiovasc Surg. 2010;139:174–180. doi: 10.1016/j.jtcvs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Bursi F, Barbieri A, Politi L, Di Girolamo A, Malagoli A, Grimaldi T, et al. Perioperative red blood cell transfusion and outcome in stable patients after elective major vascular surgery. Eur J Vasc Endovasc Surg. 2009;37:311–318. doi: 10.1016/j.ejvs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt AE, Adamski J. Education Committee of the Academy of Clinical Laboratory Physicians and Scientists. Pathology consultation on transfusion-related acute lung injury (TRALI) Am J Clin Pathol. 2012;138:498–503. doi: 10.1309/AJCPFF6JKXM7BYOI. [DOI] [PubMed] [Google Scholar]
- 23.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–937. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz DM, McCullough JN, Shander A, Klein JJ, Bodian CA, Goldweit RS, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90:451–458. doi: 10.1016/j.athoracsur.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 25.Galas FR, Almeida JP, Fukushima JT, Osawa EA, Nakamura RE, Silva CM, et al. Blood transfusion in cardiac surgery is a risk factor for increased hospital length of stay in adult patients. J Cardiothorac Surg. 2013;8:54. doi: 10.1186/1749-8090-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens RS, Shah AS, Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg. 2013;95:1122–1129. doi: 10.1016/j.athoracsur.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Gu J, Hu J, Zhang HW, Xiao ZH, Fang Z, Qian H, et al. Time-dependent changes of plasma inflammatory biomarkers in type A aortic dissection patients without optimal medical management. J Cardiothorac Surg. 2015;10:3. doi: 10.1186/s13019-014-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurabayashi M, Okishige K, Azegami K, Ueshima D, Sugiyama K, Shimura T, et al. Reduction of the PaO2/FiO2 ratio in acute aortic dissection-relationship between the extent of dissection and inflammation. Circ J. 2010;74:2066–2073. doi: 10.1253/circj.cj-10-0336. [DOI] [PubMed] [Google Scholar]