Abstract
Objectives:
To estimate normal linear dimensions and volume of spleen in Jordanians using ultrasonography, and to correlate splenic volume with age and body parameters: height, weight, body surface area (BSA), and body mass index (BMI).
Methods:
A prospective pilot study was conducted on 205 volunteers (115 males and 90 females) not known to have any conditions likely to be associated with splenomegaly. The study was performed at the Radiology Department, Jordanian University Hospital, Amman, Jordan, between December 2013 and August 2014. All linear dimensions of spleen were measured, and splenic volume (index) was calculated using the standard prolate ellipsoid formula (length × width × depth × 0.523). The splenic volume was then analyzed with age and body parameters using the Pearson’s correlation coefficient.
Results:
The mean (± SD) splenic dimensions were 10.72±1.37 cm in length, 7.40±1.52 cm in width, 4.40±1.47 cm in depth, and 184.15±79.56 cm3 in volume. Men had larger spleens than women (p<0.0001). Age had no significant effect on spleen volume (r=0.11, p=0.12). There was a significant moderate positive correlation (p<0.0001), using Pearson’s correlation coefficient, between the spleen volume, and other parameters (height, weight, BSA, and BMI), with correlation coefficients exceeding 0.3.
Conclusion:
A local reference of spleen dimensions was established with a different range of values reported previously.
The spleen is the largest organ in the reticulo endothelial system.1 Spleen size is important in the evaluation of gastrointestinal and hematological diseases for both radiologists and clinicians.2 A normal spleen weighs 150-200 g, and is 10.9 ± 1.4 cm long, 4.0 ± 0.45 cm deep, and 6.8 ± 0.71 cm in diameter. It is located in the left hypogastric quadrant of the abdomen beneath the 9th to the 11th intercostal spaces. It is a crescent shaped structure, with a convex outer margin, and indented inner margin.3 Splenomegaly is a well-known manifestation of several diseases that may involve the liver, immune system, and hematopoietic system.4,5 The reliability of clinical palpation is imprecise; the normal spleen is usually not palpable, whereas a non-palpable spleen is not always normal sized.6 The spleen volume can be measured by various techniques such as radiography, scintigraphy, CT, MRI, and ultrasonography.3,7 Ultrasonography is the first imaging method to assess splenomegaly.8,9 It is a non-invasive, commonly available, and an affordable imaging method without the risk of ionizing radiation. Current knowledge of spleen size is based on different populations or derived from autopsy studies.10 So far, established normal limits of spleen dimensions remain scanty in the Jordanian population, and the ultrasound data from the previous studies demonstrated that racial differences could affect the splenic volume; this necessitates the establishment of normative data of spleen dimensions for different areas.11,12
The aim of this prospective pilot screening survey was to establish reference values of splenic dimensions and volume in a population of adult healthy Jordanians, and to determine the relationship of splenic volume with gender, age, body height, weight, body surface area (BSA), and body mass index (BMI). This study was conducted as a first step in an attempt to improve the study design prior to performance of a full scale investigation of splenic volumes in a Mediterranean population.
Methods
The study was performed at the Radiology Department, Jordanian University Hospital, Amman, Jordan, between December 2013 and August 2014. Two hundred and 5 healthy volunteers (115 males and 90 females) were included in this study, and written informed consent was taken for each case. Ethical approval was obtained from the Academic Research Council of the Faculty of Medicine at the University of Jordan according to ethical principles of Helsinki Declaration. The procedures completely comply with the current laws of the Hashemite Kingdom of Jordan.
Exclusion criteria
Subjects underwent physical examination and completed a short standardized interview questionnaire to exclude any previous or current conditions that might involve the size of the spleen. For our study group, the following exclusion criteria were used: a) clinical or laboratory evidence of infection (subjects who had fever either at the time of the examination or within at least 4 weeks prior to the examination), b) hematopoietic diseases, c) genetic diseases (thalassemia and sickle cell anemia), d) lymphadenopathy, e) liver diseases (cirrhosis or portal hypertension), f) renal failure, g) history of splenic trauma, h) non-traumatic benign splenic lesions (infarctions, lobulations, cysts, accessory spleen, and hemangioma), i) malignant lesions, and j) pregnancy.
In order to exclude blood diseases and thalassemic traits, all enrolled volunteers had normal complete blood count (CBC), normal range of hemoglobin was 12-19 gram/deciliter, and normal range of mean corpuscular volume (MCV) was 80-100 femtoliters. Of the 300 volunteers, 205 (68%) were subjected to abdominal ultrasound examination. Ninety five adults were excluded from the study for the following reasons: hematopoietic disorders (mostly anemia; 47 cases), infections (32 cases), and benign splenic lesions (hemangiomas and cysts; 9 cases). Seven volunteers were excluded due to incomplete data.
Study design and ultrasonographic examinations
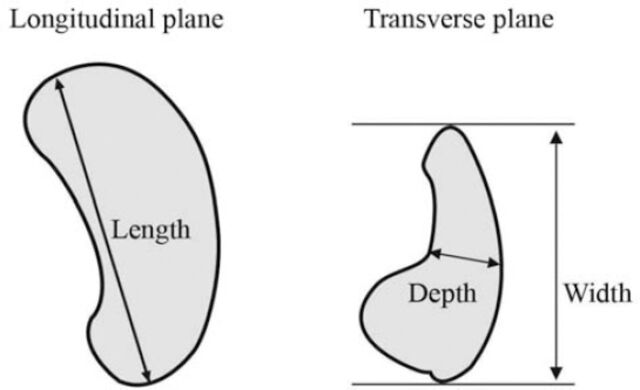
Baseline data including age, gender, height, and weight were recorded for all participants. The BSA and BMI were calculated by the following formulas: BSA = (height [cm] x weight [kg]/3600)½ and BMI = weight [kg]/height [m2]. All ultrasonographic examinations were performed by 3 experienced senior radiologists. The examinations were performed using Acuson S2000 Siemens ultrasound machine equipped with 3.4 MHz curvi-linear probe (Erlangen, Germany). The subjects were placed and examined in the supine and/or right posterior oblique positions, and the spleen was scanned during suspended respiration. The splenic length (in centimeters) is defined as the maximum distance between the most superomedial and the most inferolateral points on a longitudinal plane. The splenic width, defined as the maximum anteroposterior dimension, was measured on a transverse plane. The splenic depth is defined as the mediolateral distance from the hilum to the capsule, being measured on the same transverse plane (Figure 1). To express spleen volume, the splenic index was calculated using the standard prolate ellipsoid formula (length × width × depth × 0.523); this formula is frequently used for estimating the volume of many irregularly shaped organs. All measurements showed excellent intra-observer and inter-observer reliability, with correlation coefficients ranging from 0.77 to 0.89.
Figure 1.
Schematic diagram showing the method for measuring splenic length, width, and depth (in centimeters) on longitudinal and transverse planes.
Statistical analysis
The data was entered into a spreadsheet and analyzed using the IBM SPSS Statistics for Windows, version 19 (IBM Corp, Armonk, NY, USA). The means (± standard deviation), ranges, 5th percentiles, 95th percentiles, and the 95% confidence intervals for the mean (in order to include the true population mean in 95% of the cases) were all calculated. Differences of continuous variables between 2 independent groups were assessed with the 2-tailed t test. Relationship between splenic index and each of the 5 variables: age, height, weight, BSA, and BMI was assessed with the Pearson’s correlation coefficient (r) to measure the linear correlation (dependence) between 2 variables X and Y. Pearson’s r values between 0 and 0.3 indicate a weak positive relationship, between 0.3 and 0.7 indicate a moderate positive relationship, and values between 0.7 and 1.0 indicate a strong positive relationship; where r for positive correlation takes on values ranging from 0 to 1. The significance threshold was set at 0.05. The XY scatter plots were generated by Microsoft Excel 2010 and the figures were processed by Adobe Illustrator CC 2014.
Results
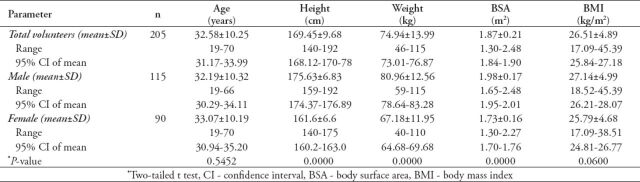
The anthropometric data (mean± standard deviation) is shown in Table 1. A statistically significant difference between males and females was observed in height, weight, and BSA (p<0.0001). There was no significant difference between the groups in term of age and BMI (p>0.05).
Table 1.
Anthropometric data of males and females in a Jordanian population.
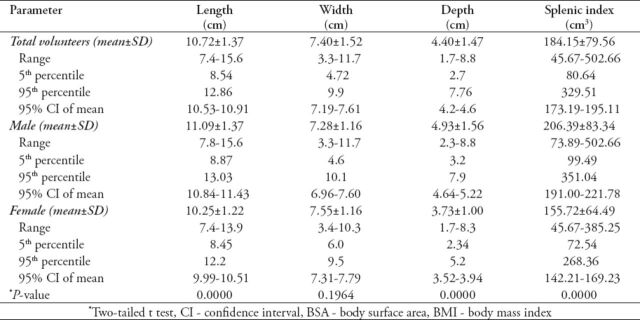
Different linear dimensions and volume of the spleen among males and females in Jordanian population were shown in Table 2. Comparison between mean splenic dimensions among males and females (from 2-tailed t-test determination) showed a statistically significant difference (p<0.0001) for splenic length, depth, and volume, but not the width. The overall data from the studied groups showed a relatively narrow 95% confidence interval of the means.
Table 2.
Different linear dimensions and volume of the spleen among males and females in a Jordanian population.
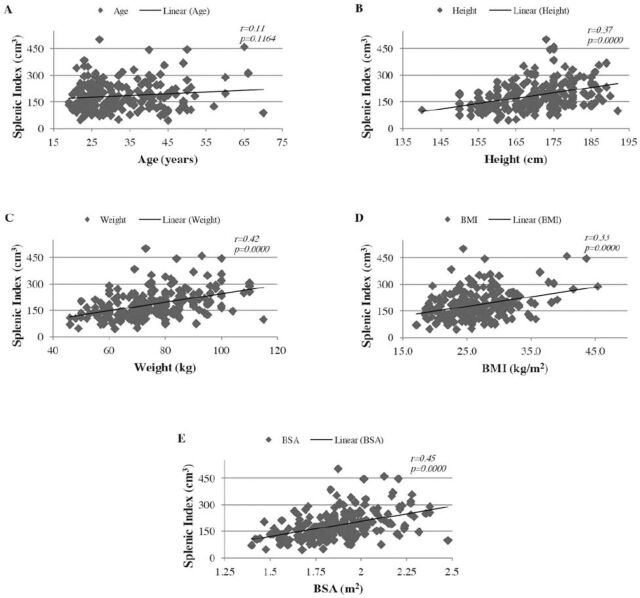
Pearson’s coefficient between splenic index and age showed a very weak positive relationship, which was statistically not a significant correlation (p>0.05) (Figure 2A). A moderate positive linear relationship was found between the splenic index and body height, weight, BSA, and BMI (r: >0.3). This correlation was statistically extremely significant (p<0.0001) (Figures 2B-2E).
Figure 2.
Scatter plots showing the correlation between splenic index: A) age, B) height, C) weight, D) body mass index (BMI), and E) body surface area (BSA). Regression lines represent the lines of best fit. r: Pearson correlation coefficient, p-values were calculated using the 2-tailed t test, n=205.
Discussion
The wide range of normal spleen size values reported in the literature makes the establishment of normal ranges more difficult. The Jordanian population is a cosmopolitan society of approximately 9.5 million people where the vast majority is Jordanian, and the rest are refugees and immigrants from nearby countries. Only Jordanian adults were included in this study. In our study population, the means of splenic dimensions were fairly similar to those recorded by Turkish and Nigerian populations.11,13 Taking the upper limits readings into consideration, our values were still higher than the data from Indians for example,14,15 implicating that ethnicity could be attributed in part to the wide ranges of normative data registered by different populations. A recent study performed on Saudi Arabian adults to estimate splenic volume using 3-dimensional abdominal CT scan images, yielded slightly higher measurements than the values recorded in this study.16 In the latter study, the same ellipsoid formula was used to calculate the spleen volume, the lower values of sonography measurements in our study were most probably due to overlapping ribs or bowel gas. Spleen length at the hilum is considered the most reproducible linear measurement of spleen size.17 Splenomegaly is considered as moderate if the biggest dimension is 11-20 cm, and severe if the biggest dimension is greater than 20 cm.18 However, we recorded a spleen length of 15.6 cm as the upper limit of normality in Jordanians. Therefore, caution is required in defining splenomegaly in our population.
A likely decrease in the size of the spleen due to ageing reported in previous literature was not evident in our study.19,20 However, our findings were in agreement with the results described in Africans and Indians studies.12,13 Moderate positive relationships between splenic volume and height, weight, BSA, and BMI were observed; this was similar to the data from spleen sonography and autopsy.10,14,15 Graphic representation of the data showed some variability of the spleen volume by height, weight, BMI, and BSA, it also showed unmistakable trend for spleen volume to increase in parallel with the increase in the body parameters. The body weight and height might show variations in different ethnic origins. In addition, variations in the body fat for the same BMI might also be caused by variations of physical activity, diet, and ethnicity. So the variations of body parameters could be attributed to different splenic measurements in different areas. Previous studies showed that the longitudinal measurements of the spleen were best correlated only with body height.19,21 On the other hand, studies of African adults and Turkish males found no correlation between spleen volume and body parameters.11,12 From a physiological perspective, our findings would make more sense; as patients with a bigger body habitus will have a larger blood volume requiring larger spleens for filtration.
In agreement with other studies,12,13 gender differences in normal splenic volume were found to be significant. As there is a positive correlation between the body parameters and the splenic volume, we would expect a larger average spleen volume in men on the basis of their larger body size and probably due to the genetic factors. A clinical dilemma might arise in patients with a spleen volume bigger or smaller than the values recorded in this study; one then needs to decide whether this can be accepted as normal for a particular patient owing to his/her body habitus.
Study limitations
The main limitation was the small sample size, which certainly has affected the generalizability of our estimates. A larger study sample is required in order to improve the accuracy of our measurements. The narrow 95% confidence intervals for the means of our values imply that there is a smaller chance of obtaining a mean value outside that interval; therefore, our accuracy is statistically high. Additionally, it must be emphasized that the exclusion criteria in our study did not include a history of familial Mediterranean fever (FMF). A recent study performed on Turkish migrants with FMF in Germany showed a larger splenic volume, in patients with FMF compared to healthy controls using sonography.22 Due to clinical phenotype among some patients who are heterozygous for FMF, it can potentially be missed or stay undiagnosed. Further studies should highlight FMF as a potential cause of moderate splenomegaly in our Mediterranean population. Furthermore, a previous study on Iranians suggested an average increase in splenic volume in thalassemic carriers by 29.4%.23 Thalassemia is most common in people of Mediterranean origin. The normal complete blood count and mean corpuscular volume do not rule out a possible increase in splenic volume in thalassemic heterozygotes, which can be better evaluated by studies in progress.
Therefore, further studies are needed with larger study populations and more ethnic backgrounds to explore wider environmental and genetic influences that might determine the splenic volume.
In conclusion, a local reference of spleen dimensions was established in this study with a different range of values reported previously. Setting a higher cut off point for defining splenomegaly in Jordanians should be considered. The higher linear measurements of spleen in our population can be explained in part by differences in body habitus.
Acknowledgment
We thank Mr. Mohamad Rammoni for technical assistance and Mrs. Hala Shahrori for the graphics and artwork.
Footnotes
Related Articles
Emran RS, Anwar IM, Trudel M, Bhatti AA. Concurrent splenic lymphangiomatosis and Proteus syndrome. Saudi Med J 2013; 34: 960-962.
Alamri NF, Alhariqi BA. Portal hypertension secondary to splenic arteriovenous fistula. Saudi Med J 2012; 33: 904-907.
References
- 1.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 2.Niederau C, Sonnenberg A, Muller JE, Erckenbrecht JF, Scholten T, Fritsch WP. Sonographic measurements of the normal liver, spleen, pancreas, and portal vein. Radiology. 1983;149:537–540. doi: 10.1148/radiology.149.2.6622701. [DOI] [PubMed] [Google Scholar]
- 3.Benter T, Kluhs L, Teichgraber U. Sonography of the spleen. J Ultrasound Med. 2011;30:1281–1293. doi: 10.7863/jum.2011.30.9.1281. [DOI] [PubMed] [Google Scholar]
- 4.Manzella A, Borba-Filho P, D’Ippolito G, Farias M. Abdominal manifestations of lymphoma: spectrum of imaging features. ISRN Radiol. 2013;2013:483069. doi: 10.5402/2013/483069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancox SH, Smith BC. Liver disease as a cause of thrombocytopenia. QJM. 2013;106:425–431. doi: 10.1093/qjmed/hcs239. [DOI] [PubMed] [Google Scholar]
- 6.Kumar L, Bansal AK. Splenomegaly: A clinical approach. In: Munjal YP, Sharma SK, editors. Api textbook of medicine. Mumbai (IN): The Association of Physicians of India; 2012. pp. 926–927. [Google Scholar]
- 7.Sutherland T, Temple F, Galvin A, Hennessy O. Contrast-enhanced ultrasound of the spleen: an introduction and pictorial essay. Insights Imaging. 2011;2:515–524. doi: 10.1007/s13244-011-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yildiz AE, Ariyurek MO, Karcaaltincaba M. Splenic anomalies of shape, size, and location: pictorial essay. Scientific World Journal. 2013;2013:321810. doi: 10.1155/2013/321810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue Y, Nakajima A, Mizukami S, Hata H. Effect of breath holding on spleen volume measured by magnetic resonance imaging. PLoS One. 2013;8:e68670. doi: 10.1371/journal.pone.0068670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caglar V, Kumral B, Uygur R, Alkoc OA, Ozen OA, Demirel H. Study of volume, weight and size of normal pancreas, spleen and kidney in adults autopsies. Forensic Medicine and Anatomy Research. 2014;2:63–69. [Google Scholar]
- 11.Serter S, Ceylan C, Tunçyürek Ö, Örgüç S, Pabuçcu Y. Sonographic evaluation of spleen size and prevalence of accessory spleen in a healthy male Turkish population. Turk J Hematol. 2010;27:25–28. [PubMed] [Google Scholar]
- 12.Mustapha Z, Tahir A, Tukur M, Bukar M, Lee WK. Sonographic determination of normal spleen size in an adult African population. Eur J Radiol. 2010;75:e133–e135. doi: 10.1016/j.ejrad.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Ehimwenma O, Tagbo MT. Determination of normal dimension of the spleen by ultrasound in an endemic tropical environment. Niger Med J. 2011;52:198–203. doi: 10.4103/0300-1652.86141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A, Ansari H, Das JK, Chandra N. Ultrasonographic measurement of splenic length in relation with height in Bihari adult population. A prospective study. J Anat Soc India. 2011;60:188–189. [Google Scholar]
- 15.Arora N, Sharma PK, Sahai A, Singh R. Sonographic measurement of the spleen: splenic length in adults and its correlation with different parameters. J Anat Soc India. 2013;62:57–61. [Google Scholar]
- 16.Siddiqui MA, Ali AHA, Bedewi MA, Serhan OO. Estimation of standard splenic volume in saudi arabian adult population: using 3D reconstruction of abdominal CT scan images. Open Journal of Internal Medicine. 2014;4:7–12. [Google Scholar]
- 17.Poddar U, Jagadisan B. Measuring liver and spleen by ultrasonography. Indian Pediatr. 2010;47:475–476. doi: 10.1007/s13312-010-0086-2. [DOI] [PubMed] [Google Scholar]
- 18.Elmakki A. Hypersplenism: review article. Journal of Biology, Agriculture and Healthcare. 2012;2:89–97. [Google Scholar]
- 19.Asghar A, Naaz S, Agrawal D. Estimation of standard splenic index (SI) in Indian population: A CT scan based study. Indian Journal of Basic and Applied Medical Research. 2014;3:332–337. [Google Scholar]
- 20.Arora N, Sharma PK, Sahai A, Singh R. Sonographic measurements of the spleen in relation to age;A prospective study in North Indian Adults. J Anat Soc India. 2010;59:177–181. [Google Scholar]
- 21.Asghar A, Agrawal D, Yunus SM, Sharma PK, Zaidi SH, Sinha A. Standard splenic volume estimation in north Indian adult population: Using 3d reconstruction of abdominal CT scan images. Anat Res Int. 2011;2011:707325. doi: 10.1155/2011/707325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornek A, Kurucay M, Henning BF, Pagonas N, Schlottmann R, Schmidt WE, et al. Sonographic assessment of spleen size in Turkish migrants with Familial Mediterranean fever in Germany. J Ultrasound Med. 2014;33:1991–1997. doi: 10.7863/ultra.33.11.1991. [DOI] [PubMed] [Google Scholar]
- 23.Karimi M, Bagheri MH, Tahmtan M, Shakibafard A, Rashid M. Prevalence of hepatosplenomegaly in beta thalassemia minor subjects in Iran. Eur J Radiol. 2009;69:120–122. doi: 10.1016/j.ejrad.2007.09.027. [DOI] [PubMed] [Google Scholar]