Abstract
Objectives:
To compare the influence of posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) on adjacent segment degeneration.
Methods:
The study was carried out in the Traumatology and Orthopedics Laboratory, Department of Traditional Chinese Medicine, Medical School, Jinan University, Guangzhou, China, between December 2013 and November 2014. A normal, healthy finite element model of L3-5 was developed, a PLIF and a TLIF model were modified from the normal model, and interbody fusions were performed in the L4-5 segment. An 800 N compressive loading plus 10 Nm moments simulating flexion, extension, lateral bending, and axial rotation were imposed on the L3 superior endplate. Intradiscal pressure and intersegmental rotation in L3-4 were investigated.
Results:
The values of intradiscal pressure and intersegmental rotation in the PLIF or TLIF model were higher than those in the normal, healthy model, but the values in the TLIF model were relatively lower than those in the PLIF model in all directions.
Conclusion:
Posterior lumbar interbody fusion has more adverse influence on the superior adjacent segment than TLIF.
Both transforaminal lumbar interbody fusion (TLIF) and posterior lumbar interbody fusion (PLIF) are standard techniques of lumbar fusion to treat degenerative lumbar disorders.1,2 Many clinical studies have been performed to compare the surgical results of the 2 techniques, most of which suggested TLIF and PLIF had no significant difference in clinical outcomes.3,4 In addition, Zhang et al5 found in a meta-analysis that patients undergoing PLIF had a higher incidence of complications than those undergoing TLIF. In a retrospective study of 163 patients, Hey and Hee6 found a reduced risk of vessel and nerve injury, shortened operating time, and reduced intraoperative bleeding in TLIF. Subsequently, TLIF became an optimal selection when spine surgeons developed treatment strategies. However, in terms of adjacent segment degeneration (ASD), and the long-term complications after lumbar fusion, few studies have been performed to compare the influence of PLIF and TLIF on the adjacent segment. In a unique biomechanical study performed using human cadavers, Sim et al7 found PLIF and TLIF had similar biomechanical properties regarding range of motion, intradiscal pressure, and laminar strain at adjacent segments. In our opinion, these 2 techniques have differences in cage selection and excision of posterior elements, which may affect the conduction of stress, and produce different influences on adjacent segment. In addition, Sim et al’s7 study was an immediate test after cage placement and instrumentation fixation, but ASD usually occurs after solid fusion and a study using samples with solid fusion, may be better in clarifying the issues. The limitations in clinical study make discrete characterization of the effects of lumbar interbody fusion on the adjacent segment significantly difficult. In addition, specimens for cases and controls in clinical studies are difficult to obtain and standardize.8 By contrast, the finite element technique, which is highly reproducible and repeatable, can mitigate these problems. A finite element model can be adjusted in material properties, loading mode or structural shape, to simulate normal, degenerative, fusion or other different situations. Compared with other experimental methods, a finite element method presents many advantages, which facilitates a comparative study among models with different biomechanical situations.8 Therefore, we developed a 3-dimensional finite element model of L3-5 for the normal, healthy spine, along with a PLIF and a TLIF model, our aim was to compare the biomechanical influence of PLIF and TLIF on adjacent segments.
Methods
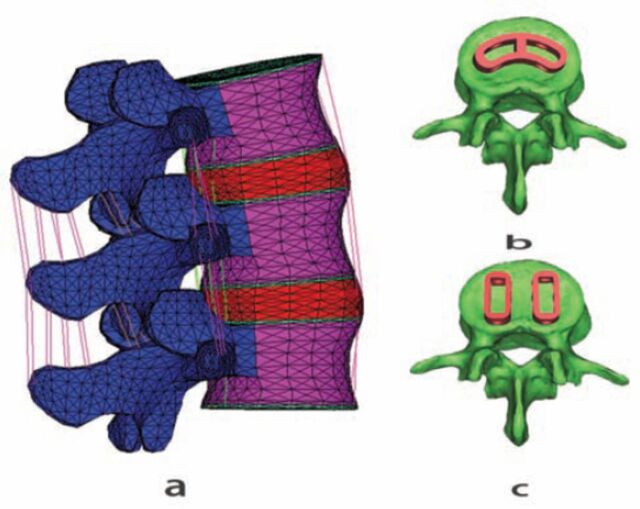
The study was carried out in the Traumatology and Orthopedics Laboratory, Department of Traditional Chinese Medicine, Medical School, Jinan University, Guangzhou, China, between December 2013 and November 2014. A normal, healthy model of L3-5 was created (Figure 1) and validated in previous studies.8 A PLIF and a TLIF model were modified from the normal, healthy model. Both TLIF and PLIF were performed at L4-5. We assumed the elements of L3-4 segments including intervertebral disc, facet joints, endplates, and vertebral bodies were normal in all models. To mimic PLIF and TLIF, L4-5 disc of the normal, healthy model was removed and 2 25-mm long ogival interbody cage (OIC) PLIF PEEK cages (Stryker, South Allendale, New Jersey, USA) one 30-mm long adaptive vertebral (AVS) PEEK Spacer TLIF cage (Stryker, South Allendale, New Jersey, USA) were inserted in the disc space. All the cages were filled with a cancellous bone and solid fusion was assumed between cages and vertebral bodies. A TLIF was performed on the left side. At the L4-5 segment in the TLIF model, the left facet joint, and ligamentum flavum were removed completely, the left superior and inferior lamina were removed partially, but the posterior elements, contralateral facet joint, supraspinous ligaments, and interspinous ligaments were preserved. In the PLIF model, the spinous process, supraspinous ligament, interspinous ligament, ligamentum flavum ligament were removed, laminectomy and partial facetectomy were performed. The posterior fixation instrumentations were removed in both models to facilitate the study. The material properties in all models were defined according to previous literature.8-10
Figure 1.
The normal finite element model of L3-5 A) adaptive vertebral cage B) in the transforaminal lumbar interbody fusion and ogival interbody cage, C) in the posterior lumbar interbody fusion.
The degrees of freedom of L5 inferior surface were completely fixed in all directions, and 10Nm flexion, 10Nm extension, 10Nm lateral bending, and 10Nm axial rotation moment under 800N compressive loading were imposed on L3 superior endplate.9 The maximum load was achieved in 5 load steps in each model, intradiscal pressure, and intersegmental rotation in L3-4 segment were investigated.
Results
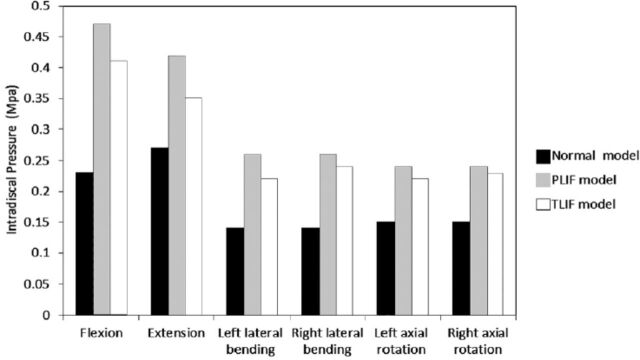
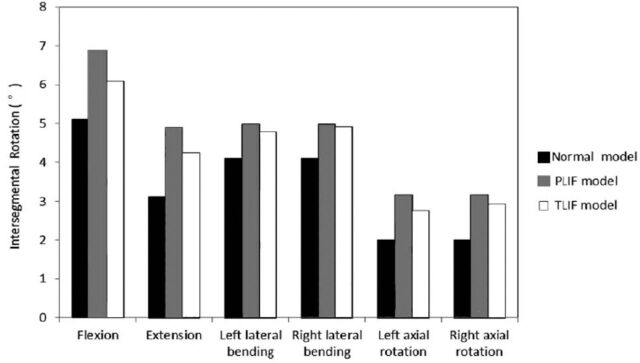
In the PLIF, and normal, healthy model, intradiscal pressure and intersegmental rotation in left lateral bending and axial rotation were equal to those in the right lateral bending and axial rotation. However, the values were different in the TLIF model. The intradiscal pressure of L3-4 in different loading directions and models are displayed in Figure 2. In all directions, the intradiscal pressure in the PLIF model was the highest, and in the normal model was the lowest. The intradiscal pressures in PLIF model increased 104.3%, 55.6%, 85.7%, 85.7%, 60.0% and 60.0%, and the values in TLIF increased 78.3%, 29.6%, 57.1%, 71.4%, 46.7% and 53.3% in flexion, extension, left lateral bending, right lateral bending, left axial rotation and right axial rotation respectively, compared with the normal, healthy model. From TLIF to PLIF, the intradiscal pressure of L3-4 segment increased 12.8% in flexion, 16.7% in extension, 15.4% in left lateral bending, 7.7% in right lateral bending, 8.3% in left axial rotation, and 4.1% in right axial rotation. The intersegmental rotations of L3-4 in different models and loading directions are displayed in Figure 3. The value of intersegmental rotation in the normal model was the lowest, and the values in the PLIF model was the largest in all directions. The intersegmental rotation in PLIF increased 34.9%, 57.7%, 21.5%, 21.5%, 57.5% and 57.5%, and the value in TLIF increased 19.2%, 36.5%, 16.6%,19.5%, 37.5% and 46.5% in flexion, extension, left lateral bending, right lateral bending, left axial rotation and right axial rotation, compared with the normal, healthy model. From TLIF to PLIF, the intersegmental rotation increased 11.6% in flexion, 13.5% in extension, 4% in the left lateral bending, 1.6% in the right lateral bending, 12.7% in the left axial rotation and 6.9% in the right axial rotation.
Figure 2.
Intradiscal pressure in different loading directions and models.
Figure 3.
Intersegmental rotations in different loading directions and models.
Discussion
In the current study, we performed an analysis of the biomechanical influence of TLIF and PLIF on ASD using a finite element technique. To date, limited studies have been carried out to determine which surgical method results in more ASD in the English literature. Adjacent segment degeneration is a well-recognized, long-term complication of lumbar fusion.11,12 Lumbar fusion results in decreased elasticity and increased stiffness of lumbar segment, which aggravates the stress concentration and disc degeneration at the adjacent segments. Adjacent segment degeneration has been confirmed,8,13 and the authors suggested that range of motion and intradiscal pressure were increased within adjacent segments. In addition, lumbar fusion has more influence on the superior adjacent segment than the inferior segment. Subsequently, we focus on the influence of TLIF and PLIF on the superior adjacent segment in the current study. We found in flexion, extension, axial rotation, and lateral bending the values of intersegmental rotation, and the intradiscal pressure at L3-4 were higher in the PLIF or TLIF model compared with the normal and healthy model. This confirmed the occurrence of ASD in lumbar fusion, demonstrating both PLIF and TLIF can promote ASD adversely.
In the current study, both PLIF and TLIF models were modified from the normal, healthy model, but the cages used as well as the extent of resection of posterior elements in TLIF and PLIF were different, which lead to different influence on stress conduction. Facet joints and the posterior ligamentous system play an important role in loading distribution. The facet joints can control and stabilize the torsional forces, resulting in limitations on the motion of the lumbar segment, especially in lateral bending and axial rotation, and subsequently affect the stress at the adjacent segment.13 In addition, Ekman et al14 found a significantly higher incidence of ASD in patients with laminectomy compared with patients without, suggesting laminectomy may be of pathogenetic importance in the development of ASD. In TLIF, we found the intradiscal pressure and intersegmental rotation in left lateral bending and left axial rotation were not equal to those in right lateral bending and right axial rotation, and the values on the right side were relatively higher. We attribute it to the complete facetectomy on the left side, the asymmetric resection of posterior elements resulted in the difference of stress conduction between the left and right side. This confirms that facet joints and the posterior ligamentous system have important influence on stress distribution, even after lumbar fusion. Moreover, we found the intradiscal pressure and intersegmental rotation in flexion, extension, lateral bending and axial rotation in PLIF were relatively larger than those in TLIF model. The result indicates the different influence on the adjacent segment between TLIF and PLIF, demonstrating a trend that PLIF exerts more adverse influence on adjacent segment than TLIF. In our opinion, the different influence can be attributed to the different cages used and resection extent of posterior elements. In the PLIF model, 2 × 25-mm long OIC PLIF PEEK cages was used, which supply a larger fusion area and higher stiffness of L4-5 segment, and subsequently affected adjacent disc more adversely. The difference in some directions, especially in lateral bending or axial rotation, was small, but the main plane of motion in the lumbar spine is in flexion and extension.8 Subsequently, we believe that PLIF can promote ASD more adversely than TLIF.
Study limitations
First, the present models did not account for the mechanical effect of muscle contraction and the loading conditions were not truly physiologic, which may not be completely representative of the clinical situation. Second, the issue of routine implant removal after a successful fusion is controversial in spinal surgery.15 The internal fixations may be removed after solid fusion, or kept in the body permanently in some patients. In the current study, the posterior instrumentations were removed in both TLIF and PLIF models to facilitate the analysis. While, the internal fixations kept in body may affect adversely the occurrence and development of ASD.
Despite the limitations, the study showed that the values of intradiscal pressure and intersegmental rotation in the adjacent superior segment of the TLIF were relatively lower than those in the PLIF model in all directions, suggesting the PLIF has more adverse influence on ASD than TLIF. The study may help surgeons better understand the 2 interbody fusion techniques, and select a surgical mode from the perspective of biomechanics.
References
- 1.Barbagallo GM, Albanese V, Raich AL, Dettori JR, Sherry N, Balsano M. Lumbar lateral interbody fusion (LLIF): Comparative effectiveness and safety versus PLIF/TLIF and predictive factors affecting LLIF outcome. Evid Based Spine Care J. 2014;5:28–37. doi: 10.1055/s-0034-1368670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee GW, Lee SM, Ahn MW, Kim HJ, Yeom JS. Comparison of posterolateral lumbar fusion and posterior lumbar interbody fusion for patients younger than 60 Years with isthmic spondylolisthesis. Spine (Phila Pa 1976) 2014;39:E1475–E1480. doi: 10.1097/BRS.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 3.Audat Z, Moutasem O, Yousef K, Mohammad B. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J. 2012;53:183–187. [PubMed] [Google Scholar]
- 4.Kunze B, Drasseck T, Kluba T. Posterior and transforaminal lumbar interbody fusion (PLIF/TLIF) for the treatment of localised segment degeneration of lumbar spine. Z Orthop Unfall. 2011;149:312–316. doi: 10.1055/s-0030-1250689. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Yuan Z, Zhou M, Liu H, Xu Y, Ren Y. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:367. doi: 10.1186/1471-2474-15-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hey HW, Hee HT. Lumbar degenerative spinal deformity: Surgical options of PLIF, TLIF and MI-TLIF. Indian J Orthop. 2010;44:159–162. doi: 10.4103/0019-5413.62066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sim HB, Murovic JA, Cho BY, Lim TJ, Park J. Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine. 2010;12:700–708. doi: 10.3171/2009.12.SPINE09123. [DOI] [PubMed] [Google Scholar]
- 8.Tang S, Rebholz BJ. Does anterior lumbar interbody fusion promote adjacent degeneration in degenerative disc disease?A finite element study. J Orthop Sci. 2011;16:221–228. doi: 10.1007/s00776-011-0037-3. [DOI] [PubMed] [Google Scholar]
- 9.Tang S, Meng X. Does disc space height of fused segment affect adjacent degeneration in ALIF?a finite element study. Turk Neurosurg. 2011;21:296–303. doi: 10.5137/1019-5149.JTN.4018-10.0. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Tang H, Guan X, Jiang F, Xu N, Ju W, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Neurosurgery. 2013;72(1 Suppl):21–26. doi: 10.1227/NEU.0b013e3182742a69. [DOI] [PubMed] [Google Scholar]
- 11.Xu JP, Yi HL, Li M, Shi ZC, Li JF, Zhao YC, et al. [Clinical application of Wallis interspinous dynamic stabilization in treating adjacent segment degeneration (ASD) after lumbar spinal fusion] Zhongguo Gu Shang. 2013;26:1005–1009. Chinese. [PubMed] [Google Scholar]
- 12.Ren C, Song Y, Liu L, Xue Y. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol. 2014;24(Suppl1):S245–S253. doi: 10.1007/s00590-014-1445-9. [DOI] [PubMed] [Google Scholar]
- 13.Tang S. Does TLIF aggravate adjacent segmental degeneration more adversely than ALIF? A finite element study. Turk Neurosurg. 2012;22:324–328. doi: 10.5137/1019-5149.JTN.5284-11.1. [DOI] [PubMed] [Google Scholar]
- 14.Ekman P, Moller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175–1186. doi: 10.1007/s00586-009-0947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavridis SI, Bucking P, Schaeren S, Jeanneret B, Schnake KJ. Implant removal after posterior stabilization of the thoraco-lumbar spine. Arch Orthop Trauma Surg. 2010;130:119–123. doi: 10.1007/s00402-009-0962-1. [DOI] [PubMed] [Google Scholar]