Abstract
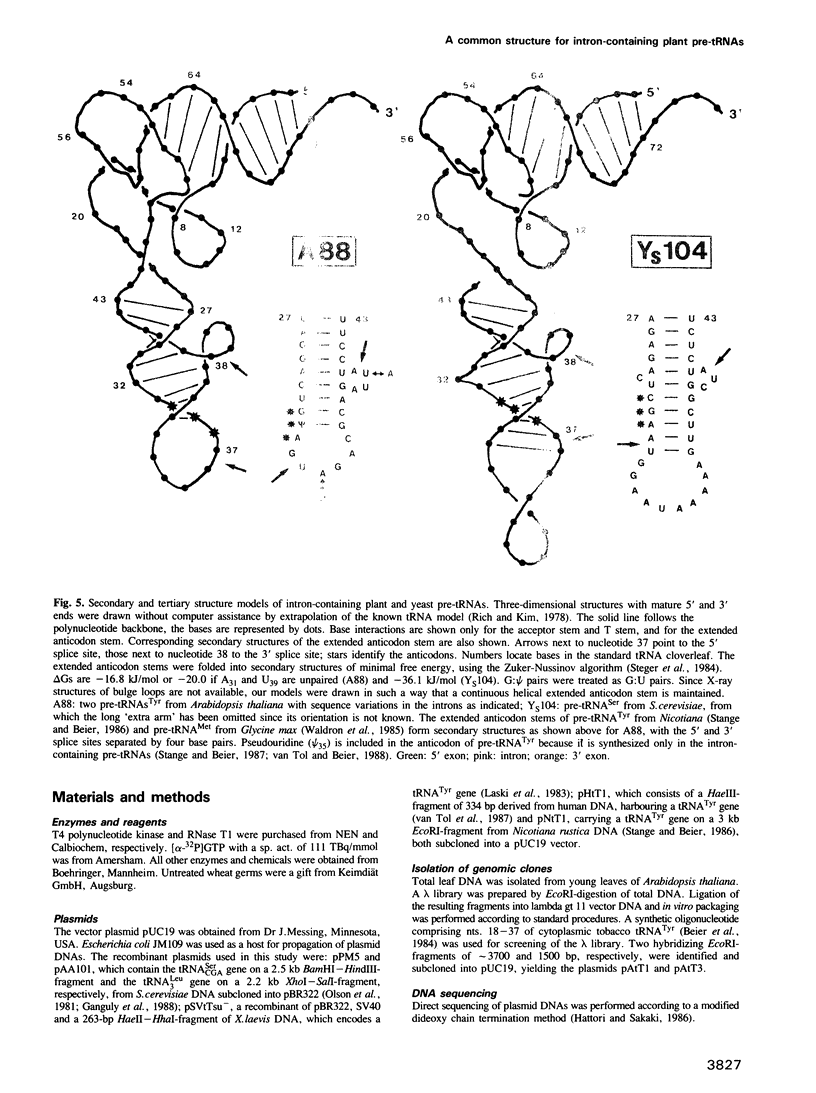
Intron-containing pre-tRNAs from organisms as different as yeast, Nicotiana, Xenopus and man are efficiently spliced and processed in a HeLa cell extract. They are also correctly processed in a wheat germ extract; however, the intron is removed only from the tobacco pre-tRNA. To determine whether plant pre-tRNA introns have any specific structural and/or sequence feature we have cloned two intron-containing tRNATyr genes from the plant Arabidopsis. Comparison of these genes, of the Nicotiana tRNATyr gene and of a Glycine max tRNAMet gene reveals that plant introns from three different species have no sequence homology and are only 11 to 13 nucleotides long. Thus, short length may be one important feature of plant introns. Furthermore, the 5' and 3' splice sites are separated by 4 bp in the extended anticodon stems of these pre-tRNA structures. In contrast, yeast and vertebrate introns are rather variable in length and the splice sites are separated by 5 or 6 bp. These differences in distance and relative helical orientation of the splice sites in plant pre-tRNAs versus pre-tRNAs from other organisms are obviously tolerated by the vertebrate splicing endonuclease, but not at all by the plant enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Goh S. H., Hall B. D. The promoter sequence of a yeast tRNAtyr gene. Cell. 1983 Sep;34(2):655–664. doi: 10.1016/0092-8674(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domdey H., Jank P., Sänger L., Gross H. J. Studies on the primary and secondary structure of potato spindle tuber viroid: products of digestion with ribonuclease A and ribonuclease T1, and modification with bisulfite. Nucleic Acids Res. 1978 Apr;5(4):1221–1236. doi: 10.1093/nar/5.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Shatkin A. J. Origin of splice junction phosphate in tRNAs processed by HeLa cell extract. Cell. 1983 Feb;32(2):547–557. doi: 10.1016/0092-8674(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Gamulin V., Mao J., Appel B., Sumner-Smith M., Yamao F., Söll D. Six Schizosaccharomyces pombe tRNA genes including a gene for a tRNALys with an intervening sequence which cannot base-pair with the anticodon. Nucleic Acids Res. 1983 Dec 20;11(24):8537–8546. doi: 10.1093/nar/11.24.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Sharp P. A., RajBhandary U. L. Saccharomyces cerevisiae SUP53 tRNA gene transcripts are processed by mammalian cell extracts in vitro but are not processed in vivo. Mol Cell Biol. 1988 Jan;8(1):361–370. doi: 10.1128/mcb.8.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Gabius H. J., Peebles C. L., Abelson J. An RNA ligase from wheat germ which participates in transfer RNA splicing in vitro. J Biol Chem. 1983 Jul 10;258(13):8365–8373. [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Greer C. L., Söll D., Willis I. Substrate recognition and identification of splice sites by the tRNA-splicing endonuclease and ligase from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jan;7(1):76–84. doi: 10.1128/mcb.7.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. D., Clarkson S. G., Tocchini-Valentini G. Transcription initiation of eucaryotic transfer RNA genes. Cell. 1982 May;29(1):3–5. doi: 10.1016/0092-8674(82)90083-6. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Fire A. Z., RajBhandary U. L., Sharp P. A. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983 Oct 10;258(19):11974–11980. [PubMed] [Google Scholar]
- Lee M. C., Knapp G. Transfer RNA splicing in Saccharomyces cerevisiae. Secondary and tertiary structures of the substrates. J Biol Chem. 1985 Mar 10;260(5):3108–3115. [PubMed] [Google Scholar]
- MacPherson J. M., Roy K. L. Two human tyrosine tRNA genes contain introns. Gene. 1986;42(1):101–106. doi: 10.1016/0378-1119(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Mattoccia E., Baldi M. I., Carrara G., Fruscoloni P., Benedetti P., Tocchini-Valentini G. P. Separation of RNA transcription and processing activities from X. laevis germinal vesicles. Cell. 1979 Nov;18(3):643–648. doi: 10.1016/0092-8674(79)90119-3. [DOI] [PubMed] [Google Scholar]
- Melton D. A., De Robertis E. M., Cortese R. Order and intracellular location of the events involved in the maturation of a spliced tRNA. Nature. 1980 Mar 13;284(5752):143–148. doi: 10.1038/284143a0. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Beckman J. S., Abelson J., Kang H. S., Söll D., Schmidt O. In vitro transcription and processing of a yeast tRNA gene containing an intervening sequence. Cell. 1979 Jun;17(2):399–406. doi: 10.1016/0092-8674(79)90166-1. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Lee M. C., Knapp G. Transfer RNA splicing in Saccharomyces cerevisiae: defining the substrates. Nucleic Acids Res. 1984 Dec 21;12(24):9367–9382. doi: 10.1093/nar/12.24.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. V., Page G. S., Sentenac A., Piper P. W., Worthington M., Weiss R. B., Hall B. D. Only one of two closely related yeast suppressor tRNA genes contains an intervening sequence. Nature. 1981 Jun 11;291(5815):464–469. doi: 10.1038/291464a0. [DOI] [PubMed] [Google Scholar]
- Rich A., Kim S. H. The three-dimensional structure of transfer RNA. Sci Am. 1978 Jan;238(1):52–62. doi: 10.1038/scientificamerican0178-52. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Venegas A., Rutter W. J. Yeast tRNA3Leu gene transcribed and spliced in a HeLa cell extract. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5963–5967. doi: 10.1073/pnas.78.10.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
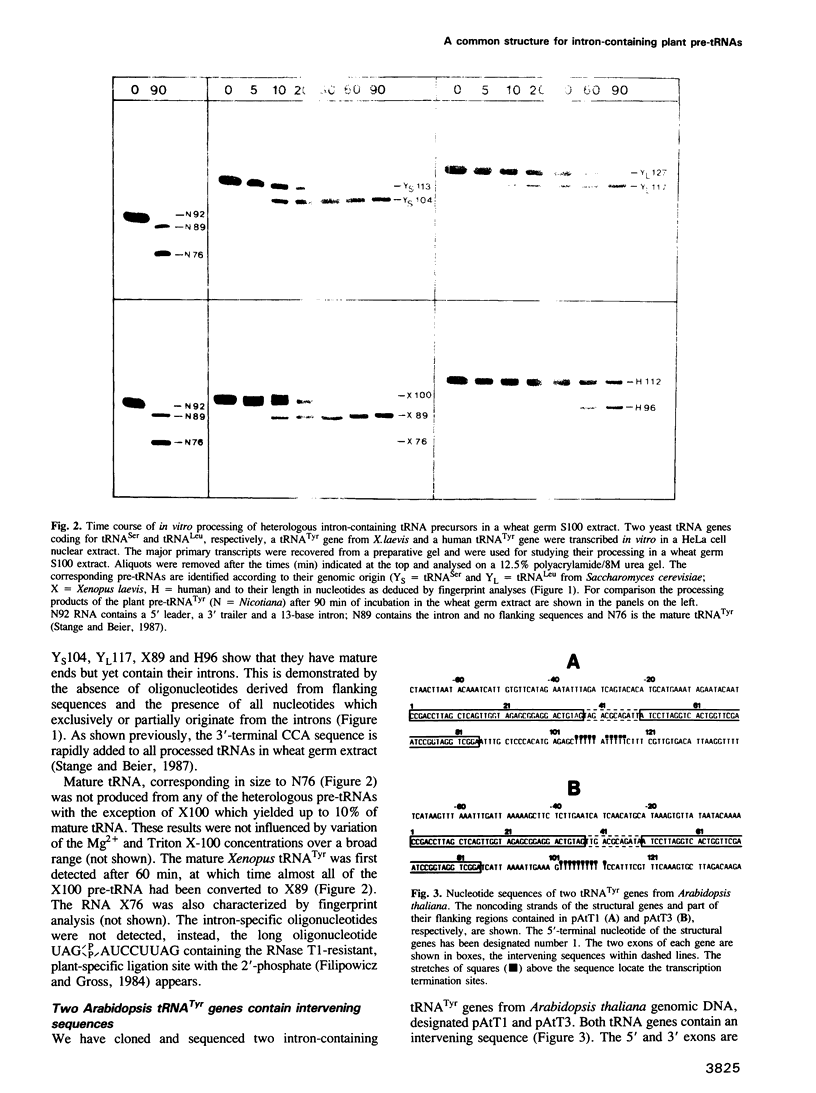
- Stange N., Beier H. A cell-free plant extract for accurate pre-tRNA processing, splicing and modification. EMBO J. 1987 Sep;6(9):2811–2818. doi: 10.1002/j.1460-2075.1987.tb02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier H. A gene for the major cytoplasmic tRNATyr from Nicotiana rustica contains a 13 nucleotides long intron. Nucleic Acids Res. 1986 Nov 11;14(21):8691–8691. doi: 10.1093/nar/14.21.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Strobel M. C., Abelson J. Intron mutations affect splicing of Saccharomyces cerevisiae SUP53 precursor tRNA. Mol Cell Biol. 1986 Jul;6(7):2674–2683. doi: 10.1128/mcb.6.7.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Smith M., Hottinger H., Willis I., Koch T. L., Arentzen R., Söll D. The sup8 tRNALeu gene of Schizosaccharomyces pombe has an unusual intervening sequence and reduced pairing in the anticodon stem. Mol Gen Genet. 1984;197(3):447–452. doi: 10.1007/BF00329941. [DOI] [PubMed] [Google Scholar]
- Swerdlow H., Guthrie C. Structure of intron-containing tRNA precursors. Analysis of solution conformation using chemical and enzymatic probes. J Biol Chem. 1984 Apr 25;259(8):5197–5207. [PubMed] [Google Scholar]
- Waldron C., Wills N., Gesteland R. F. Plant tRNA genes: putative soybean genes for tRNAasp and tRNAmet. J Mol Appl Genet. 1985;3(1):7–17. [PubMed] [Google Scholar]
- van Tol H., Beier H. All human tRNATyr genes contain introns as a prerequisite for pseudouridine biosynthesis in the anticodon. Nucleic Acids Res. 1988 Mar 25;16(5):1951–1966. doi: 10.1093/nar/16.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol H., Stange N., Gross H. J., Beier H. A human and a plant intron-containing tRNATyr gene are both transcribed in a HeLa cell extract but spliced along different pathways. EMBO J. 1987 Jan;6(1):35–41. doi: 10.1002/j.1460-2075.1987.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]