Abstract
In the present review we have analyzed the clinical applications of endoscopic ultrasound-guided-fine-needle-aspiration (EUS-FNA) and the methodological aspects obtained by cell-block procedure (CBP) in the diagnostic approach to the gastrointestinal neoplastic pathology. CBP showed numerous advantages in comparison to the cytologic routine smears; in particular, better preservation of cell architecture, achievement of routine haematoxylin-eosin staining equivalent to histological slides and possibility to perform immunohistochemistry or molecular analyses represented the most evident reasons to choose this method. Moreover, by this approach, the differential diagnosis of solid gastrointestinal neoplasias may be more easily achieved and the background of contaminant non-neoplastic gastrointestinal avoided. Finally, biological samples collected by EUS-FNA CBP-assisted should be investigated in order to identify and quantify further potential molecular markers.
Keywords: Endoscopic ultrasound-guided-fine-needle-aspiration, Cell-block procedure, Gastrointestinal tract, Immunohistochemistry, Diagnosis
Core tip: Cell-block procedure (CBP) represents the most suitable complement in diagnostic cytopathology of many gastrointestinal lesions. Hence this method allows high quality morphological evaluation and immunocytochemical analyses. On this way, the differential diagnosis of solid gastrointestinal neoplasms may be more easily achieved and the background of contaminant non-neoplastic gastrointestinal avoided, with an evident gain compared to the traditional cytological techniques. In the present review, the application of CBP in gastrointestinal solid lesions approached by endoscopic ultrasound-guided-fine-needle-aspiration, the methodological aspects and the accuracy of this diagnostic process are analyzed and discussed.
INTRODUCTION
Endoscopic ultrasound-guided-fine-needle-aspiration (EUS-FNA) represents a useful diagnostic procedure in the field of gastrointestinal pathology[1-3]. It is performed by using a curved linear array video-echo-endoscope equipped with various needles which provide cytological samples; in this way, the ability to obtain cytologic material is greatly increased due to direct visualization, with a consequent better opportunity to perform an accurate diagnosis. Since its introduction, EUS-FNA emerged as a minimally-invasive, safe and accurate technique for the diagnosis of various luminal, submucosal and extra luminal gastrointestinal neoplasms[4].
The European Society of Gastrointestinal Endoscopy published the guidelines for EUS-guided sampling, with comments on the technical prerequisites for maximizing the diagnostic yield of this procedure[5]. However, the acquisition of diagnostic samples should be approached in different ways depending on the type of the lesion. Moreover, the actual efficacy of EUS-FNA partly depends on the site, size and characteristics of the target tissue as well as on the expertise, training and interaction between endosonographer and cytopathologist[6,7].
Cell-block procedure (CBP) is a diagnostic tool which has been carried out by using different procedural steps and protocols over the years[7-11]. This technique presents several advantages compared to the cytologic routine smear: preservation of cell architecture, achievement of routine haematoxylin-eosin staining equivalent to that of surgical samples and, finally, the possibility to perform ancillary methods, such as immunohistochemistry or molecular analyses[7,8,12,13]. In particular, CBP allows the availability of an adequate number of serial sections, with increased possibility to detect malignant cells and contaminating or reactive non-neoplastic elements[6,7,13].
Aims of the present review are to discuss the application of CBP in gastrointestinal solid lesions approached by EUS-FNA and to analyze the methodological aspects and accuracy of this diagnostic process.
Methodological aspects of EUS-FNA
One of the most debated issues on EUS-FNA relates to the number of needle passes required to provide adequate diagnostic material. The presence of a well trained cytopathologist, able to evaluate the quality of samples, is probably crucial in order to decrease the number of unsatisfactory results and to reduce the need for additional passes. Indeed, the prompt cytopathology response may be useful for the endosonographer to know whether the needle aspirate is diagnostic or not[2,4,14-19]. Although it has been repeatedly reported that on-site cytological evaluation improves the diagnostic yield and accuracy of EUS-FNA, other factors, such as the localization, nature, presentation, size and sonography characteristics of the lesion, may influence the number of needle passes[2-4,20]. In detail, the percentage of adequate specimens and sensitivity of EUS-FNA are lower in intra-parietal lesions of the gastrointestinal tract (GI) compared to those of lesions in other sites[1,21,22]. In addition, the diagnostic yield and accuracy for EUS-FNA also depend on the size of the lesion and they are significantly lower in lesions less than 10 mm in size[1,23,24]. On the whole, two to five needle passes are considered to be sufficient to obtain enough diagnostic material for a correct diagnosis by EUS-FNA[2,3,20,22,25,26]. The needle size is another relevant factor. 19-G needle seems to be the most adequate to provide higher amount of diagnostic material, especially when the cytopathologist is not present in the endoscopy room. Nonetheless the 22-G or 25-G are the most commonly used needles for the cytological sampling of gastrointestinal lesions because of their easier penetration without any further complications[2,16,27,28].
Finally, a special technical training in EUS-FNA should be mandatory, as recommended by the American Society of Gastrointestinal Endoscopy which codifies the minimum number of cases that should be analyzed depending on the site of lesion[29-31].
Needle-based confocal laser endomicroscopy (CLE) is a novel endoscopic method, in which imaging is based on tissue illumination and detection of tissue-reflected fluorescence; interestingly this technique gives high-quality images which are similar to those obtained by traditional histology[32-34]. The development of tissue specific contrast agents might further extend the application of CLE to pancreatic masses, either solid or cystic, intra-parietal or submucosal gastric and esophageal tumours, biliary tract and ampullary lesions[2,33,35].
Methodological aspects and advantages of CBP
CBP has been extensively used in cytology as a helpful tool to achieve a definitive diagnosis[8-10,36,37]. CBP may be carried out by using different protocols based on various fixatives and embedding techniques[8,10,38-40].
In the manual traditional method, following the rapid on-site evaluation of specimen adequacy and preliminary cytological diagnosis by quick stains, the needles and syringes used in the procedure are rinsed with 10 mL of 50% ethanol into a special container in order to recover further material. All content is centrifuged in a 10 mL disposable centrifuge tube at 4000 rpm for 6 min to create 1 or 2 pellets; the supernatant fluid is decanted and the pelleted material obtained by sedimentation is immediately fixed in a freshly prepared solution of 4% neutral buffered formalin for 45 min. Then, the cell pellets are placed in a cassette and stored at 80% ethanol until they are ready for processing in an automatic tissue processor[36].
CBP may be based on thrombin or albumin methods. In the former, six drops of discarded human plasma and six drops of thromboplastin-DS are added to the cell sediment in order to form a clot, while in the latter 3-4 drops of 22% bovine albumin and 95% ethanol are added to the cell sediment to form a precipitate[9,41]. Whatever is the method, clots or/and precipitates are embedded in paraffin at 56 °C to realize cell blocks which are cut into 3 μm thick sections routinely stained with H and E or mounted on poly-lysine-coated glasses for immunocytochemical and molecular procedures.
A novel automated method for cell block production is the CellientTM Automated Cell Block System. Compared to the traditional manual method, the automated one allows to achieve higher cellularity and better cellular presentation in terms of architecture and details; in addition it is faster and more reliable due to lack of operator dependency[9,39,42]. Gorman and coll. showed that Cellient cell blocks gives an adequate cellularity in all the analyzed cases, while formalin and thrombin cell blocks show a progressively decreasing adequacy[37]. The main drawback of Cellient system is methanol-based fixation, which may have negative impact on the following immunohistochemical analysis[8,9]. Indeed weaker staining intensity for ER, PR, MIB-1 and HER2 was shown by using this procedure[8,37,43,44]. However this issue may be overcome by formalin pre-fixation prior to Cellient[9]. Thirty minutes pre-fixation seems to be preferable to longer fixation to ensure good morphological quality[9].
On the whole, CBP allows the collection of higher quantity of diagnostic material. Hence it may be relevant in reducing the false negative diagnoses in EUS-FNA, which may depend, not only on erroneous interpretation of the cytological samples, but also on the availability of low cytological material. In addition it was shown that CBP greatly increases the diagnostic accuracy of EUS-FNA[7,22]. CBP also represents the most appropriate method to obtain cytological preparations for subsequent immunocytochemistry. Indeed immuno-stains on CBP sections show minimal background and appear similar to those observed in surgical pathology material. In addition, numerous serial sections may be obtained from a single cell block, allowing the evaluation of a large spectrum of antigens, also in archival samples. The number of antibodies that can be applied in routinely CBP has been expanding over the years[2,3,7-9,13,37]. The possibility to test serial sections with different antibodies may allow to identify and discriminate gastrointestinal hyperplastic or reactive contaminating cells from well differentiated tumour cells[7,13,45].
CLINICAL APPLICATION OF EUS-FNA CBP-ASSISTED IN GI TRACT
Subepithelial/intramural neoplasms of the gastrointestinal wall
Although conventional endoscopy, CT scan and MRI may identify subepithelial/intramural lesions in the gastrointestinal wall, they can not reveal the nature and origin of those lesions. A wide range of subepithelial tumours, such as leiomyomas, neurinomas, granular cells tumours, gastrointestinal stromal tumours (GISTs), neuroendocrine tumours, leiomyosarcomas and lymphomas, may involve the GI tract[1,6,46] and many of those neoplastic entities exhibited overlapping cytological features[6,46], being composed by monomorphic, uniform spindle shaped cells with eosinophilic cytoplasm, vesicular elongated nuclei characterized by finely granular chromatin, sometimes dispersed and inconspicuous nucleoli (Figure 1A). For this reason, the use of immunocytochemistry, which is easily applicable to CBP, may be helpful for the differential diagnosis. In detail, the coexistence of smooth muscle actin and desmin stains strongly supports the muscle origin of the lesion, while positivity for CD-34, CD-117 (Figure 1B) or S-100 suggests other diagnostic hypotheses, such as inflammatory fibroid polyp, GIST or schwannoma[6,46,47]. The assessment of the growth fraction by using Ki-67 labeling index (Figure 2A) may further discriminate the benign or malignant nature of intra-parietal neoplasias, and may allow distinction among leiomyoma, leiomyosarcoma, spindle cells amelanotic melanoma or undifferentiated sarcomatoid carcinoma[6,46,48].
Figure 1.
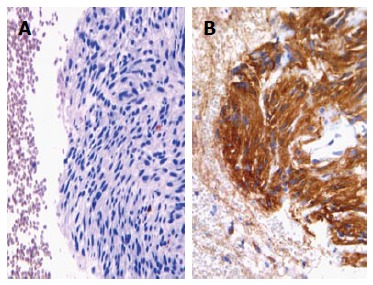
Cell block from gastrointestinal stromal tumour exhibiting aggregates of spindle cells with elongated nuclei (haematoxylin-eosin, × 200) (A), with an evident immunoreactivity for CD117 (immunoperoxidase, × 200, Mayer’s Haemalum nuclear counterstain) (B).
Figure 2.
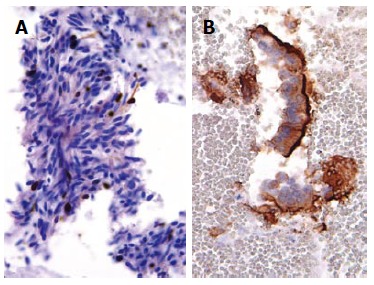
Spindle cells of gastric gastrointestinal stromal tumour documented only a sporadic nuclear Ki67 immunopositivity (immunoperoxidase, × 400, Mayer’s Haemalum nuclear counterstain) (A) in benign contaminant gastrointestinal cells, the apical cytoplasm showed a peculiar CD10 immunoreactivity (immunoperoxidase, × 400, Mayer’s Haemalum nuclear counterstain) (B).
The great efficacy of EUS-FNA associated with the higher accuracy obtained by CBP are helpful to achieve the correct preoperative diagnosis of a sub-epithelial mass which is relevant to establish the operative planning and type of surgery, and to avoid unnecessary procedures for extensive malignant lesions[6,46,49]. In addition, periodic follow-up with EUS is considered to be more acceptable to evaluate eventual changes in tumour size in those patients who refused surgery[49-51].
Solid pancreatic masses
The pre-operative correct diagnosis of ductal pancreatic adenocarcinoma is crucial for patients management and prognosis, and to reduce costs due to unwarranted procedures[1,13,52,53]. The cytological detection of pancreatic ductal adenocarcinoma is usually not difficult for the experienced cytopathologist; indeed this neoplasia is characterized by distinctive cytological features, such as the presence of groups of atypical cells with irregular roundish hyperchromatic dense nuclei, evident nucleoli, mitotic figures and absence of the honeycomb benign pattern[13]. Frequently, pancreatic smears exhibited a hemorrhagic background with clusters or small aggregates of epithelial cells, occasionally arranged in glandular or pseudo-papillary structures. Nevertheless, in a subset of carcinomas the cytological diagnosis may be hard to achieve, due to the presence of extensive necrosis, associated inflammation, contaminating intestinal epithelial cells or limited sampling[7,13,54]. In those cases, again CBP appears as a significant tool for the pathologist, either the microscopic evaluation and application of immunostainings in serial sections. In fact, it has been shown that carcinoembryonic antigen was expressed in neoplastic pancreatic elements of great majority of ductal adenocarcinomas[13]. However, carbohydrate antigen (CA 19-9) represented the most widely used biomarker for pancreatic cancer, even if it showed limitations in differential diagnosis between pancreatic neoplasms, being positive also in solid pseudopapillary tumour and not only in cancer[55-61]. An intriguing challenge, even for the expert cytopathologist, is represented by the distinction between well differentiated pancreatic neoplastic cells and gastrointestinal epithelial contaminating elements, sampled by EUS-FNA through the stomach or the duodenum[7,13,55-58]. Several efforts were made to solve this crucial diagnostic point[7,13,20,55-58]. Firstly, it was reported that a mucin panel comprising four antibodies (MUC1, MUC2, MUC5AC, MUC6) may be helpful in differentiating normal/reactive gastro-duodenal cells from neoplastic pancreatic elements[55]. Successively, the utility of immunocytochemistry against CD10 was highlighted (Figure 2B); indeed this antigen is expressed at the apical membrane of the benign contaminant gastrointestinal cells, but not in the neoplastic elements of well differentiated pancreatic adenocarcinoma[7,13,59,60]. The absence of CD10 stain in pancreatic adenocarcinomas has been also documented in surgical histological samples[59,60]. However, CD10 expression has been evidenced in 100% of solid pseudo-papillary pancreatic neoplasms[61-63] and in 30% of pancreatic endocrine tumours with focal staining[7,63,64]. As a consequence, CD10 immunostaining alone cannot be used for the differential diagnosis of pancreatic lesions[7]. An immunohistochemical panel against CK7, CDX2, chromogranin A and synaptophysin is useful for the differential diagnosis among invasive ductal carcinomas, endocrine tumours and acinar cell tumours of the pancreas[12,20,65]. Finally, a further analysis of p53 immunoreactivity may be of diagnostic help in pancreatic pathology (Figure 3); indeed immunocytochemical positivity for mutant p53 protein with long half-life has been recorded in 50%-70% of pancreatic carcinomas, but not in chronic pancreatitis[66-68].
Figure 3.
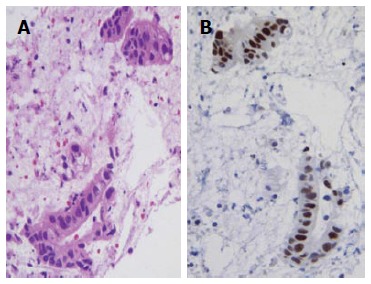
Well differentiated pancreatic carcinoma with a pseudo-glandular pattern (haematoxylin-eosin, × 400) (A), a nuclear strong p53 immunostaining was encountered in neoplastic elements (immunoperoxidase, × 400, Mayer’s Haemalum nuclear counterstain) (B).
Solid hepatic lesions
A variety of hepatocellular nodules (hyperplastic, benign, dysplastic and malignant) and secondary tumors can be detected in the liver and subjected to EUS-FNA, especially when they were confined to left hepatic lobe[3,69,70]. In particular, while a significant rate of lesions smaller than 1 cm in diameter is missed by CT and MRI, EUS shows excellent diagnostic accuracy in the identification of hepatic lesions less than 0.5 cm in size[69-72]. It is noteworthy that most of < 1 cm hepatic lesions are non-malignant, whereas the large majority of lesions exceeding 2 cm are represented by hepatocellular carcinomas (HCCs); hence in the group of lesions greater than 2 cm a diagnosis of non-malignancy should induce the suspicion of a diagnostic error[73]. Although nodular precursors such as liver regenerative (LRN) or low-grade dysplastic (LGDN) and high grade dysplastic (HGDN) nodules are related to hepatocarcinogenesis, they should be discriminated from adenomas and differentiated HCCs. LGDN category also includes the so-called LRN and it shows mild increase in cell density with a monotonous pattern and bland cytological atypia[73]. On the other hand, HGDN always exhibit more marked cytological atypia and irregular trabecular pattern[73]. Discrimination of well differentiated and hypovascular HCCs from dysplastic nodules may be particularly challenging; in those cases, CBP associated with EUS-FNA or EUS-guided biopsy are warranted, as recently acknowledged[74]. Several immunomarkers were proposed for the distinction between well differentiated HCC and non-malignant lesions[75]. Specifically, Glypican 3 appeared as a good tissue marker with 77% sensitivity and 96% specificity for HCC[74]. In addition, Heat Shock Protein 70 was reported as the most abundantly up-regulated gene in early HCC, and the protein for which it encodes can be detected by immunocytochemistry in up to 78% of the cases with 95% specificity[74]. Finally, Glutamine Synthetase is overexpressed in malignant hepatocytes with diffuse and strong pattern in 50% of HCCs[74,76]. The combined use of the aforementioned was proposed in order to increase the diagnostic accuracy in cases with dubious morphology[76], and so the availability of serial consecutive sections obtained from CBP applied to EUS-FNA could represent the gold standard. With regards to cytokeratins (CK) profile, CK8 and 18 are expressed in both normal and neoplastic hepatocytes, while about 70% of HCC are negative for CK7, CK19, and CK20[73,77]. Furthermore, the combined use of CK7 and CK20 may help to identify the origin of adenocarcinomas occurring in GI tract; in particular, CK7 and CK20 expression in cholangiocarcinomas (CC) varies along the biliary tract, with higher sensitivity of CK7+/CK20- profile in peripheral CC compared to non-peripheral ones (Figure 3)[73,77]. On the other hand, CK7+/CK20+ profile supports the diagnosis of pancreatic adenocarcinoma, while CK7-/CK20+ is the typical pattern of colonic cancer[73,77].
Gallbladder and biliary tract lesions
Approach by EUS-FNA of the lesions of biliary tract, and mainly of the hilar ones, may avoid the risk of unnecessary extensive surgery[78,79]. Indeed the sensitivity and specificity of obtaining diagnostic samples in biliary neoplasms is variable by endoscopic-retrograde cholangiography[3]. Moreover, the endoscopic retrograde cholangiopancreaticography (ERCP), used at times for hilar cholangiocarcinomas, has frequently inconclusive diagnosis[80]. Consequently, a morphological diagnosis on cytological samples provided by EUS-FNA and submitted to CBP may allow to recognize the nature of malignant biliary lesions (Figure 4) and to change the preplanned surgical approach. Generally, tumour cells appear in loosely structured groups or disorder flat sheets exhibiting as cytologic atypia that varies depending upon tumour grade; occasionally, tumour cells may exhibit cytoplasmic vacuolization and focal mucin secretion. What’s more, regional lymph nodes may be evaluated for metastasis by EUS-FNA in patients with unresectable hilar carcinomas[81,82].
Figure 4.
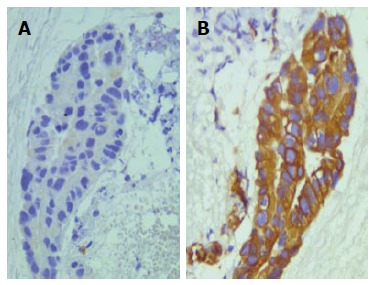
Peripheral cholangiocarcinoma with a papillary pattern (haematoxylin-eosin, × 400) (A), in a serial section obtained from CBP neoplastic elements exhibited an evident cytokeratin 7 immunoreaction (B) (immunoperoxidase, × 400, Mayer’s Haemalum nuclear counterstain).
A sensitivity and accuracy of 95% have been recorded for EUS-FNA in distal biliary malignancies[7,83] and similar values have been reported in patients with obstructive jaundice due to nodular lesion such as epithelial and non-epithelial tumours, lymphomas and metastases[84-86].
In gallbladder masses, the CBP-assisted EUS-FNA procedure has been used either for diagnostic and staging purposes, with rates of sensitivity and specificity ranging between 80% and 100%, especially in lesions of the gallbladder wall[87-91].
In ampullary tumours, EUS-FNA has higher diagnostic accuracy in the distinction between benign and malignant tumours compared to other operative procedures such as biopsy or brushing cytology during ERCP[92,93]. In addition, it is of help in the identification of patients with low or high grade dysplasia or affected by adenocarcinomas[93].
In this anatomical district, some very severe complications such as bile peritonitis and cholangitis have been described[1]; they probably represent a consequence of inadvertent needle penetration inside intrahepatic or common bile ducts as well as gallbladder. By contrast, bleeding is mild and self-limited, even when patients were taken aspirin or anti-inflammatory drugs, in absence of portal hypertension[1].
CONCLUSION
The clinical applications of EUS-FNA and the methodological advantages obtained by CBP in the diagnosis of solid neoplasms of the GI tract were reviewed. Although on-site cytological evaluation during the ultra-sonographic needle aspirative procedure may increase the diagnostic yield of EUS-FNA, in our opinion CBP represents its most appropriate diagnostic complement. Indeed this method allows high quality morphological microscopic evaluation and multiple immunocytochemical analyses. By this approach, the differential diagnosis of neoplasms may be more easily achieved, and the background of contaminant non-neoplastic gastrointestinal avoided, which represent evident advantages compared to the traditional cytological techniques. Finally, the identification and quantification of potential molecular markers may represent a promising field to be further investigated on the same biological samples collected by EUS-FNA CBP-assisted.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 15, 2015
First decision: June 24, 2015
Article in press: August 3, 2015
P- Reviewer: Tse GM, Zou XP S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743–759. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Costache MI, Iordache S, Karstensen JG, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound. 2013;2:77–85. doi: 10.4103/2303-9027.117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammoud GM, Almashhrawi A, Ibdah JA. Usefulness of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of hepatic, gallbladder and biliary tract Lesions. World J Gastrointest Oncol. 2014;6:420–429. doi: 10.4251/wjgo.v6.i11.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrone MC, Arcidiacono PG. Basic technique in endoscopic ultrasound-guided fine needle aspiration for solid lesions: How many passes? Endosc Ultrasound. 2014;3:22–27. doi: 10.4103/2303-9027.124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 6.Todaro P, Crinò SF, Pallio S, Fazzari C, Consolo P, Tuccari G. Gastrointestinal stromal tumours of the stomach: Cytological and immunocytochemical diagnostic features of two cases diagnosed by endoscopic ultrasound-guided fine needle aspiration. Oncol Lett. 2013;5:1862–1866. doi: 10.3892/ol.2013.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieni A, Todaro P, Crino SF, Barresi V, Tuccari G. Endoscopic ultrasound-guided fine-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic efficacy of cell-block immunocytochemistry. Hepatobiliary Pancreat Dis Int. 2015;14:305–312. doi: 10.1016/s1499-3872(15)60367-8. [DOI] [PubMed] [Google Scholar]
- 8.Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, Bonfiglio TA. Cellient™ automated cell block versus traditional cell block preparation: a comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol. 2011;39:730–736. doi: 10.1002/dc.21457. [DOI] [PubMed] [Google Scholar]
- 9.Prendeville S, Brosnan T, Browne TJ, McCarthy J. Automated Cellient(™) cytoblocks: better, stronger, faster? Cytopathology. 2014;25:372–380. doi: 10.1111/cyt.12159. [DOI] [PubMed] [Google Scholar]
- 10.Kruger AM, Stevens MW, Kerley KJ, Carter CD. Comparison of the Cellient(™) automated cell block system and agar cell block method. Cytopathology. 2014;25:381–388. doi: 10.1111/cyt.12216. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Yu X, Zheng Y, Yang Y, Xie J, Zhou X. Value of fine needle aspiration cell blocks in the diagnosis and classification of lymphoma. Int J Clin Exp Pathol. 2014;7:7717–7725. [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Kamada H, Fujimori T, Aritomo Y, Ono M, Masaki T. Molecular Biologic Approach to the Diagnosis of Pancreatic Carcinoma Using Specimens Obtained by EUS-Guided Fine Needle Aspiration. Gastroenterol Res Pract. 2012;2012:243524. doi: 10.1155/2012/243524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigliar E, Troncone G, Bracale U, Iaccarino A, Napolitano V, Bellevicine C. CD10 is useful to identify gastrointestinal contamination in endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) cytology from pancreatic ductal adenocarcinoma. Cytopathology. 2015;26:83–87. doi: 10.1111/cyt.12148. [DOI] [PubMed] [Google Scholar]
- 14.Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist’s perspective. Am J Clin Pathol. 2003;120:351–367. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 15.Vilmann P, Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: equipment and technique. J Gastroenterol Hepatol. 2006;21:1646–1655. doi: 10.1111/j.1440-1746.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal S, Friedel D, Gupta M, Ogden L, Stavropoulos SN. Endoscopic-ultrasound-guided fine-needle aspiration and the role of the cytopathologist in solid pancreatic lesion diagnosis. Patholog Res Int. 2012;2012:317167. doi: 10.1155/2012/317167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VandenBussche CJ, Olson MT, Adams C, Ali SZ. Cytotechnologist performance for screening microfollicular atypia in indeterminate thyroid fine-needle aspirates. Acta Cytol. 2014;58:432–438. doi: 10.1159/000367882. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh J, Varadarajulu S. How can we get the best results with endoscopic ultrasound-guided fine needle aspiration? Clin Endosc. 2012;45:132–137. doi: 10.5946/ce.2012.45.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshinaga S, Suzuki H, Oda I, Saito Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23 Suppl 1:29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 21.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 22.Jenssen C, Möller K, Wagner S, Sarbia M. [Endoscopic ultrasound-guided biopsy: diagnostic yield, pitfalls, quality management] Z Gastroenterol. 2008;46:897–908. doi: 10.1055/s-2008-1027419. [DOI] [PubMed] [Google Scholar]
- 23.Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171–177. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 24.Jhala NC, Jhala D, Eloubeidi MA, Chhieng DC, Crowe DR, Roberson J, Eltoum I. Endoscopic ultrasound-guided fine-needle aspiration biopsy of the adrenal glands: analysis of 24 patients. Cancer. 2004;102:308–314. doi: 10.1002/cncr.20498. [DOI] [PubMed] [Google Scholar]
- 25.Möller K, Papanikolaou IS, Toermer T, Delicha EM, Sarbia M, Schenck U, Koch M, Al-Abadi H, Meining A, Schmidt H, et al. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–69. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Mitra V, Nayar MK, Leeds JS, Wadehra V, Haugk B, Scott J, Charnley RM, Oppong KW. Diagnostic performance of endoscopic ultrasound (EUS)/endoscopic ultrasound--fine needle aspiration (EUS-FNA) cytology in solid and cystic pancreatic neuroendocrine tumours. J Gastrointestin Liver Dis. 2015;24:69–75. doi: 10.15403/jgld.2014.1121.vmi. [DOI] [PubMed] [Google Scholar]
- 27.Sodikoff JB, Johnson HL, Lewis MM, Garud SS, Bharmal SJ, Keilin SA, Siddiqui MT, Cai Q, Willingham FF. Increased diagnostic yield of endoscopic ultrasound-guided fine needle aspirates with flow cytometry and immunohistochemistry. Diagn Cytopathol. 2013;41:1043–1051. doi: 10.1002/dc.22903. [DOI] [PubMed] [Google Scholar]
- 28.Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–1034. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 29.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–37. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 30.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–708. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 31.Barthet M, Gasmi M, Boustiere C, Giovannini M, Grimaud JC, Berdah S. EUS training in a live pig model: does it improve echo endoscope hands-on and trainee competence? Endoscopy. 2007;39:535–539. doi: 10.1055/s-2007-966336. [DOI] [PubMed] [Google Scholar]
- 32.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 33.De Palma GD. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770–5775. doi: 10.3748/wjg.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphris J, Swartz D, Egan BJ, Leong RW. Status of confocal laser endomicroscopy in gastrointestinal disease. Trop Gastroenterol. 2012;33:9–20. doi: 10.7869/tg.2012.3. [DOI] [PubMed] [Google Scholar]
- 35.Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–1060. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 37.Gorman BK, Kosarac O, Chakraborty S, Schwartz MR, Mody DR. Comparison of breast carcinoma prognostic/predictive biomarkers on cell blocks obtained by various methods: Cellient, formalin and thrombin. Acta Cytol. 2012;56:289–296. doi: 10.1159/000337436. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez N, Selvaggi SM. Utility of cell blocks in the diagnosis of thyroid aspirates. Diagn Cytopathol. 2006;34:89–92. doi: 10.1002/dc.20385. [DOI] [PubMed] [Google Scholar]
- 39.Akalin A, Lu D, Woda B, Moss L, Fischer A. Rapid cell blocks improve accuracy of breast FNAs beyond that provided by conventional cell blocks regardless of immediate adequacy evaluation. Diagn Cytopathol. 2008;36:523–529. doi: 10.1002/dc.20846. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86–90. doi: 10.1002/dc.20963. [DOI] [PubMed] [Google Scholar]
- 41.Nigro K, Tynski Z, Wasman J, Abdul-Karim F, Wang N. Comparison of cell block preparation methods for nongynecologic ThinPrep specimens. Diagn Cytopathol. 2007;35:640–643. doi: 10.1002/dc.20713. [DOI] [PubMed] [Google Scholar]
- 42.Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356–371. doi: 10.1111/cyt.12174. [DOI] [PubMed] [Google Scholar]
- 43.Hanley KZ, Birdsong GG, Cohen C, Siddiqui MT. Immunohistochemical detection of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast carcinomas: comparison on cell block, needle-core, and tissue block preparations. Cancer. 2009;117:279–288. doi: 10.1002/cncy.20034. [DOI] [PubMed] [Google Scholar]
- 44.Shabaik A, Lin G, Peterson M, Hasteh F, Tipps A, Datnow B, Weidner N. Reliability of Her2/neu, estrogen receptor, and progesterone receptor testing by immunohistochemistry on cell block of FNA and serous effusions from patients with primary and metastatic breast carcinoma. Diagn Cytopathol. 2011;39:328–332. doi: 10.1002/dc.21389. [DOI] [PubMed] [Google Scholar]
- 45.Toll AD, Bibbo M. Identification of gastrointestinal contamination in endoscopic ultrasound-guided pancreatic fine needle aspiration. Acta Cytol. 2010;54:245–248. doi: 10.1159/000325029. [DOI] [PubMed] [Google Scholar]
- 46.Todaro P, Crinò SF, Ieni A, Pallio S, Consolo P, Tuccari G. Intraparietal esophageal leiomyomas diagnosed by endoscopic ultrasound-guided fine-needle aspiration cytology: Cytological and immunocytochemical features in two cases. Oncol Lett. 2014;8:123–126. doi: 10.3892/ol.2014.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu GQ, Qian JJ, Chen MH, Ren GP, Chen HT. Endoscopic ultrasonography for the diagnosis and selecting treatment of esophageal leiomyoma. J Gastroenterol Hepatol. 2012;27:521–525. doi: 10.1111/j.1440-1746.2011.06929.x. [DOI] [PubMed] [Google Scholar]
- 48.Maheshwari V, Alam K, Varshney M, Jain A, Asif Siddiqui F, Bhargava S. Fine-needle aspiration diagnosis of GIST: a diagnostic dilemma. Diagn Cytopathol. 2012;40:834–838. doi: 10.1002/dc.21734. [DOI] [PubMed] [Google Scholar]
- 49.Sung HJ, Cho YK, Park EY, Moon SJ, Lim CH, Kim JS, Park JM, Lee IS, Kim SW, Choi MG, et al. Performance and clinical role of endoscopic ultrasound fine needle aspiration for diagnosing gastrointestinal intramural lesions. Clin Endosc. 2013;46:627–632. doi: 10.5946/ce.2013.46.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang G, Zhao H, Yang F, Li J, Li Y, Liu Y, Liu J, Wang J. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study on 40 cases. Dis Esophagus. 2009;22:279–283. doi: 10.1111/j.1442-2050.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 51.Choi SH, Kim YT, Han KN, Ra YJ, Kang CH, Sung SW, Kim JH. Surgical management of the esophageal leiomyoma: lessons from a retrospective review. Dis Esophagus. 2011;24:325–329. doi: 10.1111/j.1442-2050.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 52.Bluen BE, Lachter J, Khamaysi I, Kamal Y, Malkin L, Keren R, Epelbaum R, Kluger Y. Accuracy and Quality Assessment of EUS-FNA: A Single-Center Large Cohort of Biopsies. Diagn Ther Endosc. 2012;2012:139563. doi: 10.1155/2012/139563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baek HW, Park MJ, Rhee YY, Lee KB, Kim MA, Park IA. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic lesions. J Pathol Transl Med. 2015;49:52–60. doi: 10.4132/jptm.2014.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H, Mandich D, Cartun RW, Ligato S. Expression of K homology domain containing protein overexpressed in cancer in pancreatic FNA for diagnosing adenocarcinoma of pancreas. Diagn Cytopathol. 2007;35:700–704. doi: 10.1002/dc.20739. [DOI] [PubMed] [Google Scholar]
- 55.Giorgadze TA, Peterman H, Baloch ZW, Furth EE, Pasha T, Shiina N, Zhang PJ, Gupta PK. Diagnostic utility of mucin profile in fine-needle aspiration specimens of the pancreas: an immunohistochemical study with surgical pathology correlation. Cancer. 2006;108:186–197. doi: 10.1002/cncr.21913. [DOI] [PubMed] [Google Scholar]
- 56.Mitsuhashi T, Ghafari S, Chang CY, Gu M. Endoscopic ultrasound-guided fine needle aspiration of the pancreas: cytomorphological evaluation with emphasis on adequacy assessment, diagnostic criteria and contamination from the gastrointestinal tract. Cytopathology. 2006;17:34–41. doi: 10.1111/j.1365-2303.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 57.Layfield LJ, Jarboe EA. Cytopathology of the pancreas: neoplastic and nonneoplastic entities. Ann Diagn Pathol. 2010;14:140–151. doi: 10.1016/j.anndiagpath.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value of EUS-FNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology. 2011;22:174–178. doi: 10.1111/j.1365-2303.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 59.Erhuma M, Köbel M, Mustafa T, Wulfänger J, Dralle H, Hoang-Vu C, Langner J, Seliger B, Kehlen A. Expression of neutral endopeptidase (NEP/CD10) on pancreatic tumor cell lines, pancreatitis and pancreatic tumor tissues. Int J Cancer. 2007;120:2393–2400. doi: 10.1002/ijc.22252. [DOI] [PubMed] [Google Scholar]
- 60.Salla C, Konstantinou P, Chatzipantelis P. CK19 and CD10 expression in pancreatic neuroendocrine tumors diagnosed by endoscopic ultrasound-guided fine-needle aspiration cytology. Cancer. 2009;117:516–521. doi: 10.1002/cncy.20048. [DOI] [PubMed] [Google Scholar]
- 61.Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361–1371. doi: 10.1097/00000478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Handra-Luca A, Fléjou JF, Rufat P, Corcos O, Belghiti J, Ruszniewski P, Degott C, Bedossa P, Couvelard A. Human pancreatic mucinous cystadenoma is characterized by distinct mucin, cytokeratin and CD10 expression compared with intraductal papillary-mucinous adenoma. Histopathology. 2006;48:813–821. doi: 10.1111/j.1365-2559.2006.02444.x. [DOI] [PubMed] [Google Scholar]
- 63.Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: a limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132:831–839. doi: 10.1309/AJCPVT8FCLFDTZWI. [DOI] [PubMed] [Google Scholar]
- 64.Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251–258. doi: 10.1016/j.humpath.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, Yatabe Y. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int. 2010;60:358–364. doi: 10.1111/j.1440-1827.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 66.Itoi T, Takei K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Nakamura K, Moriyasu F, Tsuchida A, Kasuya K. Immunohistochemical analysis of p53 and MIB-1 in tissue specimens obtained from endoscopic ultrasonography-guided fine needle aspiration biopsy for the diagnosis of solid pancreatic masses. Oncol Rep. 2005;13:229–234. [PubMed] [Google Scholar]
- 67.Jahng AW, Reicher S, Chung D, Varela D, Chhablani R, Dev A, Pham B, Nieto J, Venegas RJ, French SW, et al. Staining for p53 and Ki-67 increases the sensitivity of EUS-FNA to detect pancreatic malignancy. World J Gastrointest Endosc. 2010;2:362–368. doi: 10.4253/wjge.v2.i11.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheng W, Dong M, Zhou J, Li X, Liu Q, Dong Q, Li F. The clinicopathological significance and relationship of Gli1, MDM2 and p53 expression in resectable pancreatic cancer. Histopathology. 2014;64:523–535. doi: 10.1111/his.12273. [DOI] [PubMed] [Google Scholar]
- 69.Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743–749. doi: 10.1055/s-2003-41593. [DOI] [PubMed] [Google Scholar]
- 70.Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Lee TH, Cha SW, Kim SG, et al. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J Gastroenterol Hepatol. 2015;30:1161–1166. doi: 10.1111/jgh.12922. [DOI] [PubMed] [Google Scholar]
- 71.Awad SS, Fagan S, Abudayyeh S, Karim N, Berger DH, Ayub K. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601–604; discussion 604-605. doi: 10.1016/s0002-9610(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 72.Singh P, Mukhopadhyay P, Bhatt B, Patel T, Kiss A, Gupta R, Bhat S, Erickson RA. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367–373. doi: 10.1097/MCG.0b013e318167b8cc. [DOI] [PubMed] [Google Scholar]
- 73.Roncalli M, Terracciano L, Di Tommaso L, David E, Colombo M. Liver precancerous lesions and hepatocellular carcinoma: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S361–S372. doi: 10.1016/S1590-8658(11)60592-6. [DOI] [PubMed] [Google Scholar]
- 74.International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 75.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, Iavarone M, Colombo M, Jang JJ, Yu E, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50:746–754. doi: 10.1016/j.jhep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Rullier A, Le Bail B, Fawaz R, Blanc JF, Saric J, Bioulac-Sage P. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol. 2000;24:870–876. doi: 10.1097/00000478-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616–623. doi: 10.1016/j.cgh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 79.Nayar MK, Manas DM, Wadehra V, Oppong KE. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862–1865. doi: 10.5754/hge10531. [DOI] [PubMed] [Google Scholar]
- 80.Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45–51. doi: 10.1046/j.1572-0241.2003.04006.x. [DOI] [PubMed] [Google Scholar]
- 81.Gleeson FC, Clayton AC, Zhang L, Clain JE, Gores GJ, Rajan E, Smyrk TC, Topazian MD, Wang KK, Wiersema MJ, et al. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437–1440. doi: 10.1016/j.cgh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Pollack MJ, Gholam PM, Chak A. EUS-FNA in unresectable cholangiocarcinoma: a novel indication. Gastrointest Endosc. 2008;67:444–445. doi: 10.1016/j.gie.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104. doi: 10.1016/j.gie.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 84.Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 85.Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:244–252. doi: 10.1097/MOG.0b013e32835005bc. [DOI] [PubMed] [Google Scholar]
- 86.Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532–537. doi: 10.1097/MCG.0b013e3182745d9f. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson BC, Pitman MB, Brugge WR. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest Endosc. 2003;57:251–254. doi: 10.1067/mge.2003.86. [DOI] [PubMed] [Google Scholar]
- 88.Sadamoto Y, Kubo H, Harada N, Tanaka M, Eguchi T, Nawata H. Preoperative diagnosis and staging of gallbladder carcinoma by EUS. Gastrointest Endosc. 2003;58:536–541. doi: 10.1067/s0016-5107(03)01961-8. [DOI] [PubMed] [Google Scholar]
- 89.Varadarajulu S, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration in the evaluation of gallbladder masses. Endoscopy. 2005;37:751–754. doi: 10.1055/s-2005-870161. [DOI] [PubMed] [Google Scholar]
- 90.Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–213. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 91.Meara RS, Jhala D, Eloubeidi MA, Eltoum I, Chhieng DC, Crowe DR, Varadarajulu S, Jhala N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17:42–49. doi: 10.1111/j.1365-2303.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 92.Ogura T, Yamao K, Hara K, Mizuno N, Hijioka S, Imaoka H, Sawaki A, Niwa Y, Tajika M, Kondo S, et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J Gastroenterol. 2013;48:640–646. doi: 10.1007/s00535-012-0664-2. [DOI] [PubMed] [Google Scholar]
- 93.Roberts KJ, McCulloch N, Sutcliffe R, Isaac J, Muiesan P, Bramhall S, Mirza D, Marudanayagam R, Mahon BS. Endoscopic ultrasound assessment of lesions of the ampulla of Vater is of particular value in low-grade dysplasia. HPB (Oxford) 2013;15:18–23. doi: 10.1111/j.1477-2574.2012.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


