Abstract
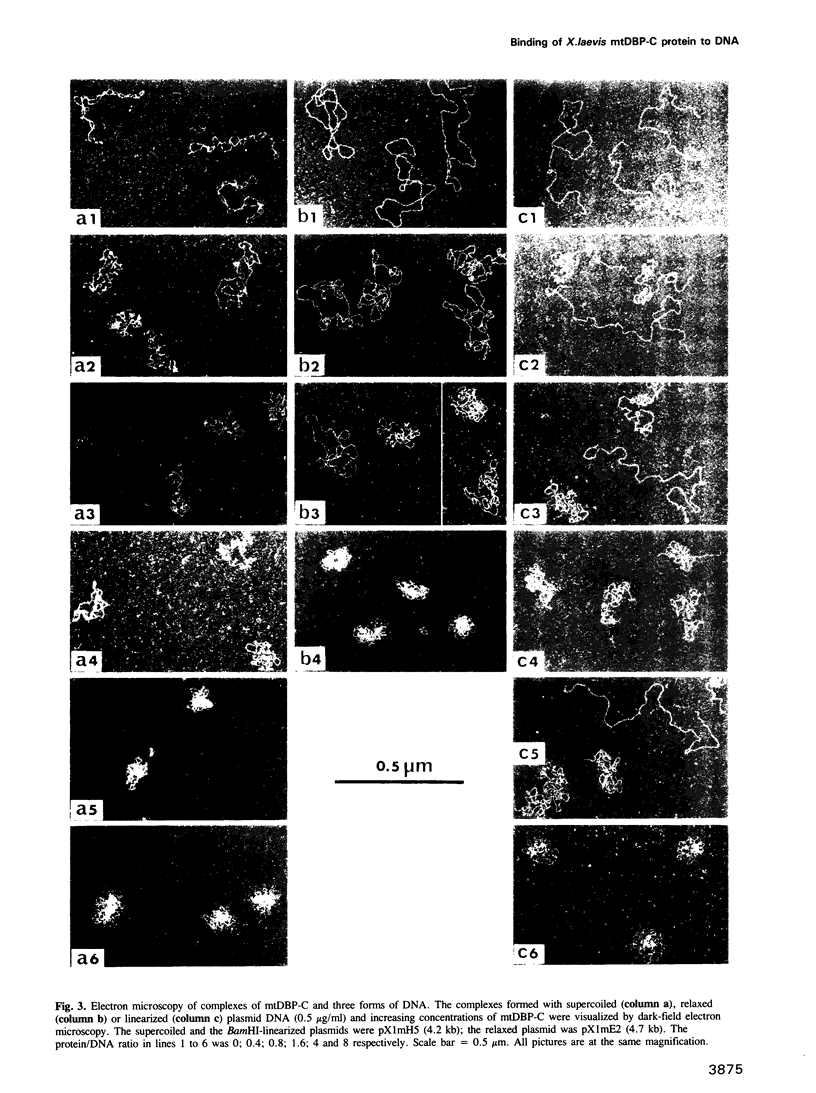
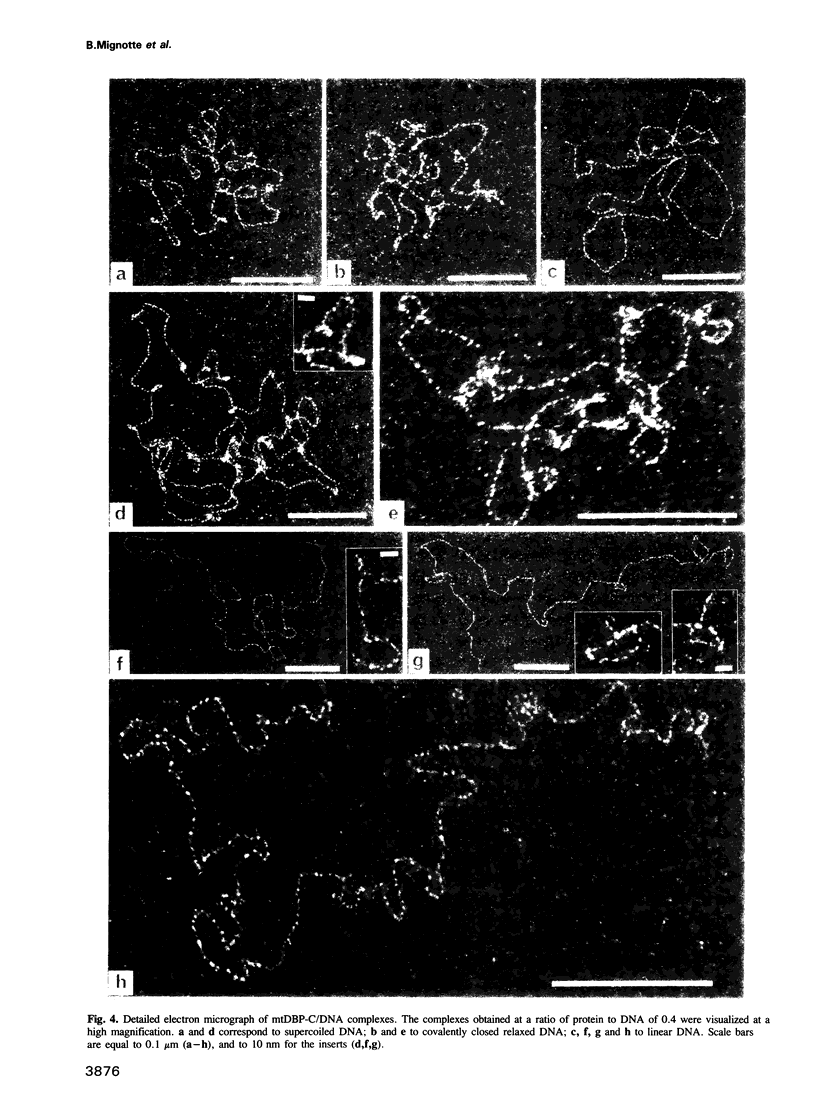
The binding of the Xenopus laevis mitochondrial protein mtDBP-C to DNA was studied by equilibrium density banding, agarose gel electrophoresis and electron microscopy. The results obtained show that the mtDBP-C binds cooperatively to DNA irrespective of whether the DNA is supercoiled, relaxed or linear and it induces the formation of superhelical turns locally leading to the formation of a highly folded structure. It appears that this protein could be involved in the compaction of DNA in the mitochondrial nucleoid.
Full text
PDF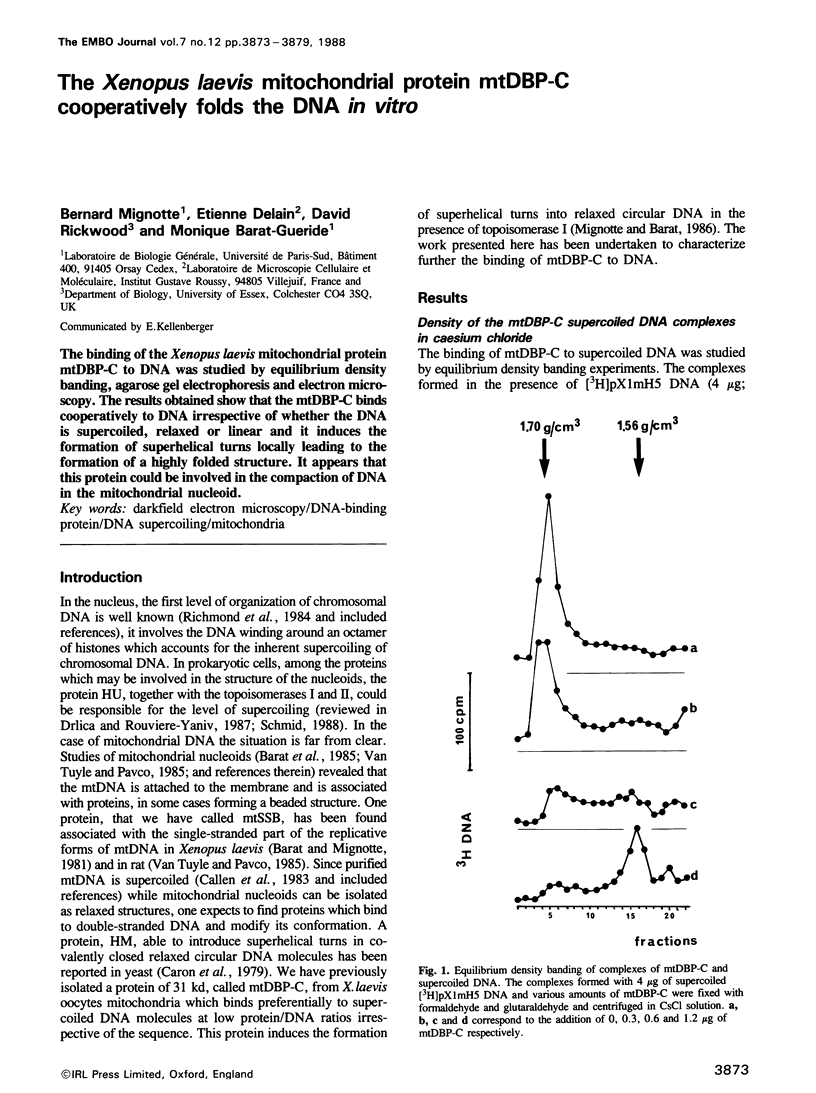



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barat M., Mignotte B. A DNA binding protein from Xenopus laevis oocyte mitochondria. Chromosoma. 1981;82(4):583–593. doi: 10.1007/BF00295014. [DOI] [PubMed] [Google Scholar]
- Barat M., Rickwood D., Dufresne C., Mounolou J. C. Characterization of DNA-protein complexes from the mitochondria of Xenopus laevis oocytes. Exp Cell Res. 1985 Mar;157(1):207–217. doi: 10.1016/0014-4827(85)90163-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Gadaleta G., Pepe G., Saccone C., Sbisà E. Structural conservation and variation in the D-loop-containing region of vertebrate mitochondrial DNA. J Mol Biol. 1986 Dec 5;192(3):503–511. doi: 10.1016/0022-2836(86)90272-x. [DOI] [PubMed] [Google Scholar]
- Callen J. C., Tourte M., Dennebouy N., Mounolou J. C. Changes in D-loop frequency and superhelicity among the mitochondrial DNA molecules in relation to organelle biogenesis in oocytes of Xenopus laevis. Exp Cell Res. 1983 Jan;143(1):115–125. doi: 10.1016/0014-4827(83)90114-3. [DOI] [PubMed] [Google Scholar]
- Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon N. E., Kornberg A. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):424–428. doi: 10.1073/pnas.81.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Duguet M., Bonne C., de Recondo A. M. Single-strand deoxyribonucleic acid binding protein from rat liver changes the helical structure of deoxyribonucleic acid. Biochemistry. 1981 Jun 9;20(12):3598–3603. doi: 10.1021/bi00515a045. [DOI] [PubMed] [Google Scholar]
- Fuller F. B. The writhing number of a space curve. Proc Natl Acad Sci U S A. 1971 Apr;68(4):815–819. doi: 10.1073/pnas.68.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet I., Zivanovic Y., Prunell A., Revet B. Chromatin reconstitution on small DNA rings. I. J Mol Biol. 1988 Mar 20;200(2):253–266. doi: 10.1016/0022-2836(88)90238-0. [DOI] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng. 1978;7:19–35. doi: 10.1146/annurev.bb.07.060178.000315. [DOI] [PubMed] [Google Scholar]
- Mignotte B., Barat M. Characterization of a Xenopus laevis mitochondrial protein with a high affinity for supercoiled DNA. Nucleic Acids Res. 1986 Aug 11;14(15):5969–5980. doi: 10.1093/nar/14.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Barat M., Marsault J., Mounolou J. C. Mitochondrial DNA-binding proteins that bind preferentially to supercoiled molecules containing the D-loop region of Xenopus laevis mtDNA. Biochem Biophys Res Commun. 1983 Nov 30;117(1):99–107. doi: 10.1016/0006-291x(83)91546-2. [DOI] [PubMed] [Google Scholar]
- Mignotte B., Dunon-Bluteau D., Reiss C., Mounolou J. C. Sequence deduced physical properties in the D-loop region common to five vertebrate mitochondrial DNAs. J Theor Biol. 1987 Jan 7;124(1):57–69. doi: 10.1016/s0022-5193(87)80252-7. [DOI] [PubMed] [Google Scholar]
- Mignotte B., Marsault J., Barat-Gueride M. Effects of the Xenopus laevis mitochondrial single-stranded DNA-binding protein on the activity of DNA polymerase gamma. Eur J Biochem. 1988 Jun 15;174(3):479–484. doi: 10.1111/j.1432-1033.1988.tb14123.x. [DOI] [PubMed] [Google Scholar]
- Moyne G., Freeman R., Saragosti S., Yaniv M. A high-resolution electron microscopy study of nucleosomes from simian virus 40 chromatin. J Mol Biol. 1981 Jul 15;149(4):735–744. doi: 10.1016/0022-2836(81)90355-7. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Schmid M. B. Structure and function of the bacterial chromosome. Trends Biochem Sci. 1988 Apr;13(4):131–135. doi: 10.1016/0968-0004(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., Pavco P. A. The rat liver mitochondrial DNA-protein complex: displaced single strands of replicative intermediates are protein coated. J Cell Biol. 1985 Jan;100(1):251–257. doi: 10.1083/jcb.100.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Murcia G., Jongstra-Bilen J., Ittel M. E., Mandel P., Delain E. Poly(ADP-ribose) polymerase auto-modification and interaction with DNA: electron microscopic visualization. EMBO J. 1983;2(4):543–548. doi: 10.1002/j.1460-2075.1983.tb01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]