Abstract
The diets of populations in industrialized nations have shifted to dramatically increased consumption of ω6 polyunsaturated fatty acids (PUFA), with a corresponding decrease in the consumption of ω3 PUFA. This dietary shift may be related to observed increases in obesity, chronic inflammation, and comorbidities in the human population. We examined the effects of ω3:ω6 fatty acid ratios in the context of constant total dietary lipid on the growth, total body fat, and responses of key inflammatory markers in adult zebrafish (Danio rerio). Zebrafish were fed diets in which the ω3:ω6 PUFA ratios were representative of those in a purported ancestral diet (1:2) and more contemporary Western diets (1:5 and 1:8). After 5 mo, weight gain (fat free mass) of zebrafish was highest for those that received the 1:8 ratio treatment, but total body fat was lowest at this ratio. Measured by quantitative real-time RT–PCR, mRNA levels from liver samples of 3 chronic inflammatory response genes (C-reactive protein, serum amyloid A, and vitellogenin) were lowest at the 1:8 ratio. These data provide evidence of the ability to alter zebrafish growth and body composition through the quality of dietary lipid and support the application of this model to investigations of human health and disease related to fat metabolism.
Abbreviations: LC-PUFA, long-chain PUFA; PUFA, polyunsaturated fatty acids
Most animals require specific (essential) dietary fatty acids, and deficiencies in these fatty acids typically exert a negative effect on their health at some level. The ω3 and ω6 families of fatty acids are essential polyunsaturated fatty acids (PUFA) or long-chain PUFA (LC-PUFA) for many animals, including humans; however, consensus regarding the recommended dietary levels of these PUFA has not been achieved for any species, including humans. Several studies have proposed that a disproportionately high intake of ω6 PUFA and LC-PUFA promotes inflammation, resulting in chronic inflammatory diseases associated with metabolic syndrome.10,22 This ‘high’ intake is difficult to describe accurately because both individual as well as regional diversity in the dietary intake of ω3 and ω6 fatty acids exist globally. Over the last century, diets in the western hemisphere have shifted to a dramatically increased consumption of total lipids. This increase in total fat consumption is associated with increases in ω6 PUFA and ω6 LC-PUFA intakes and corresponding decreases in ω3 PUFA and ω3 LC-PUFA.16 The shift in the dietary ω3:ω6 ratio, toward ω6 and away from ω3 fatty acids, in industrialized societies has been proposed to be the major factor contributing to inflammatory diseases.22 This proinflammatory effect is often attributed to the production of arachidonic acid metabolites, which act as potent proinflammatory and plaque forming molecules, from ω6 fatty acids, like linoleic acid.7 However, many antiinflammatory mediators also are produced during the metabolism of ω6. Several studies support a possible association between a reduced risk of coronary heart disease and increased dietary ω6 PUFA.7 The American Heart Association Science Advisory Panel has stated, “At present, there is little direct evidence that supports a net proinflammatory, proatherogenic effect of linoleic acid (18:2 ω6) in humans.”11 The authors of a recent review19 concluded that reducing the intake of dietary ω6 fatty acid did not change the levels of arachidonic acid in the plasma, serum, or erythrocytes of adults who consumed western-type, high-fat diets. Other scientists18 have suggested that specific proportional combinations of ω3 and ω6 fatty acids may actually decrease the concentrations of proinflammatory cytokines.
Zebrafish continue to gain popularity as an animal model for cardiovascular disease.4 For example, blood vessel plaques formed in zebrafish that consumed a high-cholesterol (4%) diet, mimicking atherosclerosis in humans.24 Recent advances in the area of zebrafish nutrition25 allow the use of formulated diets, wherein the levels of specific nutrients, such as fatty acids, can be modified to evaluate response. The current study evaluated the effects of different dietary ω3:ω6 fatty acid ratios on weight gain, body composition, and inflammatory response proteins in the zebrafish.
Materials and Methods
Danio rerio (AB strain) were obtained from the Nutrition Obesity Research Center Aquatic Animal Research Core at the University of Alabama at Birmingham. All animal protocols were reviewed and approved by the University of Alabama at Birmingham IACUC. Embryos were collected randomly from a mass spawn of adult zebrafish previously fed the mixed-protein diet (see Diet section). At 5 d after fertilization, hatched larvae were fed rotifers (Branchionus plicatilis) enriched with Nannochloropsis (RotiGrow Omega, Reed Mariculture, Campbell, CA) ad libitum 3 times daily until 28 d after fertilization, at which point 250 fish of mixed sex were randomly introduced into 1.8-L tanks (10 fish per tank, 15 tanks), representing 5 replicates of each of the 3 dietary treatments. A photograph of all fish in each tank was recorded (DS FIL or D70 camera, Nikon, Tokyo, Japan), and the length of each fish was determined through image analysis (NIS Elements 3.1, Nikon). Prior to the initiation of the experiment, averages were calculated for each replicate for all dietary treatments, and ANOVA indicated no significant difference among the treatments (7.00 ± 0.02 mm). All zebrafish were maintained at 28 °C in a recirculating system (Aquaneering, San Diego, CA). Flow rates were adjusted to provide at least 2 complete water-volume changes hourly within each tank. Municipal tap water was filtered through a 5-μm sediment filter, followed by charcoal, reverse osmosis, and cation–anion exchange resin (Kent Marine, Franklin, WI) prior to the addition of synthetic sea salts (Instant Ocean) to obtain a final conductivity of 1500 μS/cm for the system water source. Throughout the experiment, the fish tanks were rotated across rack positions weekly to reduce potentially confounding effects due to environmental noise, light, vibration, or other sources. Uneaten feed and debris was removed weekly from the tank by siphon as needed. Sodium bicarbonate was used to adjust and maintain the pH of the system water at approximately 7.4. At least 20% of system water was replaced weekly. Total ammonia nitrogen, nitrite, and nitrate were measured colorimetrically (Mars Fish Care, Chalfont, PA) and, except for nitrate, remained below detectable limits.
Diets.
The ingredient composition of each of the experimental diets is provided in Table 1. Each diet was produced by using a single base mix of defined ingredients. The proportional amounts of menhaden oil (Omega Protein, Houston, TX) and corn oil (JM Smuckers, Orville, OH) differed to obtain a final fatty acid ratio (ω3:ω 6) of 1:2, 1:5, or 1:8 that included both PUFA and LC-PUFA. These ratios represent combined PUFA and LC-PUFA for ω3 and ω6 fatty acids and were chosen to reflect the variation in ω6 fatty acids reported for human Paleolithic ancestral populations and those characteristic of a westernized diet as consumed in many industrialized countries.22 In the current study, the primary dietary ω6 PUFA was linoleic acid (18:2ω6), and the primary dietary ω3 PUFA was α-linolenic acid (18:3ω3). The ω3 LC-PUFA were eicosapentanoic acid (20:5ω3) and docosahexaenoic acid (22:6ω3). Diets were mixed in an orbital mixer (KitchenAid, St Joseph, MO), extruded (KPEXTA, KitchenAid), air-dried to approximately 10% moisture content, and then stored at 4 °C until fed. Diets were analyzed for ω3 and ω6 fatty acid composition (Eurofins Laboratories, DesMoines, IA), and results are presented in Table 2. For feeding, diets were ground to a fine powder (250 to 500 μm sieved) and weighed aliquots were manually (dispensed at ca. 900, 1300, and 1700 during the light cycle in surplus quantities). Fish were fed at a minimum of 3% to 5% body weight daily and observed to consume the diets throughout the water column.
Table 1.
Diet ingredients, as percentages of the total diet (as fed).
| ω3:ω6 ratio of diet |
|||
| 1:2 | 1:5 | 1:8 | |
| Fish protein hydrolysate | 20 | 20 | 20 |
| Vitamin-free casein | 25 | 25 | 25 |
| Isolated soy protein | 5 | 5 | 5 |
| Refined soy lecithin | 4 | 4 | 4 |
| Wheat gluten | 10 | 10 | 10 |
| Menhaden oil (ω3)a | 3.337 | 1.0768 | 0.041 |
| Corn oil (ω6)a | 3.663 | 5.9232 | 6.959 |
| Canthaxanthin | 2.31 | 2.31 | 2.31 |
| Cholesterol | 3 | 3 | 3 |
| Ascorbylpalmitate | 0.04 | 0.04 | 0.04 |
| Vitamin mix BML2 | 4 | 4 | 4 |
| Mineral mix BTm | 3 | 3 | 3 |
| Betaine | 0.15 | 0.15 | 0.15 |
| KH2PO4 | 1.15 | 1.15 | 1.15 |
| Sodium alginate | 5.58 | 5.58 | 5.58 |
| Dextrin | 9.77 | 9.77 | 9.77 |
These were the primary sources of ω3 and ω6 in the diet; other ingredients in the diet may contribute to the actual ω3:ω6 ratios. Final ratios are based on chemical analysis of each diet as reported in Table 2.
Table 2.
Total contents (%) of ω3 and ω6 fatty acids in zebrafish diets, according to chemical analysis Eurofins Analysis Center, Des Moines, IA.
| ω3:ω6 ratio of diet |
|||
| Fatty acid | 1:2 | 1:5 | 1:8 |
| C08:0 Octanoic (caprylic) | <0.01 | <0.01 | <0.01 |
| C10:0 Decanoic (capric) | <0.01 | <0.01 | <0.01 |
| C11:0 Undecanoic (hendecanoic) | <0.01 | <0.01 | <0.01 |
| C12:0 Dodecanoic (lauric) | 0.03 | 0.03 | 0.02 |
| C14:0 Tetradecanoic (myristic) | 0.31 | 0.17 | 0.10 |
| C14:1 Tetradecenoic (myristoleic) | <0.01 | <0.01 | <0.01 |
| C15:0 Pentadecanoic | 0.03 | 0.02 | 0.01 |
| C15:1 Pentadecenoic | <0.01 | <0.01 | <0.01 |
| C16:0 Hexadecanoic (palmitic) | 1.66 | 1.56 | 1.51 |
| C16:1 Hexadecenoic (palmitoleic) | 0.39 | 0.20 | 0.10 |
| C17:0 Heptadecanoic (margaric) | 0.03 | 0.02 | 0.01 |
| C17:1 Heptadecenoic (margaroleic) | <0.01 | <0.01 | <0.01 |
| C18:0 Octadecanoic (stearic) | 0.34 | 0.32 | 0.31 |
| C18:1 Octadecenoic (oleic) | 1.91 | 2.28 | 2.43 |
| C18:2 Octadecadienoic (linoleic) | 3.38 | 4.43 | 5.02 |
| C18:3 Octadecatrienoic (linolenic) | 0.27 | 0.26 | 0.25 |
| C18:4 Octadecatetraenoic | 0.12 | 0.06 | 0.03 |
| C20:0 Eicosanoic (arachidic) | 0.03 | 0.03 | 0.03 |
| C20:1 Eicosenoic (gadoleic) | 0.16 | 0.14 | 0.12 |
| C20:2 Eicosadienoic | 0.02 | 0.01 | 0.01 |
| C20:3 Eicosatrienoic | <0.01 | <0.01 | <0.01 |
| C20:4 Eicosatetraenoic (arachidonic) | 0.10 | 0.06 | 0.04 |
| C20:5 Eicosapentaenoic | 0.42 | 0.22 | 0.11 |
| C21:5 Heneicosapentaenoic | 0.02 | 0.01 | <0.01 |
| C22:0 Docosanoic (behenic) | 0.03 | 0.03 | 0.02 |
| C22:1 Docosenoic (erucic) | 0.13 | 0.12 | 0.11 |
| C22:2 Docosadienoic | <0.01 | <0.01 | <0.01 |
| C22:3 Docosatrienoic | <0.01 | <0.01 | <0.01 |
| C22:4 Docosatetraenoic | <0.01 | <0.01 | <0.01 |
| C22:5 Docosapentaenoic | 0.12 | 0.06 | 0.04 |
| C22:6 Docosahexaenoic | 0.56 | 0.32 | 0.21 |
| C24:0 Tetracosanoic (lignoceric) | 0.03 | 0.04 | 0.03 |
| C24:1 Tetracosenoic (nervonic) | 0.04 | 0.03 | 0.03 |
| C18:2 Octadecadienoic ω6 | 3.34 | 4.42 | 4.97 |
| C18:3 Octadecatrienoic ω3 | 0.26 | 0.25 | 0.24 |
| C18:3 Octadecatrienoic ω6 | 0.02 | <0.01 | <0.01 |
| C18:4 Octadecatetraenoic ω3 | 0.10 | 0.05 | 0.03 |
| C20:2 Eicosadienoic ω6 | 0.02 | 0.01 | 0.01 |
| C20:3 Eicosatrienoic ω3 | 0.01 | <0.01 | <0.01 |
| C20:3 Eicosatrienoic ω6 | <0.01 | <0.01 | <0.01 |
| C20:4 Eicosatetraenoic ω3 | 0.06 | 0.03 | 0.02 |
| C20:4 Eicosatetraenoic ω6 | 0.04 | 0.02 | 0.02 |
| C20:5 Eicosapentaenoic ω3 | 0.42 | 0.22 | 0.11 |
| C21:5 Heneicosapentaenoic ω3 | 0.02 | 0.01 | <0.01 |
| C22:2 Docosadienoic ω6 | <0.01 | <0.01 | <0.01 |
| C22:3 Docosatrienoic ω3 | <0.01 | <0.01 | <0.01 |
| C22:4 Docosatetraenoic ω6 | <0.01 | <0.01 | <0.01 |
| C22:5 Docosapentaenoic ω3 | 0.10 | 0.06 | 0.04 |
| C22:5 Docosapentaenoic ω6 | 0.02 | <0.01 | <0.01 |
| C22:6 Docosahexaenoic ω3 | 0.56 | 0.32 | 0.21 |
| Total content of ω3 fatty acids | 1.54 | 0.94 | 0.65 |
| Total content of ω6 fatty acids | 3.44 | 4.47 | 5.01 |
Wet and dry weight and total body lipid of fish.
After 5 mo of feeding the experimental diets, 10 male and 10 female fish were removed randomly from each diet group, blotted on absorbent towels, and weighed individually to the nearest 0.01 g. All fish then were euthanized as indicated by the lack of detection of opercular movement after submersion in ice water (approximately 5 min). Livers were dissected from male fish (n = 5 per treatment) and stored in RNAlater (Ambion, Austin, TX) at –30 °C for RNA extraction at a later time. Total dry weights and body lipid were determined for individual female fish (n = 10 per treatment). To determine dry weight, fish were dried at 55 °C to a constant weight (48 h) and reweighed to determine moisture content. Dried fish were ground by using a Wiley mill, and a subsample was removed for total lipid determination according to the method of Folch and colleagues.9 Adiposity was calculated as total body dry lipid (mg) divided by total body fat-free tissue mass (mg).
Expression analysis of inflammatory response genes.
Vitellogenin is upregulated during the inflammatory response in zebrafish.13 However, in female fish, it also is a key component in the reproductive cycle (egg production). Therefore, to eliminate any possible confounding effects of vitellogenin expression due the reproductive cycle, we assessed inflammatory response genes in male fish only. Livers of 3 fish selected randomly from each diet treatment were pooled to ensure sufficient tissue (approximately 30 mg wet) for each replicate. Three replicates (each consisting of 3 livers) were obtained for each treatment. Total RNA was isolated by using the RNAqueous RNA isolation kit (Ambion). Equal amounts of total RNA from each pooled sample were used to prepare cDNA (RETROscript, Ambion) according to the manufacturer's protocol. A real-time PCR assay was performed by using the IQ SYBR Green Supermix (BioRad, Philadelphia, PA) in the BioRad MiniOpticon RT–PCR detection system. RT–PCR primer pairs used to amplify the genes for C-reactive protein, vitellogenin (Vg1), and serum amyloid A are described in Table 3. PCR reactions were performed according to the manufacturer's protocol, using 2 μg cDNA and primer concentrations of 0.5 μM in a total volume of 25 μL. PCR conditions comprised 95 °C for 3 min followed by 40 cycles of 94 °C for 10 s, 62 °C for 1 min.13 Reactions for each sample were performed in duplicate. Zebrafish β-actin was used as a control to normalize the starting quantity of RNA. Gene expression levels are represented as the fold increase among exposure levels and are representative of results from independent experiments.
Table 3.
Primers for real-time PCR analysis of zebrafish inflammatory genes
| Gene | Forward primer (3’’to 5′) | Reverse primer (3’ to 5’) | Amplicon size (bp) | Reference (PMID) |
| C-reactive protein | GGG TGG ACG GTC AAC GCA GT | ACG GTG CCG CCA GGA CGA AT | 70 | 16630661 |
| Vitellogenin 1 | CTT CTG GAT GCT CTT CCT GCT GT | TCT GAA TGA ACT CGG GAG TGG TA | 100 | 20006406 |
| Serum amyloid A | CGG GGT CCT GGG GGC TAT TG | GTT GGG GTC TCC GCC GTT TC | 141 | 16630661 |
| β-actin | CGA GCA GGA GAT GGG AAC C | CAA CGG AAA CGC TCA TTG C | 102 | 20006406 |
Statistics.
Data are reported as mean ± SEM. Data were tested for homogeneity of variance by Levene test; means for wet and dry weights and percentages lipid and adiposity were compared by using ANOVA with arc-sine transformation of data where appropriate. When significance among responses to dietary treatment existed, the Tukey post-hoc test was used to identify significant differences between treatments. All statistical analysis was conducted by using SPSS version 21 (IBM, Armonk, NY).
Results
Wet weights.
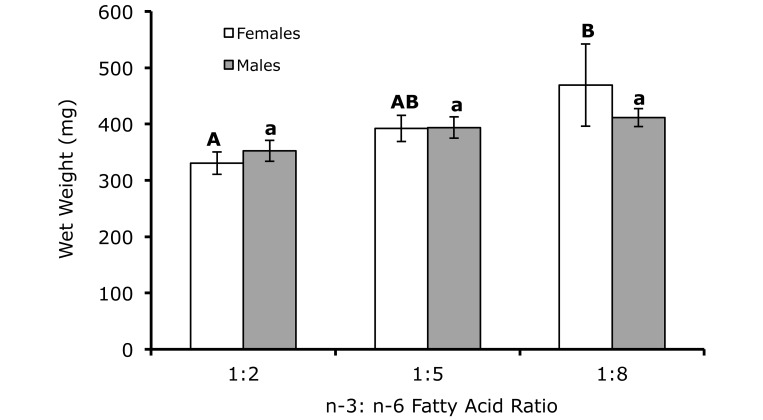
Survival exceeded 95% in all 3 dietary treatment groups. All diets supported weight gain. Wet weights for female fish were 469 ± 73, 392 ± 23, and 330 ± 20 mg for the 1:8, 1:5, and 1:2 dietary treatments, respectively. All female fish were reproductively mature, as evidenced by the presence of eggs. As total dietary ω6 increased, wet weight in female fish increased (P = 0.07; Figure 1); increases in wet weights of male fish were not significant.
Figure 1.
Wet weights for male (n = 10 per treatment) and female (n = 10 per treatment) zebrafish fed diets varying in the ratio of ω3:ω6 fatty acids. Different letters indicate significant (P < 0.05) differences within sex. Male wet weights increased nominally (P = 0.07) with increasing ω6 fatty acids.
Body composition.
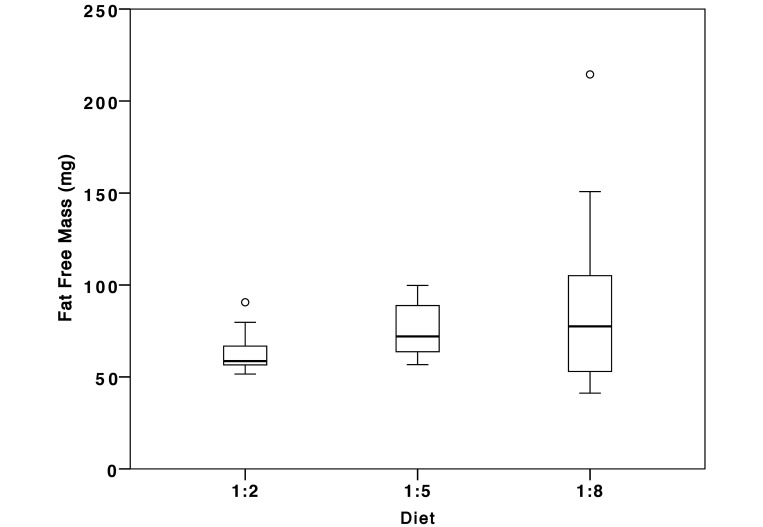
At 5 mo (the end point of the experiment), total body dry weights for female fish were 30% higher in those fed the 1:8 diet than the 1:2 diet (121 ± 2.2 mg compared with 85.4 ± 5.7 mg dry weights, respectively). Percentage moisture did not differ significantly among female fish representing the different dietary treatments. The percentage of total body lipid in fish fed the 1:8 diet did not differ from that of fish fed the 1:5 diet (Table 4). Adiposity was lowest for those that consumed the 1:8 diet (P = 0.048, Table 4). Variance in the fat-free mass increased significantly as the proportion of ω6 increased (P < 0.011; Figure 2).
Table 4.
Characteristics (mean ± SEM) of female zebrafish fed diets differing in the dietary ratio of ω3:ω6 fatty acids (n= 10/treatment)
| Dietary ω3:ω6 ratio | Wet weight (mg) | Dry weight (mg) | % moisture | % lipid (% dry matter) | Adiposity (mg dry lipid / mg fat-free tissue) |
| 1:2 | 330 ± 20a | 84 ± 0.57a | 74.6 ± 0.54 | 24.2 ± 2.1a | 0.33 ± 0.037a |
| 1:5 | 392 ± 23a,b | 102 ± 0.67a,b | 74.2 ± 0.52 | 25.1 ± 1.4ab | 0.34 ± 0.025ab |
| 1:8 | 469 ± 73b | 121 ± 2.20b | 75.2 ± 0.44 | 21.8 ± 0.9b | 0.28 ± 0.014b |
Within a column, values with different superscripted letters differ significantly (P < 0.05) from each other.
Figure 2.
Fat free mass (FFM) for female zebrafish (n = 10) fed diets varying in the ratio of ω3:ω6 fatty acids. Fat mass did not vary significantly across treatments; however, there was increased variance in fat free mass with increasing level of ω6 fatty acid in the diet (P < 0.011). Open circles represent suspected outliers as identified by SPSS.
Proinflammatory response.
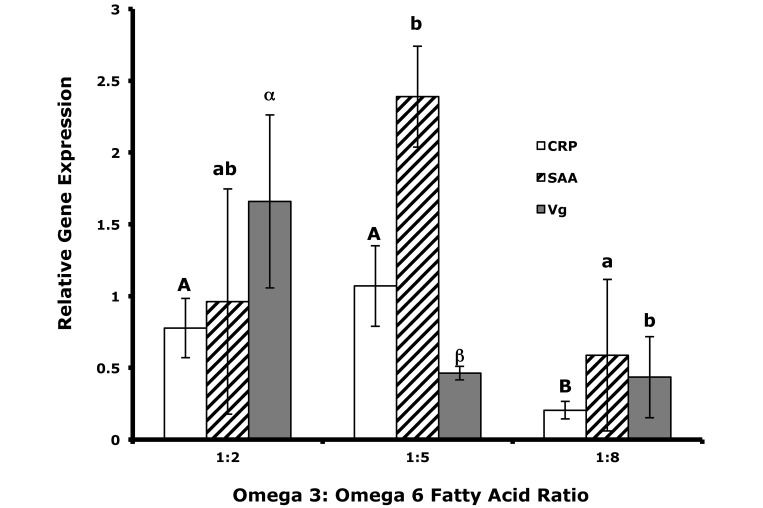
The expression levels of all 3 proinflammatory genes varied widely among treatment groups (Figure 3). The expression of C-reactive protein was significantly (P < 0.05) lower in the 1:8 dietary treatment compared with both the 1:2 and 1:5 diets. The expression of vitellogenin was significantly (P < 0.05) higher in fish fed the 1:2 diet compared with both the 1:5 and 1:8 diets but did not differ significantly between the 1:5 and 1:8 diet treatments. The expression of serum amyloid A was significantly (P < 0.05) higher for the 1:5 dietary treatment compared with the 1:8 diet treatment, but there was no significant differences between the 1:2 diet treatment and the 1:5 or 1:8 diets.
Figure 3.
Relative gene expression levels for C reactive protein (CRP), serum amyloid A (SAA), and vitellogenin (VG) in male zebrafish livers (n = 5 per treatment) from fish fed 5 mo on diets with ω3:ω6 ratios of 1:2, 1:5, or 1:8. Different letters (case-sensitive) indicate significant (P < 0.05) differences in the expression level for each gene across diet treatments.
Discussion
Several 18- to 22-carbon PUFA and LC-PUFA of the ω6 and ω3 families are termed ‘essential fatty acids’ because they cannot be synthesized by most animals and must therefore be obtained from the diet.6 Dietary requirements for these essential fatty acids vary across phyla and have been reported for approximately 30 fish species.17 Fish have a species-dependent requirement for fatty acids that is influenced by environmental factors such as temperature: coldwater species have a higher requirement for ω3 family of PUFA and LC-PUFA, whereas warmwater species, such as zebrafish, require both ω3 and ω6 fatty acids.14,15 However, including ω3 LC-PUFA in the diet of warmwater species such as hybrid tilapia and channel catfish can significantly improve growth.5 Essential fatty acids are also important in reproduction; for example, the essential fatty acid content of broodstock diets has been shown to affect the fatty acid composition of eggs in Eurasian perch1 and Nile tilapia.20 In addition, insufficient dietary ω6 (PUFA and LC-PUFA) for zebrafish leads to decreased growth and quality of eggs.13 The complex chemical nature and multiple functional roles of lipids make it difficult to define specific dietary lipid requirements for many fish species.
In the current study, gene expression levels for the proinflammatory response proteins (C-reactive protein, serum amyloid A, and vitellogenin) decreased when combined PUFA and LC-PUFA dietary ω6 fatty acids were proportionally higher relative to combined PUFA and LC-PUFA ω3 fatty acids at the evaluated levels of total lipid. In female fish, this relative increase in dietary ω6 fatty acids resulted in larger, leaner zebrafish with reduced adiposity. However, fat-free mass was highly variable, suggesting significant individual variation in the response to the proportionally higher level of ω6 fatty acids.
In fish and humans, ω3 and ω6 fatty acids compete for many of the same elongation enzymes that form both antiinflammatory and proinflammatory products. The antiinflammatory effects of ω3 fatty acids have been well documented in humans.2 Men and women who consume comparatively high levels of ω3 PUFA have lower cardiovascular morbidity and mortality.8 Dietary supplementation with ω3 decreases insulin resistance, triglyceride levels, heart rate, and blood pressure.3 The effects of dietary ω6 PUFA and LC-PUFA on inflammation are not as clear. One study12 suggested that ω6 PUFA increase inflammatory signals associated with cardiovascular disease. One potentially inflammatory product from the elongation of ω6 linoleic acid is arachidonic acid (20:4 ω6), which is a substrate for the production of proinflammatory, vasoconstrictive, and proaggregatory products such as prostaglandin E2, thromboxane A2, and leukotriene B4.11 Although this pathway has been hypothesized to link high dietary levels of ω6 PUFA and LC-PUFA to inflammation, few studies have identified a direct connection between dietary ω6 PUFA and LC-PUFA and inflammation in humans. In contrast, studies have shown that arachidonic acid serves as a precursor for a group of potent antiinflammatory–antiaggregatory products21 such as prostacyclin, lipoxin A4, and epoxyeicosatrienoic acids.11 In human diets in which the dietary fat content does not exceed 30% of the consumed energy, ω6 fatty acids may provide substantial health benefits.10
An increase in the level of dietary ω6 fatty acids relative to that of ω3 fatty acids has been identified as a possible factor contributing to the increase in obesity and the inflammatory diseases associated with metabolic syndrome among people in industrialized countries.22 However, in human diets, the effects of changing ratios of ω3:ω6 PUFA and LC-PUFA are often confounded by changing levels of total dietary lipid, which may sometimes mask the effects of ω3:ω6 ratios from PUFA and LC-PUFA.7 In the current study, we maintained total dietary lipid at approximately 12% as we varied the ω3:ω6 PUFA ratios. This level of fat has been shown to promote growth and survival in zebrafish.23 The American Heart Association currently recommends that no more than 30% of the total daily energy should be consumed as lipid10,11. Given the current results from zebrafish, the qualitative composition of the lipid contributing to that 30% level may play a critical role in influencing overall health, including terminal weight gain, fat content (obesity), and inflammation.
The specific ratio of ω3 and ω6 PUFA and LC-PUFA required in human and most animal diets continues to be debated. The mounting data suggest that the relationship between inflammation and dietary ratios of ω3:ω6 from PUFA and LC-PUFA is more complex than simply the ratios themselves. Because relatively small amounts of these dietary fatty acids are required, the total amount of dietary fatty acids consumed, possibly influenced by the ratio of ω3:ω6 consumed or the proportional contribution from PUFA and LC-PUFA sources, may be more important to cardiovascular health than is the ratio of ω3:ω6 from PUFA and LC-PUFA.26 One study18 found that in humans, dietary ω6 fatty acids do not inhibit the antiinflammatory effects of dietary ω3 fatty acids and that the lowest levels of inflammation occurred when ω6 and ω3 fatty acids both were included in the diet. This observation is supported by the data we collected in the current zebrafish experiment, which shows that changes in combined PUFA and LC-PUFA ratios alone are sufficient to cause significant changes in physiologic outcomes. Therefore, the greatest benefits from both ω3 and ω6 PUFA and LC-PUFA may be achieved only when both are present in the diet in correct proportions. Fully understanding the implication of lipid source on physiologic outcomes require additional data regarding how ω3:ω6 ratios from PUFA and LC-PUFA in association with diets of high lipid content, predominantly containing either saturated or unsaturated fats, determine physiologic outcomes.
Acknowledgments
We thank Nazia Mojiba for her technical assistance in molecular techniques and Mr Jeff Barry (UAB Nutrition Obesity Research Center Aquatic Animal Models Core) for feed preparation. This work was supported in part by a pilot/feasibility grant from the UAB Nutrition Obesity Research Center to MLP funded by grant no. P30DK056336.
References
- 1.Abi-ayad SMEA, Melard C, Kestemont P. 1997. Effects of ω3 fatty acids in Eurasian perch broodstock diet on egg fatty acid composition and larvae stress resistance. Aquaculture international 5:161–168. [Google Scholar]
- 2.Calder PC. 2006. ω3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier YA, Portois L, Malaisse WJ. 2006. ω3 fatty acids and the metabolic syndrome. Am J Clin Nutr 83:1499S–1504S. [DOI] [PubMed] [Google Scholar]
- 4.Chico TJ, Ingham PW, Crossman DC. 2008. Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc Med 18:150–155. [DOI] [PubMed] [Google Scholar]
- 5.Chou BS, Shiau SY. 1999. Both ω6 and ω3 fatty acids are required for maximal growth of juvenile hybrid tilapia. N Am J Aquac 61:13–20. [Google Scholar]
- 6.Cunnane SC. 2003. Problems with essential fatty acids:time for a new paradigm? Prog Lipid Res 42:544–568. [DOI] [PubMed] [Google Scholar]
- 7.Czernichow S, Thomas D, Bruckert E. 2010. ω6 fatty acids and cardiovascular health: a review of the evidence for dietary intake recommendations. Br J Nutr 104:788–796. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. 2006. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 91:439–446. [DOI] [PubMed] [Google Scholar]
- 9.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. [PubMed] [Google Scholar]
- 10.Fritsche KL. 2008. Too much linoleic acid promotes inflammation—doesn't it? Prostaglandins Leukot Essent Fatty Acids 79:173–175. [DOI] [PubMed] [Google Scholar]
- 11.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. 2009. ω6 fatty acids and risk for cardiovascular disease:a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119:902–907. [DOI] [PubMed] [Google Scholar]
- 12.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. 2006. Healthy intakes of ω3 and ω6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr 83:1483S–1493S. [DOI] [PubMed] [Google Scholar]
- 13.Lin B, Chen S, Cao Z, Lin Y, Mo D, Zhang H, Gu J, Dong M, Liu Z, Xu A. 2007. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection:striking similarities and obvious differences with mammals. Mol Immunol 44:295–301. [DOI] [PubMed] [Google Scholar]
- 14.Meinelt BT, Schulz C, Wirth M, Kürzinger H, Steinberg C. 1999. Dietary fatty acid composition influences the fertilization rate of zebrafish (Danio rerio Hamilton–Buchanan). J Appl Ichthyol 15:19–23. [Google Scholar]
- 15.Meinelt T, Schulz C, Wirth M, Kurzinger H, Steinberg C. 2000. Correlation of diets high in ω6 polyunsaturated fatty acids with high growth rate in zebrafish (Danio rerio). Comp Med 50:43–45. [PubMed] [Google Scholar]
- 16.Molendi-Coste O, Legry V, Leclercq IA. 2011. Why and how meet ω3 PUFA dietary recommendations? Gastroenterol Res Pract 2011:. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council (U.S.). Committee on the Nutrient Requirements of Fish and Shrimp 2011. Nutrient requirements of fish and shrimp. Washington (DC):National Academies Press. [Google Scholar]
- 18.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. 2003. Habitual dietary intake of ω3 and ω6 fatty acids in relation to inflammatory markers among US men and women. Circulation 108:155–160. [DOI] [PubMed] [Google Scholar]
- 19.Rett BS, Whelan J. 2011. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Westerωtype diets: a systematic review. Nutr Metab (Lond). 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago CB, Reyes OS. 1993. Effects of dietary lipid source on reproductive performance and tissue lipid levels of Nile tilapia Oreochromis niloticus (Linnaeus) broodstock. J Appl Ichthyol 9:33–40. [Google Scholar]
- 21.Serhan CN. 2007. Resolution phase of inflammation:novel endogenous antiinflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25:101–137. [DOI] [PubMed] [Google Scholar]
- 22.Simopoulos AP. 2008. The importance of the ω6:ω3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233:674–688. [DOI] [PubMed] [Google Scholar]
- 23.Smith DL, Jr, Barry RJ, Powell ML, Nagy TR, D'Abramo LR, Watts SA. 2013. Dietary protein source influence on body size and composition in growing zebrafish. Zebrafish 10:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. 2009. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res 104:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts SA, Powell M, D'Abramo LR. 2012. Fundamental approaches to the study of zebrafish nutrition. ILAR J 53:144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijendran V, Hayes KC. 2004. Dietary ω6 and ω3 fatty acid balance and cardiovascular health. Annu Rev Nutr 24:597–615. [DOI] [PubMed] [Google Scholar]