Abstract
Göttingen minipigs are a useful model for diseases having an inflammatory component, and the associated use of acute-phase proteins (APP) as biomarkers of inflammation warrants establishment of their reference ranges. The objective of this study was to establish reference values for selected APP in Göttingen minipigs and to investigate the effects of age, sex, and various stimuli on these ranges. Serum concentrations of C-reactive protein (CRP), serum amyloid A (SAA), haptoglobin, pig major acute-phase protein (PMAP), albumin, and porcine α-1 acid glycoprotein (PAGP) were evaluated in 4 age groups (6, 16, 24 and 40–48 wk) of male and female Göttingen minipigs. In addition, minipigs were tested under 2 housing conditions, after acute LPS challenge, and after diet-induced obesity with and without mild diabetes. Changing the pigs to a new environment induced significant increases in CRP, PMAP, haptoglobin and PAGP and a decrease in albumin. An acute LPS stimulus increased CRP, PMAP, haptoglobin, and SAA; PAGP was unchanged and albumin decreased. Obese pigs with and without diabetes showed increases in CRP and PAGP, albumin decreased, and haptoglobin and SAA were unchanged. PMAP was increased only in obese pigs without diabetes. In conclusion, reference values for CRP, PMAP, haptoglobin, SAA, PAGP and albumin were established for male and female Göttingen minipigs of different ages. These APP were influenced by age and sex, underlining the importance of considering these factors when designing and interpreting studies including aspects of inflammation. In addition, an APP response was verified after both acute and chronic stimuli.
Abbreviations: APP, acute-phase proteins; APR, acute-phase response; CRP, C-reactive protein; HFD, high-fat diet; HFD+D, high fat diet + diabetes; PAGP, porcine α1 acid glycoprotein; PMAP, pig major acute-phase protein; SAA, serum amyloid A
Inflammation is involved in a number of important and increasingly widespread human diseases, including inflammatory bowel diseases, cancers, infections, metabolic diseases like obesity and diabetes, and cardiovascular diseases like atherosclerosis.1,5,7,11,20,41 The systemic response to inflammation is the acute-phase response (APR) which, together with innate immune responses, prevents infection, clears pathogens, and contributes to inflammation resolution and the healing process. The APR has been extensively described in humans10,22 and other mammals,8,14,29,31 and in all cases, it is regulated by cytokines including IL6 and TNFα.21,30 The APR is activated by many different stimuli, including trauma, infection, stress, neoplasia, and inflammatory stimuli, resulting in significant changes in the circulating concentrations of the so-called acute-phase proteins (APP). The APP are synthetized primarily by the liver and can be divided into positive and negative APP depending on whether their concentration in plasma increases (positive) or decreases (negative) in response to a stimulus.10 In addition, they can be divided into major and minor APP, depending on the magnitude of their concentration change after a given stimulus.22 Because the concentrations of the APP change in response to a given stimulus, their serum or plasma levels can be used diagnostically as biomarkers of disease severity and progression or to evaluate the effect of various interventions.8,14,31 The APP show different kinetics after a stimulus, with C-reactive protein (CRP) and serum amyloid A (SAA) displaying rapid increases and normalization after the stimulus has been removed, whereas haptoglobin shows a later and more prolonged response.10,31 The APR may be transient and revert to normal with recovery, or it can persist, as during chronic conditions.21 Importantly, APP and their kinetics differ somewhat between species.31
To further elucidate the involvement of inflammation in human diseases, accurate animal models of inflammation, including species validated biomarkers of inflammation, are needed. Mouse models are commonly used in many research areas, but their response to several different inflammatory conditions is not comparable to that of humans, and therefore the predictive validity of these models may be limited.39 Pigs are highly comparable to humans with respect to anatomy and physiology,44 and their APR to various stimuli has been described.14,23,26 In general, the APR and the resulting changes in APP seem to be very similar in pigs compared with humans, with CRP, haptoglobin, and SAA being major positive APP and albumin being a negative APP.14 In humans, α1-acid glycoprotein (AGP) is a positive APP but has been reported to either increase,17 remain unchanged23,45 or to decrease12 in pigs, depending on the stimulus investigated. The concentrations of some of the major APP characterized in domestic pigs show significant effects of age and sex.32,34 In addition to age and sex effects, significant differences in APP between herds have been observed, most likely reflecting different pathogenic pressures in the different herds.32 Furthermore, some indications exist for possible interbreed differences in APP concentrations, although this possibility has not been investigated in detail.12
Minipigs are especially relevant in biomedical research, given their smaller size and well-defined microbiology and genetics.4 Göttingen minipigs are a useful model for several conditions involving inflammation and the APR, including infection,2 obesity,19 diabetes24 and atherosclerosis,18 and different APP have already been used as biomarkers in some of these models.2 Therefore, existing data suggest that APP commonly applied in human medicine could be relevant in Göttingen minipigs as well. However, the APR and reference values of APP, including the potential influence of age and sex indicated in other studies, have not been investigated systematically in this breed.12,32,34
The objective of the current study was to establish reference values of selected APP in normal Göttingen minipigs, including evaluation of the possible effects of age and sex. In addition, the effects of housing condition and acute and chronic inflammatory stimuli were assessed.
Materials and Methods
All animal experiments were approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark.
Animals, diets and housing conditions.
Reference values in Göttingen minipigs and influence of age and sex.
This study was performed in healthy, lean Göttingen minipigs (Ellegaard Göttingen Minipigs A/S, Dalmose, Denmark; n = 120). The study used 4 age groups (6, 16, 24 and 40 to 48 wk) each comprising 30 (15 male, 15 female) pigs. The age groups thus include animals both before and after sexual maturity, which is reached at 4 to 5 mo for female and 3 to 4 mo for male Göttingen minipigs.3 At the Ellegaard facility, the pigs were housed in groups of 2 to 15 according to animal size, were restrictively fed with a commercial minipig diet (Special Diets Services, Essex, UK) according to the breeder's guidelines without additional supplementation, and had free access to water.
The Ellegaard facility is a barrier facility that only houses minipigs bred within the facility, and no pigs are allowed to enter the facility. The barrier has HEPA-filtered ventilation with 100% fresh air, disinfection locks for diet and materials, shower locks for personnel, and a closed manure system. All diet is heat-treated, water is UV-radiated, and the bedding material is irradiated and derived from designated fields to prevent environmental contamination. Thus, the environment and minipigs at the Ellegaard facility are highly controlled and well-defined microbiologically (www.minipigs.dk).
With the pigs in dorsal recumbency, blood from the cranial caval vein was collected into uncoated tubes and stored at 5 °C overnight. The tubes were centrifuged at 1000 × g for 10 min at room temperature, and serum was stored at –20 °C.
Influence of housing condition.
Female Göttingen minipigs (Ellegaard Göttingen Minipigs A/S; age, 7 to 8 mo; n = 22) underwent blood sampling at the Ellegaard facility as described earlier and at 3 to 4 wk after transfer to Novo Nordisk A/S (Ganløse, Denmark), where they were individually housed on wood shavings and straw. The minipigs had free access to water and were fed once daily (360 g; SDS Minipig Diet); apples and yoghurt were provided daily. The Novo Nordisk facility houses both minipigs and domestic pigs. At the new facility, blood samples were left to clot for 1 h at room temperature, followed by centrifugation at 1942 × g and 4 °C for 10 min. The resulting serum was frozen on dry ice and stored at –20 °C until analysis. In addition, the pigs were observed once daily and subsequently divided into 2 groups based on their clinical symptoms after arrival to the purchaser facility: one group that had diarrhea within 1 wk before blood sampling (n = 9); the other group that were free of clinical symptoms from at least 1 wk before until 3 d after blood sampling (n = 13).
APP response to an acute inflammatory stimulus (LPS).
This study was performed using male Göttingen minipigs (Ellegaard Göttingen Minipigs A/S) approximately 10 mo of age (n = 5). The animals were housed individually at Novo Nordisk A/S (Ganløse, Denmark) and fed once daily (420 g SDS Minipig Diet) and with free access to water. After 3 wk of acclimation and at 19 wk before the LPS challenge, the pigs were implanted in the caudal caval vein with permanent intravenous catheters (Redo TPN Catheters, William Cook Europe Aps, Bjæverskov, Denmark) according to previously described principles.36 Prior to the LPS challenge, the pigs had been used in different unrelated pharmacologic studies followed by a washout period of 12 wk. For the LPS challenge (5 μg/kg), Escherichia coli serotype K-235 LPS (Sigma–Aldrich Denmark A/S, Brøndby, Denmark) was dissolved in sterile saline to a final concentration of 50 μg /mL, and each pig received 0.1 mL/kg IV. Blood was sampled from the central venous catheter before dosing and at 0.5, 1, 2, 4, 8, 12, 24, 48, 96, 168, 216, and 264 h after LPS dosing. The blood was transferred to EDTA-coated tubes that were kept on ice until centrifugation for 10 min at 1942 × g and 4 °C. The plasma was frozen on dry ice and kept at –20 °C until analysis. Only plasma from time points 0, 0.5, 12, 24, and 48 h was analyzed in the present study. In case of treatment-requiring clinical symptoms after the LPS injection, a single intramuscular injection of metacam (20 mg/mL, 0.4 mg/kg, Boehringer Ingelheim Danmark A/S, Copenhagen, Denmark) was given.
APP response to a chronic inflammatory condition (diet-induced obesity with or without hyperglycemia).
The study was performed in castrated male Göttingen minipigs (Ellegaard Göttingen Minipigs A/S). The minipigs were individually housed at the animal facility (University of Copenhagen, Frederiksberg, Denmark). Three groups were evaluated: a control group fed the SDS Minipig Diet (n = 6), a group fed a high-fat, high-fructose, and high-cholesterol diet (HFD; Europe product 5B4L, TestDiet, St Louis, MO; n = 6), and a group in which mild diabetes was induced after a minimum 18 wk of HFD-feeding (HFD+D, n = 11). Diabetes was induced by using a high-dose IV streptozotocin (125 mg/kg) combined with nicotinamide (67 mg/kg), as previously described.24 The pigs were fed twice daily with a total daily amount corresponding to 2% to 2.5% of their body weight, evaluated weekly. After 37 wk of diet-feeding, when the pigs were approximately 11 mo old, 6-mL blood samples were collected from the cranial caval vein of dorsally recumbent pigs into an uncoated tube and an EDTA-coated tube. The uncoated tubes were left at room temperature for 1 h, followed by centrifugation for 10 min at 2000 × g and 4 °C. The resulting serum was kept at –80 °C until analysis. The EDTA-coated tubes were kept on wet ice until centrifugation for 10 min at 2000 × g and 4 °C. The plasma was stored at –20 °C until plasma albumin analysis. The characteristics of the pigs approximately at the time of blood sampling with regard to body weight, body fat percentage measured by dual-energy X-ray absorptiometry, and fasting plasma glucose are shown in Table 1.
Table 1.
Background characteristics in diet-induced obese (HFD) and diet-induced obese and diabetic (HFD+D) pigs
| Control (n = 6) | HFD (n = 5) | HFD+D (n = 11) | |
| Body weight, kg | 24.0b (22.6–25.3) | 56.0c (50.7–60.7) | 53.4c (52.0–56.6) |
| Body fat, % | 17.9b (15.2–20.9) | 49.1c (46.8–51.5) | 50.6c (48.6–51.4) |
| Glucose,a mmol/L | 3.9b (3.6–6.7) | 4.0b (3.7–4.1) | 9.4c (6.7–13.8) |
| Total cholesterol, mmol/L | 1.8b (1.5–2.8) | 20.3c (11.6–31.4) | 18.9c (16.6–22.6) |
Data are presented as median and interquartile range (25th–75th percentiles). Intergroup differences were evaluated by one-way ANOVA with Tukey–Kramer posthoc testing (or Kruskal–Wallis testing for nonparametric dataa).
b,cValues with different superscript letters are significantly (P < 0.05) different.
ELISA analysis.
All analyses were performed at the National Veterinary Institute (Technical University of Denmark). The plasma or serum concentrations of CRP and haptoglobin were measured by using an inhouse sandwich ELISA, as described previously.13,43 The detection limits of these assays were 354 ng/mL for CRP and 13 μg/mL for haptoglobin.
PAGP concentrations were measured in a previously described competitive inhouse ELISA12 using a mouse monoclonal antibody specific for PAGP. Biotinylated AGP was applied immediately after the samples were added. An inhouse pig serum pool was used as a standard. The detection limit for this assay was 10 μg/mL. The SAA concentrations were measured with a commercially available sandwich ELISA kit (Phase SAA assay, Tridelta Development, Wicklow, Ireland) in accordance with the manufacturer's instructions. The detection limit was 31.25 μg/mL (porcine SAA equivalents). Likewise, the concentrations of PMAP were measured by using a commercially available kit (PigCHAMP Pro Europa, Segovia, Spain) in accordance with the manufacturer's instructions. The detection limit for this assay was 43.8 μg/mL. All samples were run in duplicate, and each assay had a calibrated standard curve. For all assays, the absorbance was read at 450 nm with the subtraction of 650 nm on an automatic plate reader (Multiskan, ThermoScientific, Waltham, MA). Only results that yielded a coefficient of variation less than 15% between duplicate samples were used; otherwise samples were retested. Furthermore, samples with optical density values at the low or high end of the calibration curve were rerun at other dilutions.
Albumin analysis.
The albumin concentrations in the serum or plasma samples were measured on a Cobas6000 (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. The assay range was from 2 to 60 g/L; samples above 60 g/L were diluted 1:3 and retested.
Calculations and statistical analyses.
Statistical analyses were performed in SAS version 9.2 or 9.3 (SAS Institute, Cary, NC). Overall, a P value less than 0.05 was considered significant. When a measured value was below the lower limit of quantification of the assay (primarily important for SAA), it was set to that value. Data were in all cases transformed when appropriate to achieve normal distribution of data and homogeneity of residuals. All post hoc comparisons were adjusted for multiple testing.
The reference values were analyzed by ANOVA, with age, sex and the interaction between age and sex as independent variables. ANOVA was followed by Tukey's multiple-comparison test, and relevant posthoc comparisons were made between all age groups of the same sex and between the 2 sexes in each age group.
The overall comparison of the 2 housing conditions was done by using paired t tests. Because many SAA data points were below detection limit of the assay (31.25 μg/mL), they were set to 31.25 μg/mL, and the paired nonparametric Wilcoxon matched-pairs signed-rank test was used. Data were analyzed both together and separately for the pigs with and without diarrhea.
In the study on acute inflammation, ANOVA with pig and time as factors was used for haptoglobin, albumin, PMAP, PAGP, and CRP. For a nonspecific comparison of time points, the ANOVA F-test for time was calculated, whereas specific comparisons at each time point compared with the 0-h sample was done by using the Dunnett multiple-comparison test. For SAA, basic assumptions for ANOVA were not fulfilled (that is, no variation at t = 0 h and t = 0.5 h because all values were set to lower limit of quantification of the assay); therefore this parameter was not included in the statistical analysis.
In the study on chronic inflammation, overall groupwise differences of the APP were evaluated by ANOVA followed by Tukey–Kramer posthoc testing. When homogeneity of residuals could not be achieved, a Kruskal–Wallis test was applied followed by Wilcoxon signed-rank posthoc testing.
Results
Reference values.
All reference values are given as medians with interquartile ranges and P values for the overall statistical model (Table 2). Posthoc group comparisons are described in the following paragraphs.
Table 2.
Serum concentrations of acute-phase proteins in male and female Göttingen minipigs of different ages
| Male |
Female |
|||||||
| 6 wk | 16 wk | 24 wk | 40–48 wk | 6 wk | 16 wk | 24 wk | 40–48 wk | |
| CRP,b μg/mL | 5.77 (4.42–7.19) | 7.21 (4.59–21.35) | 5.50 (2.27–10.02) | 3.63 (2.84–13.45) | 12.2 (5.87–17.75) | 3.28 (2.59–6.12) | 10.18 (5.83–15.80) | 8.47 (6.58–17.85) |
| Haptoglobin,c μg/mL | 6145.6 (4653.8– 7070.8) | 305.3 (21.4– 1440.6) | 248.9 (13– 1165.7) | 34.7 (13– 2203.1) | 7294.0 (4795.7–8783.8) | 2284.1 (1821.0– 3220.2) | 2521.0 (1733.9– 3693.6) | 3018.4 (1634.6–4082.3) |
| PMAP,c μg/mL | 1021.0 (771.6– 1270.3) | 708.0 (554.0– 901.6) | 682.5 (583.4– 976.5) | 740.4 (609.8–1107.8) | 792.7 (588.9– 1229.3) | 157.4 (129.5– 173.5) | 247.0 (192.5– 491.8) | 184.2 (140.3– 209.5) |
| PAGP,d,e μg/mL | 344.2 (251.5–530.3) | 409.6 (344.9–472.6) | 329.5 (264.0–380.4) | 287.0 (227.0–413.9) | 545.2 (305.2–640.8) | 563.7 (457.3–771.8) | 298.5 (222.8–443.2) | 413.0 (316.3–556.6) |
| Albumin,a g/L | 44.3 (42.7–49.8) | 41.8 (40.7–48.2) | 50.7 (45.6–54.3) | 48.6 (38.4–58.7) | 44.4 (41.9–55.5) | 49.1 (43.9–56.5) | 46.4 (44.4–58.7) | 68.1 (55.5–73.8) |
CRP, C-reactive protein; PAGP, porcine α-1 acid glycoprotein; PMAP, pig major acute-phase protein.
Values are expressed as median with interquartile range (n = 15). All dependent variables were log transformed. Intergroup differences were evaluated by two-way ANOVA (sex, age and the interaction between the two as factors) followed by Tukey's multiple-comparison test. Significant effect of age × sex: aP < 0.05, bP < 0.01, cP < 0.001; dsignificant (P < 0.001) effect of age; esignificant (P < 0.001) effect of gender.
Because most values for serum amyloid A were below the lower limit of quantification (31.25 µg/mL), no statistical analysis was performed.
CRP showed a significant interaction between age and sex. Among female minipigs, those 16 wk old had significantly lower CRP values than did all other age groups (P < 0.05 for all); no age-associated differences in CRP values were found in the male minipigs. No difference between sexes was found within any age group.
Like CRP, haptoglobin, PMAP, and albumin showed significant interaction between age and sex. Haptoglobin concentrations were significantly higher in 6-wk-old male minipigs than in male pigs in all other age groups (P < 0.001 for all). In the 16-, 24-, and 48-wk age groups, female minipigs had significantly higher haptoglobin concentrations than did age-matched male pigs (P < 0.001 for all). For PMAP, female minipigs 6 wk of age had significantly higher PMAP concentrations than did female minipigs in all other age groups (P < 0.001 for all). Moreover, 24-wk-old female minipigs had higher PMAP concentrations than did both 16-wk-old (P < 0.001) and 48-wk-old (P < 0.05) female pigs. Male minipigs that were 6 wk old had higher PMAP concentrations than did 16-wk-old male pigs, and males aged 16, 24, and 48 wk had significantly higher PMAP concentrations than did age-matched female pigs (P < 0.001 for all). Regarding albumin, female minipigs 48 wk in age had higher albumin concentrations than did female pigs in all other age groups (P < 0.05 for all). In addition, 48-wk-old female minipigs had higher albumin concentrations than did 48-wk-old male pigs (P < 0.01), whereas albumin concentrations did not differ between the sexes in any of the other age groups .
PAGP showed a significant effect of both age and sex. Overall, female minipigs had higher PAGP concentrations than did male pigs (P < 0.001), and PAGP concentrations were higher at 16 wk of age compared with 24 and 48 wk of age (P < 0.001 and P < 0.01, respectively) and higher at 6 wk of age compared with 24 wk of age (P < 0.05).
In almost all pigs, SAA values were below the detection limit of the assay and therefore were not analyzed statistically in this part of the study.
Influence of housing condition.
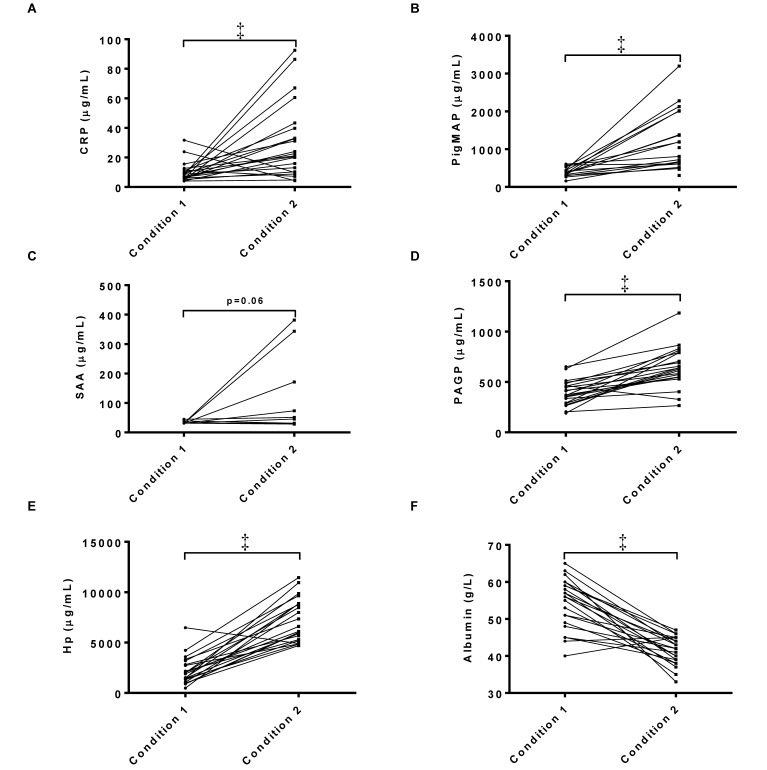
According to the overall statistical analysis, PAGP, CRP, PMAP and haptoglobin increased (P < 0.05) when pigs were exposed to a new housing condition, albumin was decreased (P < 0.05), and no significant changes occurred in SAA (P = 0.06; Figure 1). These differences were present both when data were analyzed together and when they were analyzed separately for the pigs with and without diarrhea (data not shown).
Figure 1.
Serum concentrations of (A) CRP, (B) PMAP, (C) SAA, (D) PAGP, (E) haptoglobin, and (F) albumin in pigs moved from one housing condition (condition 1) to a new facility (condition 2, n = 17–22). Note that many of the SAA values were below the lower limit of quantification for the assay (31,250 ng/mL) and therefore were set to that value. Data are shown as individual values. Paired t test (all but SAA) or Wilcoxon matched-pairs signed-rank test (SAA); ‡, P < 0.001.
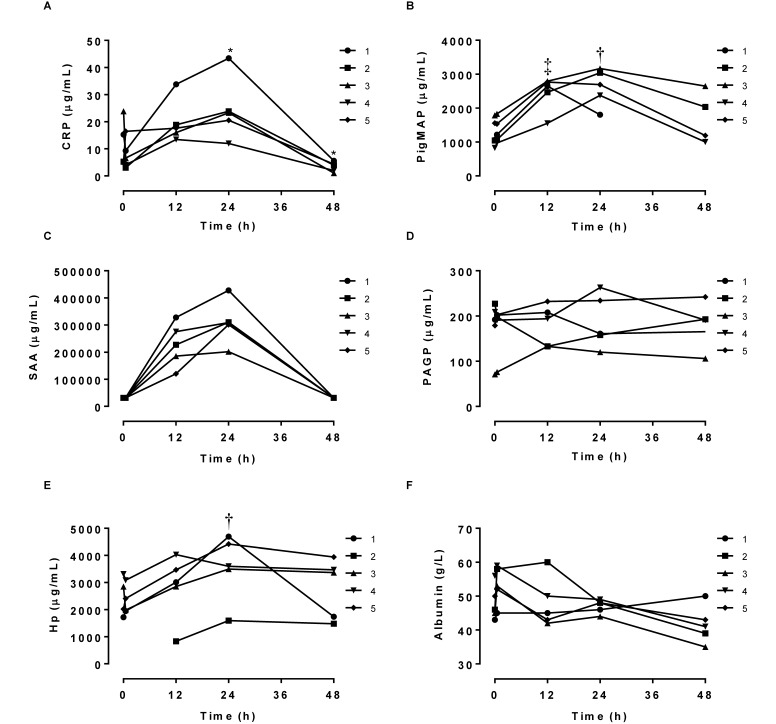
APP response to LPS, an acute inflammatory stimulus.
The LPS injection induced clinical symptoms including tremor, hyperventilation, and signs of nausea, which were treated with a single intramuscular injection of metacam. The positive APP CRP, haptoglobin, PMAP, and SAA all increased after injection, reaching significantly (P < 0.05) higher concentrations at 24 h postinjection compared with preinjection values (Figure 2). PMAP was increased (P < 0.05) at 12 h postinjection as well. CRP was lower at 48 h after injection compared with before injection. Albumin showed an overall significant decrease over time (F-test P < 0.05, Figure 2), although there were no significant differences in the post hoc analysis. No significant differences were found for PAGP. SAA could not be analyzed statistically because many values were below the assay's limit of detection; however, data in Figure 2 suggest that SAA increased over time in response to the LPS stimulus.
Figure 2.
Individual serum concentrations of (A) CRP, (B) PMAP, (C) SAA, (D) PAGP, (E) haptoglobin (Hp), and (F) albumin after challenge with LPS (n = 4 or 5). Two-way ANOVA (pig and time as factors) followed by Dunnet multiple-comparison testing (*, P < 0.05; †, P < 0.01; ‡, P < 0.001 compared with value at time 0). SAA was not evaluated statistically because the basic assumptions for ANOVA were not fulfilled.
APP response to diet-induced obesity with or without hyperglycemia, which are chronic inflammatory conditions.
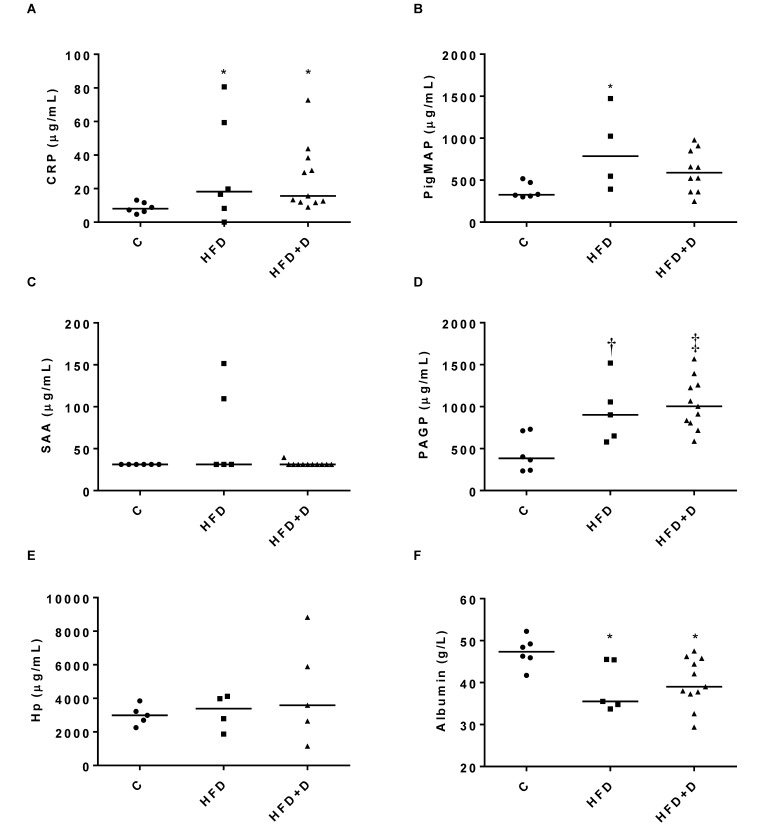
One pig in the HFD group was excluded from the study because macroscopic and microscopic findings indicated that this animal had polyarthritis nodosa, which might have affected the pig's baseline inflammatory state. PMAP was not measured in one pig in the HFD group and in one animal in the HFD+D group, because of insufficient serum for this analysis. Several of the haptoglobin values were below the lower limit of quantitation for the assay; all of these were set to this lower limit (13 µg/mL) in both the figures and the statistical analysis.
The experimental diet and subsequent obesity (Table 1) led to significant changes in the APP compared with those in control animals (P <0.05), but there were no significant differences between the HFD and HFD+D groups in any of the measured parameters (Figure 3). Compared with levels in control animals, CRP and PAGP were both increased (P <0.05) in HFD and HFD+D pigs, PMAP was increased (P <0.05) in HFD pigs and albumin was decreased (P <0.05) in both the HFD and HFD+D groups; no significant changes in haptoglobin or SAA were noted.
Figure 3.
Serum concentrations of (A) CRP, (B) PMAP, (C) SAA, (D) PAGP, (E) haptoglobin (Hp), and (F) albumin in lean control pigs (C, n = 6), obese pigs fed a high-fat, high-fructose, and high-cholesterol diet (HFD, n = 5), and pigs with mild diabetes that were fed the HFD (HFD+D, n = 11). Individual and median data (n = 5–11). SAA data were evaluated by Kruskal–Wallis testing. CRP, PAGP, haptoglobin, and PMAP were log transformed and evaluated by one-way ANOVA (*, P < 0.05; †, P < 0.01; ‡, P < 0.001). Due to insufficient serum, PMAP values are missing for one animal each in the HFD and HFD+D groups.
Discussion
In the present study, most of the evaluated APP showed significant age- and sex-associated differences in Göttingen minipigs from 6 to 48 wk of age. Changing the environment for the pigs led to induction of an APR with significant increases in CRP, PMAP, haptoglobin, and PAGP concentrations and a decrease in albumin concentration. An acute inflammatory stimulus (LPS) increased CRP, PMAP, haptoglobin and SAA; albumin tended to decrease; whereas no difference was found in PAGP. A chronic inflammatory stimulus (HFD with or without diabetes induction) increased CRP, PMAP, PAGP and decreased albumin; no significant changes were observed in haptoglobin and SAA.
An effect of age on APP has previously been described in domestic pigs at different ages ranging from 2 d to 19 wk12,34 and humans.28 In humans, the age-related increase in inflammatory markers is related both to declining sex hormone levels and changes in body composition and fat distribution,42 which may apply to other species as well. The effects of sex and sex hormones on APP concentrations are, however, not well described in the literature. In the present study, the sex-associated effect was not the same for all APP. For example for PMAP, male minipigs had higher concentrations of APP than did female pigs from 16 wk and on, whereas, for haptoglobin, the female minipigs had the highest concentrations in these same age groups. Similar effects of sex on PMAP and haptoglobin have been described in domestic pigs32 whereas, other studies found no effect of sex on CRP, haptoglobin, SAA, and PMAP.34 These differences may reflect the age interval studied, given that our present data indicate that the sex-associated differences are age-dependent or potentially related to the changes in sex hormone levels that occur at the time of sexual maturation (at 3 to 4 mo of age for male Göttingen minipigs and 4 to 5 mo of age for female minipigs of this breed). In humans, sex hormones influence components of the APR, for example IL627,35 and CRP.40 In one study,40 testosterone was negatively associated with CRP in men, whereas the use of oral contraceptives was positively correlated with CRP in women. Apart from these seemingly direct effects of sex hormones, whether of endo- or exogenous origin, secondary sex-related characteristics like fat mass and fat distribution have also been shown to contribute to the sex differences observed in CRP concentrations.40,42 Therefore, both sex hormone concentrations and secondary sex characteristics seem to play a role in the age- and sex-associated effects on the APR in humans. The same could be true in Göttingen minipigs, given that they show significant age- and sex-dependent differences in sex hormone concentrations, fat mass, and fat distribution.6 In any case, the present study underlines the need for taking age and sex into account in studies including APP responses. For instance, measuring several APP has been shown to increase the ability to detect clinical and subclinical conditions in pigs,14 and adding age and sex into this equation might further improve sensitivity.
The APR induced by changing the pigs to a new environment was expected and is considered to be a normal healthy response to a changed microbial flora. At the breeder facility (Ellegaard Göttingen Minipigs A/S), the minipigs are kept in a microbiologically defined environment with a limited number of pathogens present, potentially rendering the pigs vulnerable to changes in pathogens after transfer to a different housing facility. The APP changed in the same manner in animals with and without diarrhea, suggesting such changes are not always related to visible clinical signs. However, whether the APP concentrations would return to baseline after a longer acclimation period or whether they would remain on a new, higher level corresponding to the new environment is unknown. Regardless, the duration of the acclimation period could be important in studies where the APP themselves are investigated or are expected to influence the read-outs of the study.
After the acute LPS challenge, CRP, PMAP, haptoglobin, and SAA levels were increased, an overall decrease in albumin occurred, and no difference was found in PAGP. PAGP data are in accordance with data from studies on LPS effects on domestic pigs, in which no change in PAGP was observed.45 Likewise, CRP and haptoglobin were increased in the saliva of crossbred pigs after repeated LPS injections.9 The changes in APP concentrations observed in Göttingen minipigs are thus as expected from other published studies in pigs, and although obtained from a limited number of animals, our current results provide evidence that the APP investigated can be used as inflammatory markers in Göttingen minipigs as well. The absolute levels can, however, not be used for reference, given that the minipigs also received metacam, which might have dampened the response.
In the present study, increases in CRP, PAGP (HFD and HFD+D groups), and PMAP (HFD group only) and decreases in albumin occurred after a prolonged period (37 wk) of feeding a HFD, indicating that a chronic inflammatory state was obtained in these groups of pigs. The observed changes in APP concentrations may be due to the diet itself, to the resulting obesity, or to the subsequent metabolic changes in the 2 HFD-fed groups (Table 1). In one study,37 gene expression and serum concentrations of different cytokines and APP were measured in old lean and obese Göttingen minipigs fed a standard pig chow, and no significant differences were found in serum concentrations of CRP, SAA, and haptoglobin between the 2 groups. This result is in clear contrast to the present study, to what is seen in obese Ossabaw minipigs fed a HFD,38 and to the situation in obese humans.1,5,33 However, the contradictory findings37 may indicate that the diet-induced metabolic changes, including possible differences in liver involvement,25 rather than obesity itself, are important.
The serum concentration of PAGP has been investigated in several porcine breeds.12,23,38,45 In one study,12 PAGP was found to be a negative APP in conventional pigs exposed to different infectious challenges and turpentine injection; this finding is in contrast to the human situation15 and to earlier data from pigs with infectious diseases.17 In other studies,23,45 no change in PAGP concentrations occurred after injection with LPS or turpentine, whereas PAGP concentrations were increased in obese, diet-fed Ossabaw minipigs.38 In the present study, PAGP and other positive APP increased when the pigs were moved to a new housing condition and after a period with diet-induced chronic inflammation, indicating that PAGP is a positive APP in Göttingen minipigs. However, the PAGP concentration did not change after an acute LPS challenge despite significant increases in the other APP, perhaps indicating that PAGP is more sensitive to chronic inflammatory conditions compared with an acute inflammatory stimulus. This differential sensitivity of some APP to acute and chronic stimuli has also been described in cattle.16
In summary, reference values for CRP, PMAP, haptoglobin, SAA, PAGP, and albumin were established for Göttingen minipigs. In addition to providing evidence that Göttingen minipigs display APP responses comparable to those in other pig breeds, this study provides important information on factors that influence the APP levels in Göttingen minipigs. All the APP measured were influenced by age, sex, or both factors, with the most important changes seen in PMAP and haptoglobin between 6 and 16 wk of age. In addition, the PAGP data indicate that this APP responds differently to acute and chronic stimuli in Göttingen minipigs, thus highlighting the importance of choosing the correct biomarker depending on the stimulus applied. The current study highlights some important aspects that need to be considered when designing studies and using APP as biomarkers. Additional investigation of the influence of age and sex on APP may refine the use of these biomarkers in humans, as well.
Acknowledgments
We thank laboratory technicians Lotte Schmidt Marcher, Inge Rubach, and Pia Skaarup Andersen; veterinarian Laust Peter Gade; and the staff at Ellegaard Göttingen Minipigs A/S for their excellent technical assistance during blood sampling and albumin measurements. We thank Heidi Gertz Andersen (National Veterinary Institute, Denmark) for her excellent help with the ELISA-based protein analyses.
References
- 1.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. 2006. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12. [PubMed] [Google Scholar]
- 2.Birck MM, Pesonen E, Odermarsky M, Hansen AK, Persson K, Frikke-Schmidt H, Heegaard PM, Liuba P. 2011. Infection-induced coronary dysfunction and systemic inflammation in piglets are dampened in hypercholesterolemic milieu. Am J Physiol Heart Circ Physiol 300:H1595–H1601. [DOI] [PubMed] [Google Scholar]
- 3.Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J, Steering Group of the RETHINK Project 2010. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods 62:196–220. [DOI] [PubMed] [Google Scholar]
- 4.Bollen P, Ellegaard L. 1997. The Gottingen minipig in pharmacology and toxicology. Pharmacol Toxicol 80 Suppl 2:3–4. [DOI] [PubMed] [Google Scholar]
- 5.Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, Lopez-Soriano J, Centoni R, Ciccarone AM, Benzi L, Vitti P, Del Prato S, Pinchera A, Maffei M. 2004. Serum haptoglobin: a novel marker of adiposity in humans. J Clin Endocrinol Metab 89:2678–2683. [DOI] [PubMed] [Google Scholar]
- 6.Christoffersen B, Golozoubova V, Pacini G, Svendsen O, Raun K. 2013. The young Gottingen minipig as a model of childhood and adolescent obesity: influence of diet and sex. Obesity (Silver Spring) 21:149–158. [DOI] [PubMed] [Google Scholar]
- 7.Correale M, Brunetti ND, De Gennaro L, Di Biase M. 2008. Acute-phase proteins in atherosclerosis (acute coronary syndrome). Cardiovasc Hematol Agents Med Chem 6:272–277. [DOI] [PubMed] [Google Scholar]
- 8.Cray C, Zaias J, Altman NH. 2009. Acute-phase response in animals: a review. Comp Med 59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 9.Escribano D, Campos PH, Gutierrez AM, Le Floc'h N, Ceron JJ, Merlot E. 2014. Effect of repeated administration of lipopolysaccharide on inflammatory and stress markers in saliva of growing pigs. Vet J 200:393–397. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. [DOI] [PubMed] [Google Scholar]
- 11.Grimm G, Haslacher H, Kampitsch T, Endler G, Marsik C, Schickbauer T, Wagner O, Jilma B. 2009. Sex differences in the association between albumin and all-cause and vascular mortality. Eur J Clin Invest 39:860–865. [DOI] [PubMed] [Google Scholar]
- 12.Heegaard PM, Miller I, Sorensen NS, Soerensen KE, Skovgaard K. 2013. Pig α1-acid glycoprotein: characterization and first description in any species as a negative acute-phase protein. PLOS ONE 8:e68110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heegaard PM, Pedersen HG, Jensen AL, Boas U. 2009. A robust quantitative solid phase immunoassay for the acute-phase protein C-reactive protein (CRP) based on cytidine 5′-diphosphocholine-coupled dendrimers. J Immunol Methods 343:112–118. [DOI] [PubMed] [Google Scholar]
- 14.Heegaard PM, Stockmarr A, Pineiro M, Carpintero R, Lampreave F, Campbell FM, Eckersall PD, Toussaint MJ, Gruys E, Sorensen NS. 2011. Optimal combinations of acute-phase proteins for detecting infectious disease in pigs. Vet Res 42:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochepied T, Berger FG, Baumann H, Libert C. 2003. α1-acid glycoprotein: an acute-phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev 14:25–34. [DOI] [PubMed] [Google Scholar]
- 16.Horadagoda NU, Knox KM, Gibbs HA, Reid SW, Horadagoda A, Edwards SE, Eckersall PD. 1999. Acute-phase proteins in cattle: discrimination between acute and chronic inflammation. Vet Rec 144:437–441. [DOI] [PubMed] [Google Scholar]
- 17.Itoh H, Tamura K, Izumi M, Motoi Y, Kidoguchi K, Funayama Y. 1993. The influence of age and health status on the serum α1-acid glycoprotein level of conventional and specific pathogen-free pigs. Can J Vet Res 57:74–78. [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsson L. 1989. Comparison of experimental hypercholesterolemia and atherosclerosis in male and female minipigs of the Gottingen strain. Artery 16:105–117. [PubMed] [Google Scholar]
- 19.Johansen T, Hansen HS, Richelsen B, Malmlof R. 2001. The obese Gottingen minipig as a model of the metabolic syndrome: dietary effects on obesity, insulin sensitivity, and growth hormone profile. Comp Med 51:150–155. [PubMed] [Google Scholar]
- 20.Knutson CG, Mangerich A, Zeng Y, Raczynski AR, Liberman RG, Kang P, Ye W, Prestwich EG, Lu K, Wishnok JS, Korzenik JR, Wogan GN, Fox JG, Dedon PC, Tannenbaum SR. 2013. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc Natl Acad Sci USA 110:E2332–E2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushner I. 1993. Regulation of the acute-phase response by cytokines. Perspect Biol Med 36:611–622. [DOI] [PubMed] [Google Scholar]
- 22.Kushner I, Mackiewicz A. 1987. Acute-phase proteins as disease markers. Dis Markers 5:1–11. [PubMed] [Google Scholar]
- 23.Lampreave F, Gonzalez-Ramon N, Martinez-Ayensa S, Hernandez MA, Lorenzo HK, Garcia-Gil A, Pineiro A. 1994. Characterization of the acute-phase serum protein response in pigs. Electrophoresis 15:672–676. [DOI] [PubMed] [Google Scholar]
- 24.Larsen MO, Wilken M, Gotfredsen CF, Carr RD, Svendsen O, Rolin B. 2002. Mild streptozotocin diabetes in the Gottingen minipig. A novel model of moderate insulin deficiency and diabetes. Am J Physiol Endocrinol Metab 282:E1342–E1351. [DOI] [PubMed] [Google Scholar]
- 25.Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. 2009. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda K, Schoeniger LO, Shimada M, Winchurch RA, Buchman TG, Robotham JL. 1993. Regulation of acute-phase gene expression following surgery and endotoxin administration in the anesthetized pig. Anesthesiology 79:1324–1337. [DOI] [PubMed] [Google Scholar]
- 27.Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. 2006. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91:345–347. [DOI] [PubMed] [Google Scholar]
- 28.Milman N, Graudal N, Andersen HC. 1988. Acute-phase reactants in the elderly. Clin Chim Acta 176:59–62. [DOI] [PubMed] [Google Scholar]
- 29.Murata H, Shimada N, Yoshioka M. 2004. Current research on acute-phase proteins in veterinary diagnosis: an overview. Vet J 168:28–40. [DOI] [PubMed] [Google Scholar]
- 30.Murtaugh MP, Baarsch MJ, Zhou Y, Scamurra RW, Lin G. 1996. Inflammatory cytokines in animal health and disease. Vet Immunol Immunopathol 54:45–55. [DOI] [PubMed] [Google Scholar]
- 31.Petersen HH, Nielsen JP, Heegaard PM. 2004. Application of acute-phase protein measurements in veterinary clinical chemistry. Vet Res 35:163–187. [DOI] [PubMed] [Google Scholar]
- 32.Pineiro C, Pineiro M, Morales J, Andres M, Lorenzo E, Pozo MD, Alava MA, Lampreave F. 2009. Pig MAP and haptoglobin concentration reference values in swine from commercial farms. Vet J 179:78–84. [DOI] [PubMed] [Google Scholar]
- 33.Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, Gouillon M, Bouillot JL, Oppert JM, Basdevant A, Clement K. 2006. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 14:309–318. [DOI] [PubMed] [Google Scholar]
- 34.Pomorska-Mól M, Kwit K, Markowska-Daniel I. 2012. Major acute-phase proteins in pig serum from birth to slaughter. Bull Vet Inst Pulawy 56:553–557. [Google Scholar]
- 35.Pottratz ST, Bellido T, Mocharla H, Crabb D, Manolagas SC. 1994. 17β-estradiol inhibits expression of human interleukin-6 promoter–reporter constructs by a receptor-dependent mechanism. J Clin Invest 93:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribel U, Larsen MO, Rolin B, Carr RD, Wilken M, Sturis J, Westergaard L, Deacon CF, Knudsen LB. 2002. NN2211: a long-acting glucagon-like peptide 1 derivative with antidiabetic effects in glucose-intolerant pigs. Eur J Pharmacol 451:217–225. [DOI] [PubMed] [Google Scholar]
- 37.Rodgaard T, Skovgaard K, Moesgaard SG, Cirera S, Christoffersen BO, Heegaard PM. 2014. Extensive changes in innate immune gene expression in obese Gottingen minipigs do not lead to changes in concentrations of circulating cytokines and acute-phase proteins. Anim Genet 45:67–73. [DOI] [PubMed] [Google Scholar]
- 38.Rodgaard T, Stagsted J, Christoffersen BO, Cirera S, Moesgaard SG, Sturek M, Alloosh M, Heegaard PM. 2013. Orosomucoid expression profiles in liver, adipose tissues, and serum of lean and obese domestic pigs, Gottingen minipigs, and Ossabaw minipigs. Vet Immunol Immunopathol 151:325–330. [DOI] [PubMed] [Google Scholar]
- 39.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110:3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanahan L, Copeland WE, Worthman CM, Erkanli A, Angold A, Costello EJ. 2013. Sex-differentiated changes in C-reactive protein from ages 9 to 21: the contributions of BMI and physical–sexual maturation. Psychoneuroendocrinology 38:2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. 2013. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh T, Newman AB. 2011. Inflammatory markers in population studies of aging. Ageing Res Rev 10:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen NS, Tegtmeier C, Andresen LO, Pineiro M, Toussaint MJ, Campbell FM, Lampreave F, Heegaard PM. 2006. The porcine acute-phase protein response to acute clinical and subclinical experimental infection with Streptococcus suis. Vet Immunol Immunopathol 113:157–168. [DOI] [PubMed] [Google Scholar]
- 44.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr, Frazier KS. 2011. Swine as models in biomedical research and toxicology testing. Vet Pathol 49:344–356. [DOI] [PubMed] [Google Scholar]
- 45.Webel DM, Finck BN, Baker DH, Johnson RW. 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J Anim Sci 75:1514–1520. [DOI] [PubMed] [Google Scholar]