Abstract
The respiratory system is a primary target of the harmful effects of key air pollutants of health concern. Several air pollutants have been implicated including particulate matter (PM), ozone (O3), nitrogen dioxide (NO2) polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs). It is well known that episodes of exposure to high concentrations of outdoor air pollutants can cause acute respiratory exacerbations. However, there is now increasing evidence suggesting that significant exposure to outdoor air pollutants may be also associated with development of lung cancer and with incident cases of chronic obstructive pulmonary disease (COPD) and respiratory allergies. Here we provide a critical appraisal of the impact of air pollution on respiratory diseases and discuss strategies for preventing excessive exposure to harmful air pollutants. However, the evidence that significant exposure to air pollutants is causing COPD, lung cancer or respiratory allergies is not conclusive and therefore regulators must be aware that execution of clean air policies may not be that cost-effective and may lead to unintended consequences. Addressing the lung health effects of air pollution must be considered work in progress.
Keywords: air pollution, asthma, COPD, lung cancer, respiratory diseases
Introduction
Air pollution may be an important environmental risk factor with global, public health implications. The World Health Organization (WHO) estimates that outdoor air pollution may have caused 3.7 million premature deaths worldwide in 2012 [WHO, 2014].
Given that an adult inhales on average 10–15 m3 of air per day, it is obvious that inhalation represents the fundamental exposure mechanism to airborne pollutants in man [Phalen, 2008]. Consequently, it is not surprising that the respiratory system becomes a primary target of the harmful effects of key air pollutants of health concern including particulate matter (PM), ozone (O3) and nitrogen dioxide (NO2) [Spirić et al. 2012], as well as new and emerging pollutants such as polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) [Ferrante et al., 2012a]. Most importantly, there is now emerging evidence that co-exposures have the potential to synergistically augment the individual effects of several air pollutants [Sava and Carlsten, 2012].
The overall impact of these exposures on lung health has been the subject of intense research. Epidemiological studies suggest that significant exposure to air pollutants appears to be associated with respiratory exacerbations. Less convincing evidence is available for the causation of chronic obstructive pulmonary disease (COPD), lung cancer and respiratory allergies (Table 1).
Table 1.
Effects of common air pollutants on lung function and respiratory diseases.
| Outdoor pollutants | Lung health outcomes |
||||
|---|---|---|---|---|---|
| Lung function decrements | Lung function growth | Asthma and COPD exacerbation | Acute respiratory symptoms | Lung cancer | |
| PM10 | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation |
| PM2.5 | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation |
| UFP | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation |
| Ozone | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | |
| Nitrogen oxides (NOx) | Positive Correlation | Positive Correlation | Positive Correlation | ||
| SO2 | Positive Correlation | Positive Correlation | |||
| PAHs | Positive Correlation | ||||
| Benzene* | Positive Correlation | Positive Correlation | |||
| 1,3-Butadiene* | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | |
| Aldehydes | Positive Correlation | Positive Correlation | Positive Correlation | Positive Correlation | |
| Inorganic arsenic | Positive Correlation | ||||
| Chromium | Positive Correlation | Positive Correlation | Positive Correlation | ||
| Nickel | Positive Correlation | Positive Correlation | |||
| Cadmium | Positive Correlation | Positive Correlation | Positive Correlation | ||
| Phthalates | Positive Correlation | Positive Correlation | |||
Volatile organic compound (VOC)
COPD, chronic obstructive pulmonary disease; PAH, polycyclic aromatic hydrocarbon; PM, particulate matter; UFP, ultrafine particle.
References
Cohen, A. (2000) Outdoor air pollution and lung cancer. Environ Health Perspect 108(Suppl. 4): 743–750.
Cohen, A., Anderson, R., Ostro, B., Pandey, K., Krzyzanowski, M., Kunzli, N. et al. (2004) Mortality impacts of particulate air pollution in the urban environment. In: Ezzati, M., Lopez, A., Rodgers, A. and Murray, C. (eds), Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization, Vol. 2, Chapter 17, pp. 1354–1433 [cited in: Ezzati, M., Lopez, A., Jaakkola, J. and Knight, T. (2008) The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect 116: 845–853].
Ezzati, M., Lopez, A., Rodgers, A. and Murray, C. (eds) (2004) Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization.
Perez, L., Rapp, R. and Künzli, N. (2010) The year of the lung: outdoor air pollution and lung health. Swiss Med Wkly 140: w13129.
Schwartz, J. (1989) Lung function and chronic exposure to air pollution: a cross-sectional analysis of NHANES II. Environ Res 50: 309–321.
US EPA (2015) Health effects of ozone in the general population. Available at: http://www.epa.gov/apti/ozonehealth/population.html (accessed 8 May 2015).
Vineis, P., Forastiere, F., Hoek, G. and Lipsett, M. (2004) Outdoor air pollution and lung cancer: recent epidemiologic evidence. Int J Cancer 111: 647–652.
Particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) and O3 are amongst the most studied air pollutants of health concern [Huang, 2014]. It seems as if the size of particles is directly linked to their potential for causing health problems. Particles less than 10 μm in diameter can get deeper into the lungs and some may even get into the bloodstream [Nemmar et al. 2002]. However, based on 23 estimates for all causes mortality, a 10 μg/m3 increment in PM2.5 was associated with only a 1.04% increase in the risk of death [Atkinson et al. 2014]. Last but not least, it should be noted that specific changes in climate appears to have a negative impact on overall air quality with substantial increase in respiratory morbidity and mortality of patients with chronic lung conditions [Bernstein and Rice, 2013].
There is indication that the implementation of strategies to reduce air pollution may lead to significant health benefits. For example, the US Environmental Protection Agency (US EPA) estimated that the implementation of measures to reduce emissions from diesel engines might result in 12,000 fewer mortalities and 8900 less hospital admissions in the United States each year [US EPA, 2004]. However, while legislation to control air pollution has vastly improved air quality in many regions of the world, there still remain many countries with heavily polluted cities and increasing vehicle exhaust emissions. In China, ambient air pollution is associated with more than 300,000 deaths and 20 million cases of respiratory illnesses annually [World Bank, 2007]. Outdoor air pollution resulted in 1.2 million premature deaths in China in 2010, or nearly 40% of premature deaths worldwide due to pollution in the world [Lim et al. 2012]. Alarming levels of air pollution in China have been reported in as many as 31 Chinese cities; the median of daily PM2.5 concentration exceeds up to 5 times the air quality levels recommended by the World Health Organization (WHO) [Wang et al. 2014].
Here we provide a critical appraisal of the impact of outdoor air pollution on respiratory diseases and discuss strategies for preventing excessive exposure to harmful air pollutants.
Air quality and COPD
COPD is a progressive and debilitating disease characterized by a persistent inflammatory response that cannot be reversed and generally leads to progressive decline in lung function, respiratory failure, cor pulmonale, and death [Morjaria et al. 2010].
In order to reduce the significant economic burden of COPD [Mannino and Braman, 2007], it is important to identify each contributing factor. Cigarette smoking is currently considered as the most important cause of COPD. However, cigarette smoking is not the sole cause for COPD. Besides disease exacerbation, some authors have also suggested a role for air pollution as an important factor in the development of COPD [Salvi and Barnes, 2009; Mackay and Hurst, 2013]. However, there is insufficient evidence to prove a causal relationship because of the influence of powerful confounding factors, including tobacco smoking and climatic changes.
Only a few studies have investigated the relationship between outdoor air pollutants and objectively defined COPD [Schikowski et al. 2005; Pujades-Rodriguez et al. 2009; Schikowski et al. 2010] (Table 2). For example, a 4.5% prevalence of COPD was found in 4757 women living in the Rhine-Ruhr Basin (Germany) in a consecutive cross-sectional study conducted between 1985 and 1994. In this study, COPD and pulmonary function were markedly affected by PM10 and traffic-related exposure. For women living less than 100 m from a busy road, COPD was 1.79 times more likely than for those living farther away [Schikowski et al. 2005]. In a subsequent long-term follow up of a subgroup of 402 women, the same researchers were able to show a lower COPD prevalence in association with improved air quality (i.e. decreasing PM10 level) [Schikowski et al. 2010]. In contrast, a study from Nottingham (UK) involving a cohort of 2644 adults found no significant cross-sectional associations between living in close proximity to traffic or NO2 levels and spirometry confirmed COPD [Pujades-Rodriguez et al. 2009]. Most recently, a 35-year prospective study of more than 57,000 participants in a Danish cohort reported a significant but small positive association between long-term exposure to traffic-related air pollution [NO2 or nitrogen oxides (NOx)] and incident COPD in the period from 1993 to 2006 [Andersen et al. 2011]. This study considered extensive control of confounders, but was limited by the lack of objective spirometric measurement for the diagnosis of COPD.
Table 2.
Traffic-related exposure studies on COPD.
| Study | Date | Location | Pollutants | Subject/location | Results |
|---|---|---|---|---|---|
| Schikowski et al. [2005], Schikowski et al. [2010] | 1985–1994 | Rhine-Ruhr Basin (Germany) | PM10 | 4757 women living less than 100 m from a busy road | 4.5% prevalence of COPD |
| Pujades-Rodriguez et al. [2009] | 1991–2000 | Nottingham (UK) | NO2 | 2644 adults aged 18–70 living in close proximity to traffic | spirometry confirmed COPD |
| Andersen et al. [2011] | 1993–2006 | Denmark | NO2/NOx | 57,000 adults | incident COPD |
| Zanobetti et al. [2000] | 1986–1994 | 10 US cities | PM10 | adults aged >65 years living in a metropolitan county | 2.5% increase in hospital admissions for AECOPD |
| Dominici et al. [2006] | 1999–2002 | 204 US urban counties | PM2.5 | 11.5 million adults aged >65 years | risk of about 0.9% for COPD hospitalization |
| Medina-Ramon et al. [2006] | 1986–1999 | 36 US cities | PM10 | warm season | 1.47% increase in hospital admissions for AECOPD |
| Fusco et al. [2001] | 1995–1997 | Rome (Italy) | NO2 and O3 | residents of all ages and among children (0–14 years) | 4.3% increase in hospital admissions for AECOPD |
| Tao et al. [2014] | 2001–2005 | Lanzhou, China | PM10, SO2, NO2 | females and aged ⩾65 years | increases in hospital admissions for AECOPD |
AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; NOx, nitrogen oxides; PM, particulate matter.
If the evidence for an important association between air pollution and COPD development is not very clear, its role as an important trigger of COPD exacerbations it is widely acknowledged. Most of the published studies in this area of research have focused on associations between air pollution and hospital admissions.
In a very large study of hospital admissions related to heart and lung diseases in 10 US cities, a 2.5% increase in COPD admissions was observed for every 10 μg/m3 increase in PM10 [Zanobetti et al. 2000]. Another US study found that a sudden increase in PM2.5 was associated with a risk of about 0.9% for COPD hospitalizations [Dominici et al. 2006]. The role of O3 was addressed in a large multicity (n = 36) study in the US, in which a 2-day cumulative effect of a 5 parts per billion (ppb) increase in O3 in the course of warm sunny days was associated with a 0.27% increase in admissions for acute exacerbation of COPD (AECOPD). Likewise, a 10 μg/m3 increase in PM10 during the warm season was associated with 1.47% [95% confidence interval (CI): 0.93–2.01] immediate increase in AECOPD [Medina-Ramon et al. 2006].
A study assessing the data on admissions for COPD in 6 European cities showed that the relative risk for a 50 μg/m3 increase in daily mean level of SO2, black smoke, total suspended particulates, NO2 and O3 for AECOPD admissions was 1.02, 1.04, 1.02, 1.02 and 1.04, respectively [Anderson et al. 1997]. A study in Rome (Italy) reported that carbon monoxide (CO) and the photochemical pollutants, NO2 and O3, were important determinants for acute respiratory conditions with a 4.3% increase in COPD admissions [Fusco et al. 2001]. A recent study from a rural county of England, where the pollutant concentration is lower than that in the urban area, found that increases in ambient CO, nitric oxide (NO), NO2 and NOx concentrations were associated with increases in hospital admissions for AECOPD, similar in extent to that in the urban areas [Sauerzapf et al. 2009]. Other published European studies have investigated the effect of air pollutants on asthma and COPD admissions grouped together instead of analysing them separately, making it difficult to estimate the accurate effect on COPD admissions [Atkinson et al. 2001; Halonen et al. 2009; 2010].
A report including a systematic and quantitative assessment of 82 time-series Asiatic studies of daily mortality and hospital admissions for cardiovascular and respiratory disease observed that all-cause mortality was associated with increase in ambient PM10, total suspended particles and SO2 levels [HEI, 2010]. In addition, respiratory admissions were associated with NO2 and SO2 levels. However, COPD admissions or mortality were not separately addressed in this study. A single city study in Hong Kong focused specifically on the effect of air pollutants on hospital admissions due to AECOPD from 2000 to 2004 and included 119,225 admissions for AECOPD [Ko et al. 2007]. The study observed that the relative risk of hospital admissions for every 10 μg/m3 increase in SO2, NO2, O3, PM10 and PM2.5 was 1.007, 1.026, 1.034, 1.024 and 1.031, respectively. In Lanzhou, one of the most air-polluted city in China, stronger effects of air pollutants on respiratory hospital admissions were observed, particularly in females aged ⩾65 years [Tao et al. 2014].
In conclusion, time series studies appear to support a relationship between AECOPD and increasing ambient air pollutant levels, but the role of additional confounding factors has to be considered.
Air quality and lung cancer
Although the most common cause of lung cancer is long-term exposure to tobacco smoke, an estimated 10–25% of cases worldwide occur in never smokers [Couraud et al. 2012] and there is now increasing evidence that also exposure to air pollution may contribute to lung cancer in at-risk individuals (Table 3).
Table 3.
Traffic-related exposure studies on lung cancer.
| Study | Date | Location | Pollutants | Subject/location | Results |
|---|---|---|---|---|---|
| Vineis et al. [2006] | 1993–1998 | 10 European countries | NO2 | adults aged 35–74 residing near heavy traffic roads | 46% increase in lung cancer |
| Chiu et al. [2006] | 1994–2003 | Taiwan | PM10, SO2, NO2 | females | 28% increased risk of lung cancer |
| Edwards et al. [2006] | 2000–2004 | Teesside, northeast England | PM10, SO2, NO2 | women aged <80 years living for >25 years close to highly industrialized area | 83% increased risk of lung cancer |
| Loomis et al. [2014] | 2003–2012 | 31 provincial capital cities in China | PM2.5 | 71,000 adults | increased risk of lung cancer |
| Raaschou-Nielsen et al. [2013] | 2008–2011 | 17 separate European cohorts | PM10 | 312,944 adults | increased risk of lung cancer |
PM, particulate matter.
NO2 is a good marker of traffic-related pollution [Vineis et al. 2007; NSW Government Advisory Committee on Tunnel Air Quality, 2014]. Near-roadway (within about 50 m) concentrations of NO2 have been measured to be approximately 30–100% higher than concentrations away from roadways [US EPA, 2014]. The Health Effects Institute (HEI) identified that an exposure zone extending up to 300–500 m from a major road was the most highly affected by traffic emissions [HEI Traffic Panel, 2010]. In a European nested case-control study of non- and ex-smokers, a 46% increase in lung cancer was reported in those residing near heavy traffic roads [Vineis et al. 2006]. When individual pollutants were examined, exposure to each increment of 10 ppb NO2 produced a 14% increase in lung cancer. Exposure to concentrations greater than 30 ppb resulted in a 30% increase. These findings did not change after controlling for occupational factors and cotinine (a short-term marker of tobacco exposure). In another case-control study examining the risk of outdoor air pollution, women living in the group of Taiwan municipalities with the highest levels of air pollution had a 28% increased risk of lung cancer [Chiu et al. 2006]. Likewise, lung cancer risk among women with prolonged (>25 years) residence in a highly industrialized area of northeast England was increased by 83% [Edwards et al. 2006].
In 2013, the International Agency for Research on Cancer (IARC) classified outdoor air pollution and related PM as a class I human carcinogen [IARC, 2013]. The IARC evaluation showed an increasing risk of lung cancer with rising environmental levels of PM [Costa et al. 2014]. Data from epidemiologic studies in Asia, Europe and North America consistently show positive association between lung cancer and PM exposure and other indicators of air pollution, which persist after adjustment for important lung cancer risk factors such as tobacco smoking [Loomis et al. 2014].
Another emerging air pollutant is diesel exhaust particles (DEPs), a complex mixture of thousands of chemicals. Most DEPs have aerodynamic diameters falling within a range of 0.1 to 0.25 μm and are classified as class I human carcinogen by the IARC based on epidemiological evidence for lung cancer [Leem and Jang, 2014].
In urban settings worldwide, diesel exhaust emissions are known to contribute substantially to the PM quota of air pollution and the possible association of exposures to diesel exhaust and an increased incidence of lung cancer has been raised. However, current epidemiological evidence from population-based case-control studies [Olsson et al. 2011; Villeneuve et al. 2011] and cohort studies of bus and truck drivers [Birdsey et al. 2010; Merlo et al. 2010; Petersen et al. 2010] fails to develop any confident quantitative estimate of cancer risk due to several methodological problems. Specifically, the population-based case-control studies suffer from inherent defects in job groupings and exposure estimations, insufficient latency periods, inconsistent a posteriori subanalyses, nonsignificant exposure–response trends after adjustment for potential confounders, and failures to adjust for the rates of dieselization or for the evolution of diesel engines and fuels (and thus exposure levels) over time. In the cohort studies of bus and truck drivers, incorrect adjustments for confounding factors such as current smoking produced spuriously elevated odds ratios (ORs) that were incorrectly attributed to diesel exhaust exposure.
The European Study of Cohorts for Air Pollution Effects (ESCAPE) allowed collection and analysis of clinical and exposure data from 312,944 members enrolled in 17 separate European cohorts [Raaschou-Nielsen et al. 2013]. The association between long-term exposure to ambient air pollution and lung cancer was investigated in the 2095 incident lung cancer cases diagnosed over a follow-up period averaging 12.8 years. The meta-analyses showed a statistically significant association between risk for lung cancer and PM10 [hazard ratio (HR) 1.22 (95% CI 1.03–1.45) per 10 μg/m3]. For PM2.5. a nonsignificant HR of 1.18 (0.96–1.46) per 5 μg/m3 was reported. Of note, proxies for traffic intensities failed to show a significant association with lung cancer.
New and emerging pollutants should be also considered. VOCs, PAHs that are known to react with other common air pollutants such as O3, NOx and SO2 yielding diones, nitro- and dinitro-PAHs, sulfonic acids, and heavy metals bound to inorganic and organic compounds and/or adsorbed on PM may have mutagenic and genotoxic potential, and may induce DNA adduct formation in vitro and in vivo, thus qualifying for being probable (group 2A) or possible (group 2B) human carcinogens [Ferrante et al. 2012]. However, these have been little studied in epidemiological population studies. Due to known role of nanoparticles in inducing an intense lung inflammation, studies to clarify the role of nanoparticles on the respiratory tract are significant [Bakand et al. 2012; Ferrante et al., 2012b].
Air quality and respiratory allergies
It is widely accepted that exposure to ambient concentrations of air pollutants can cause short-term exacerbations in those who already have respiratory allergies (i.e. asthma and rhinitis). In particular, asthma exacerbations – measured as visits to emergency departments – have been frequently reported on days with higher levels of O3 and other pollutants [Weisel et al. 1995].
However, whether air pollutants play a role in the initiation of new cases of asthma in those previously free from the condition is less clear. Main attention has been concentrated on gaseous materials such as O3 and NO2, as well as PM, generated by urban traffic and industry (Table 4). The fast expansion of the global vehicular fleet during the past 50 years exposes billions of people to unhealthy and dangerous levels of motor vehicle generated air pollutants. Among the various pollutants emitted from motor vehicle exhausts, airborne PM has been long suggested to be an important contributing factor for the increased prevalence of respiratory allergies in recent years [Polosa et al. 2002; Diaz-Sanchez et al. 2003; Oliveri et al. 2011]. The largest single source of airborne PM is that derived diesel engine emissions; diesel vehicles emit up to 100 times more PM than catalyst-equipped petrol cars of corresponding performance. In cities such as Los Angeles (USA), 40% of the 10 μm particles (PM10) in the atmosphere are derived from diesel vehicle engines [Diaz-Sanchez, 2000]. DEPs have the ability to bind proteins and may serve as a potential carrier of allergens, penetrating deep into respiratory tract [Anderson et al. 2013]. In allergic subjects challenged nasally with pollen ±0.3 mg DEP, allergic antibody production is up to 50 times greater if DEP present [Diaz-Sanchez, 2000].
Table 4.
Traffic-related exposure studies on respiratory allergies.
| Study | Date | Location | Pollutants | Subject/location | Results |
|---|---|---|---|---|---|
| Gehring et al. [2010], McConnell et al. [2010] | 1996–1997 | Netherlands | PM2.5 | 3863 yearly from birth until age 8 years | significant increase in incidence and prevalence of asthma |
| Penard-Morand et al. [2010] | 1998–2000 | 6 French cities | benzene, SO2, PM10, NOx, and CO | 6683 children (9–11 years) | increased risk of asthma and allergic rhinitis |
| Yamazaki et al. [2014] | Japan | elemental carbon | 10,069 school children 6–9-year old | increased risk of asthma incidence | |
| Kunzli et al. [2009] | 1991–2002 | Switzerland | PM10 | adult aged 18–60 years | increased risk of asthma incidence |
| Young et al. [2014] | U.S. | PM2.5 | 50,884 women | increased risk of asthma incidence |
PM, particulate matter.
Although initial studies of traffic-related air pollution and asthma were conflicting due to a number of methodological flaws including misclassification of pollution exposure to the individual cases [Jenerowicz et al. 2012], subsequent studies using more refined methodologies such as land-use regression (LUR) or dispersion modelling produced some significant associations in both children and adults with chronic pollution exposure.
Two recent studies using these sophisticated techniques to investigate the association between childhood asthma and exposure to traffic-related air pollution at home, school or both have shown increased risk of childhood asthma incidence even after controlling for relevant confounders [Gehring et al. 2010; McConnell et al. 2010].
The prospective birth cohort study of 3863 children has investigated the association between traffic-related air pollution and the development of objectively diagnosed asthma and allergies during the first 8 years of life. PM2.5 levels were associated with a significant increase in incidence of asthma (OR, 1.28; 95% CI, 1.10–1.49), prevalence of asthma (OR, 1.26; 95% CI, 1.04–1.51), and prevalence of asthma symptoms (OR, 1.15; 95% CI, 1.02–1.28). Similar findings were observed for NO2. Differences in air pollution effects between different age groups were small with the exception of asthma incidence, for which associations became somewhat stronger at 6–8 years of age [Gehring et al. 2010].
In a smaller study, Carlsten and colleagues recruited infants at high familial risk for asthma and examined birth year home exposures to NO, NO2, black carbon and PM2.5 with follow up at 7 years of age. Birth year PM2.5 (interquartile range, 4.1 μg/m3) was associated with a markedly increased risk of asthma with an OR of 3.1 (95% CI, 1.3–7.4). NO and NO2 demonstrated similar associations, but black carbon did not [Carlsten et al. 2011].
A study of self-reported allergic disease, using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, and home traffic density based on distance to major roadways found approximately 1.5–3 fold prevalence ratios for heavy traffic density for wheeze, asthma, rhinitis and rhinoconjunctivitis, with no associations for children who slept in air-conditioned homes [Zuraimi et al. 2011].
Using a cross-sectional design and an enhanced ISAAC protocol for outcomes, 6683 children in the French Six Cities Study were studied with exposures based on a 3-year dispersion model for each school address to assign individual school exposures [Penard-Morand et al. 2010]. Asthma was significantly associated with benzene, SO2, PM10, NOx and CO levels; allergic rhinitis was only associated with PM10 levels. Sensitization to pollens was associated with benzene and PM10 levels. This is in accordance with the increase in the proportion of positive skin prick test and total immunoglobulin E (IgE) levels reported in traffic warden of the city of Catania (Sicily) with a well-characterized occupational history of road traffic fumes exposure [Proietti et al. 2003].
A cohort study in Japan examined the association between traffic-related air pollution and the development of asthma in school children [Yamazaki et al. 2014]. Subjects were 10,069 school children in their first through third years of compulsory education (6–9 years old). As surrogates of traffic-related air pollution, the estimation target was the annual average individual exposure of automobile exhaust-originating NOx and elemental carbon (EC). The OR (95% CI) for asthma incidence was significant at 1.07 (1.01–1.14) for each 0.1 μg/m3 EC, but not significant for NOx (OR = 1.01 (0.99–1.03) for each 1 ppb NOx.
The Swiss Cohort Study on Air Pollution and Lung Diseases in Adults was a population-based cohort of adult lung disease-free nonsmokers initiated in 1991 with 11-year follow up in 2002 [Kunzli et al. 2009]. Using a dispersion model that included hourly meteorological and emissions data on industrial, construction, heating, agricultural and forestry, and traffic emissions, the latter separated by type of vehicle (truck versus car), each participant was assigned an exposure to PM10. An HR for doctor-diagnosed asthma of 1.30 (95% CI, 1.05–1.61) new cases was found for a given (1 μg/m3 as PM10) change in traffic pollution over the 10 years; this was more frequent in those with baseline atopy or bronchial hyperreactivity.
Trupin and colleagues looked at the simultaneous effect on forced expiratory volume in 1 second (FEV1) percent predicted and an asthma severity score of diverse social and physical environmental exposures on adult asthma in 176 subjects. Their final model had an R2 value of 0.30 for FEV1 percent predicted and 0.16 for the severity of asthma score and distance to the nearest road was a significant predictor of FEV1 [Trupin et al. 2010].
A recent study estimated the association between traffic-related air pollution exposure (PM2.5) and incident adult asthma in a nationwide cohort of US women (n = 50,884) [Young et al. 2014]. For an interquartile range (IQR) difference (3.6 μg/m3) in estimated PM2.5 exposure, the adjusted odds ratio (aOR) was 1.20 (95% CI, 0.99–1.46; p = 0.063) for incident asthma. Results suggest that PM2.5 exposure may increase the risk of developing asthma in adult women.
The above mentioned studies support an association between various aspects of traffic-related air pollution and new-onset asthma or asthma exacerbation in children and adults. This relationship, at least for the acute exposure domain, has been further strengthened by the findings of recent pollution intervention studies. The Beijing Olympics of 2008 were a great opportunity for environmental researchers to study the effects of greater than usual degrees of changes in air pollution on human health [Cai and Xie, 2011]. One study examined visits for outpatient treatment of asthma at a Beijing Hospital [Li et al. 2010]. Although somewhat sparse in clinical detail, they reported a reduction from 12.5 visits per day to 7.3 visits per day, a 41.6% reduction during the Olympic Games.
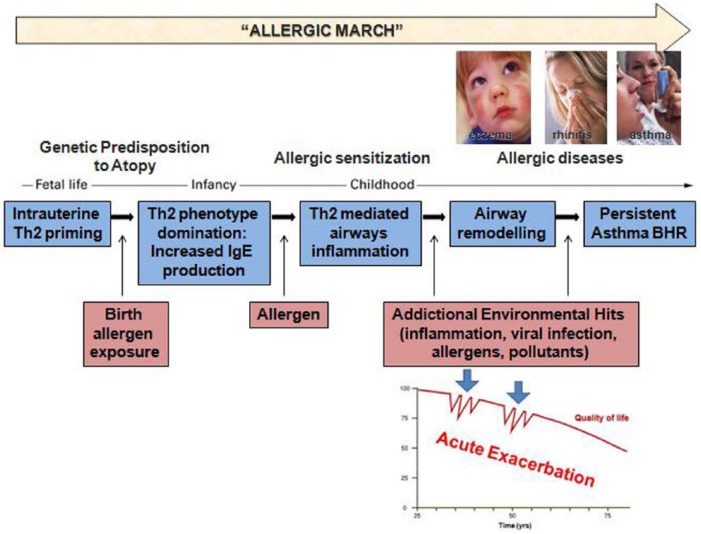
A mechanistic hypothesis to explain the interaction between air pollution and asthma has been proposed [Gowers et al. 2012] (Figure 1). Subjects with a genetic predisposition to atopy are known to develop a strong Th2 inflammatory phenotype with increased IgE production. In presence of continuous environmental exposure, these individuals might end up developing a full-blown allergic inflammatory process and to develop respiratory disorders such as allergic rhinitis and asthma [Bates and Maksym, 2011; Holgate, 2011]. When exposed to allergen, air pollutants or viruses [Willart and Lambrecht, 2009], these individuals are more likely to develop acute inflammatory responses and related respiratory symptoms (i.e. acute exacerbations).
Figure 1.
Hypothetical network of interaction between air pollution and asthma. Subjects with genetic predisposition to atopy tend to develop a prevalent Th2 immune phenotype, as a result of birth allergen exposure. Increased IgE production shapes a state of allergic sensitization, with Th2 mediated airways inflammation. Continuous environmental exposure to pollutants or/with other factors (viral infection, allergens, inflammation, etc.), inducing persistent inflammation and airway remodeling, contributes to acute exacerbations on sensitized subjects.
BHR, bronchial hyperresponsiveness; IgE, immunoglobulin E; Th2, type 2 helper T cells.
Prevention and public health measures
Air pollution may affect the health of millions of people. Current understanding of the role of air pollutants, both in isolation and in association with climate changes, on human health is unclear [Prüss-Üstün and Corvalán, 2006]. Nonetheless, effective and proportionate policies to reduce environmental air pollution may still be contemplated. The case for action to reduce air pollution can take many forms including urban planning, technological developments (e.g. the design of new vehicles that produce less pollution), introduction of warning systems and new policies. This strategy must work both at the population level, with preventive policies and at the individual level, by informing and educating the population at risk about reducing exposure to air pollutants. Individuals with heart or lung disease (such as coronary artery disease, congestive heart failure, and asthma or COPD), older adults and children should be warned about higher exposure risk when exercising outdoors. Also pregnant women are at risk of exposing their vulnerable unborn child to the harmful effects of air pollutants. It has been recently shown that exposure to road traffic-related air pollution (i.e. higher levels of benzene and NO2) during early stages of pregnancy may be associated with lung function defects in children later in life [Morales et al. 2014].
At population level, some health benefits can be obtained by implementing specific policies as for the case of congestion charges in large cities such as London and Stockholm. Likewise, Aphekom (Improving Knowledge and Communication for Decision Making on Air Pollution and Health in Europe), a multicountry EU project investigating the effects of EU legislation to reduce inner cities air pollution in several EU capitals [Aphekom Project, 2011], shows that substantial reduction in ambient SO2 levels is estimated to prevent some 2200 premature deaths valued at €192 million. These findings underscore the combined health and monetary benefits deriving from implementing effective EU policies on air pollution and ensuring compliance with them over time. Since the 1973, the EU has also developed a series of Environment Action Programmes (EAPs) to prevent and reduce the burden of air pollution-related diseases. The most recent ones, 5th EAP (1993–2000) and 6th EAP (2002–2012), are most relevant and set the scene for developing specific policies and directives to control air pollution and improve air quality in the past two decades, with a specific focus on reducing emissions from both the road traffic and industrial sector by 2020 [EEA, 2012]. However, it is important to understand the true impact to human health of specific exposure thresholds to key air pollutants before investing huge amount of taxpayers money in meaningless prevention programs. This is particularly relevant if we consider that there are more common sources of pollution that are not accounted for. For example, living with a smoker exposes an individual to levels of toxicants that are comparable with those of a heavily polluted city [Semple et al. 2014].
Conclusion
If in principle it makes sense to ensure that the air we breathe should be clean as possible, there is however no conclusive evidence that significant exposure to outdoor air pollutants is causing COPD, lung cancer or respiratory allergies. The current available literature has revealed unforeseen complexities and methodological limitations thus increasing, rather than reducing, contradictions and doubts. The extraordinary difficulty of demonstrating the benefits of salt reduction, mammography or various diets, for example, ought to serve as cautionary lessons.
Given the uncertainties about the exposure levels known to be dangerous to lung health and about mortality/morbidity estimations from air pollution, it does not seem right to spend years characterizing and debating ancillary risks of air pollutants that are almost certainly much less serious than the known risks of smoking. Nonetheless, for areas where knowledge is lacking may still be sensible to invoke the precautionary principle. But regulators must be aware that execution of clean air policies may not be that cost-effective and may lead to unintended consequences.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Elisa Marino, Dipartimento di Medicina Clinica e Sperimentale, and UOC di Medicina Interna e Immunologia Clinica, Università di Catania, Italy.
Massimo Caruso, Dipartimento di Medicina Clinica e Sperimentale, and UOC di Medicina Interna e Immunologia Clinica, Università di Catania, Italy.
Davide Campagna, Dipartimento di Medicina Clinica e Sperimentale, and UOC di Medicina Interna e Immunologia Clinica, Università di Catania, Italy.
Riccardo Polosa, UOC di Medicina Interna e Immunologia Clinica, Policlinico Universitario, University of Catania, Via S. Sofia 78, 95100, Catania, Italy.
References
- Anderson H., Favarato G., Atkinson R. (2013) Long-term exposure to outdoor air pollution and the prevalence of asthma: meta-analysis of multi-community prevalence studies. Air Qual Atmos Health 6: 57–68. [Google Scholar]
- Andersen Z., Hvidberg M., Jensen S., Ketzel M., Loft S., Sørensen M., et al. (2011) Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med 183: 455–461. [DOI] [PubMed] [Google Scholar]
- Anderson H., Spix C., Medina S., Schouten J., Castellsague J., Rossi G., et al. (1997) Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J 10: 1064–1071. [DOI] [PubMed] [Google Scholar]
- Aphekom Project (2011) Summary report of the Aphekom project 2008–2011. Saint Maurice, France: Institut de Veille Sanitaire; Available at: http://www.aphekom.org/c/document_library/get_file?uuid=5532fafa-921f-4ab1-9ed9-c0148f7da36a&groupId=10347 (accessed 7 December 2014). [Google Scholar]
- Atkinson R., Anderson H., Sunyer J., Ayres J., Baccini M., Vonk J., et al. (2001) Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air pollution and health: a European approach. Am J Respir Crit Care Med 164: 1860–1866. [DOI] [PubMed] [Google Scholar]
- Atkinson R., Kang S., Anderson H., Mills I., Walton H. (2014) Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 69: 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakand S., Hayes A., Dechsakulthorn F. (2012) Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal Toxicol 24: 125–135. [DOI] [PubMed] [Google Scholar]
- Bates J., Maksym G. (2011) Mechanical determinants of airways hyperresponsiveness. Crit Rev Biomed Eng 39: 281–296 [DOI] [PubMed] [Google Scholar]
- Bernstein A., Rice M. (2013) Lungs in a warming world: climate change and respiratory health. Chest 143: 1455–1459. [DOI] [PubMed] [Google Scholar]
- Birdsey J., Alterman T., Li J., Petersen M., Sestito J. (2010) Mortality among members of a truck driver trade association. AAOHN J 58: 473–480. [DOI] [PubMed] [Google Scholar]
- Cai H., Xie S. (2011) Traffic-related air pollution modeling during the 2008 Beijing Olympic Games: the effects of an odd-even day traffic restriction scheme. Sci Total Environ 409: 1935–1948. [DOI] [PubMed] [Google Scholar]
- Carlsten C., Dybuncio A., Becker A., Chan-Yeung M., Brauer M. (2011) Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med 68: 291–295. [DOI] [PubMed] [Google Scholar]
- Chiu H., Cheng M., Tsai S., Wu T., Kuo H., Yang C. (2006) Outdoor air pollution and female lung cancer in Taiwan. Inhal Toxicol 18: 1025–1031. [DOI] [PubMed] [Google Scholar]
- Costa S., Ferreira J., Silveira C., Costa C., Lopes D., Relvas H., et al. (2014) Integrating health on air quality assessment – review report on health risks of two major European outdoor air pollutants: PM and NO2. J Toxicol Environ Health B Crit Rev 17: 307–340. [DOI] [PubMed] [Google Scholar]
- Couraud S., Zalcman G., Milleron B., Morin F., Souquet P. (2012) LC in never smokers – a review. Eur J Cancer 48: 1299–1311. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D. (2000) Pollution and the immune response: atopic diseases – are we too dirty or too clean? Immunology 101: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Proietti L., Polosa R. (2003) Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep 3: 146–152. [DOI] [PubMed] [Google Scholar]
- Dominici F., Peng R., Bell M., Pham L., McDermott A., Zeger S., et al. (2006) Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R., Pless-Mulloli T., Howel D., Chadwick T., Bhopal R., Harrison R., et al. (2006) Does living near heavy industry cause lung cancer in women? A case-control study using life grid interviews. Thorax 61: 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Environment Agency (EEA) (2002) Air quality in Europe — 2012 report. Copenhagen: EEA Publications Office. [Google Scholar]
- Ferrante M., Cicciù F., Fiore M., Fallico R., Sciacca S. (2012a) Nanotecnologies and nanoparticles. Ig Sanità Pubbl 68: 875–883. [PubMed] [Google Scholar]
- Ferrante M., Fiore M., Oliveri Conti G., Ledda C., Fallico R., Sciacca S. (2012b) Old and new air pollutants: an evaluation on thirty years experiences. In: Haryanto B. (ed.), Air Pollution – A Comprehensive Perspective. Rijeka: Intech Open, pp. 3–26. [Google Scholar]
- Fusco D., Forastiere F., Michelozzi P., Spadea T., Ostro B., Arcà M., et al. (2001) Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J 17: 1143–1150. [DOI] [PubMed] [Google Scholar]
- Gehring U., Wilga A., Brauer M., Fischer P., de Jongste J., Kerkhof M., et al. (2010) Traffic- related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med 181: 596–603. [DOI] [PubMed] [Google Scholar]
- Gowers A., Cullinan P., Ayres J., Anderson H., Strachan D., Holgate S., et al. (2012) Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology 17: 887–898. [DOI] [PubMed] [Google Scholar]
- Halonen J., Lanki T., Tiittanen P., Niemi J., Loh M., Pekkanen J. (2010) Ozone and cause-specific cardiorespiratory morbidity and mortality. J Epidemiol Commun Health 64: 814–820. [DOI] [PubMed] [Google Scholar]
- Halonen J., Lanki T., Yli-Tuomi T., Tiittanen P., Kulmala M., Pekkanen J. (2009) Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology 20: 143–153. [DOI] [PubMed] [Google Scholar]
- HEI (2010) Outdoor air pollution and health in the developing countries of Asia: a comprehensive review. Executive summary. Boston, MA: Health Effects Institute International Scientific Oversight Committee; Available at: http://pubs.healtheffects.org/view.php?id=349 (accessed 7 December 2014). [Google Scholar]
- HEI Traffic Panel (2010) Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Boston, MA: Health Effects Institute; Available at: http://pubs.healtheffects.org/view.php?id=334 (accessed 7 January 2014). [Google Scholar]
- Holgate S. (2011) The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 242: 205–219. [DOI] [PubMed] [Google Scholar]
- Huang Y. (2014) Outdoor air pollution: a global perspective. J Occup Environ Med 56(Suppl. 10): S3–S7. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) (2013) IARC: outdoor air pollution a leading environmental cause of cancer deaths. Press release No 221. Lyon, France: IARC; Available at: http://www.iarc.fr/en/media-centre/iarcnews/pdf/pr221_E.pdf (accessed 7 December 2014). [Google Scholar]
- Jenerowicz D., Silny W., Dańczak-Pazdrowska A., Polańska A., Osmola-Mańkowska A., Olek-Hrab K. (2012) Environmental factors and allergic diseases. Ann Agric Environ Med 19: 475–481. [PubMed] [Google Scholar]
- Ko F., Tam W., Wong T., Chan D., Tung A., Lai C., et al. (2007) Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 62: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzli N., Bridevaux P., Liu L., Garcia-Esteban R., Schindler C., Gerbase M., et al. (2009) Swiss Cohort study on air pollution and lung diseases in adults. Traffic-related air pollution correlates with adult-onset asthma among neversmokers. Thorax 64: 664–670. [DOI] [PubMed] [Google Scholar]
- Leem J., Jang Y. (2014) Increase of diesel car raises health risk in spite of recent development in engine technology. Environ Health Toxicol 29: e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang W., Kan H., Xu X., Chen B. (2010) Air quality and outpatient visits for asthma in adults during the 2008 Summer Olympic Games in Beijing. Sci Total Environ 408: 1226–1227. [DOI] [PubMed] [Google Scholar]
- Lim S., Vos T., Flaxman A., Danaei G., Shibuya K., Adair-Rohani H., et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease study 2010. Lancet 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D., Huang W., Chen G. (2014) The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chin J Cancer 33: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A., Hurst J. (2013) COPD exacerbations: causes, prevention, and treatment. Immunol Allergy Clin North Am 33: 95–115. [DOI] [PubMed] [Google Scholar]
- Mannino D., Braman S. (2007) The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 4: 502–506. [DOI] [PubMed] [Google Scholar]
- McConnell R., Islam T., Shankardass K., Jerrett M., Lurmann F., Gilliland F., et al. (2010) Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 118: 1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ramon M., Zanobetti A., Schwartz J. (2006) The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol 163: 579–588. [DOI] [PubMed] [Google Scholar]
- Merlo D., Stagi E., Fontana V., Consonni D., Gozza C., Garrone E., et al. (2010) A historical mortality study among bus drivers and bus maintenance workers exposed to urban air pollutants in the city of Genoa, Italy. Occup Environ Med 67: 611–619. [DOI] [PubMed] [Google Scholar]
- Morales E., Garcia-Esteban R., De la Cruz O., Basterrechea M., Lertxundi A., de Dicastillo M., et al. (2014) Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 70: 64–73 [DOI] [PubMed] [Google Scholar]
- Morjaria J., Malerba M., Polosa R. (2010) Biologic and pharmacologic therapies in clinical development for the inflammatory response in COPD. Drug Discov Today 15: 396–405. [DOI] [PubMed] [Google Scholar]
- Nemmar A., Hoet P., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M., et al. (2002) Passage of inhaled particles into the blood circulation in humans. Circulation 105: 411–414. [DOI] [PubMed] [Google Scholar]
- NSW Government’s Advisory Committee on Tunnel Air Quality (2014) Initial report on tunnel air quality. TP03: Health Effects of Traffic-Related Air Pollution; Sydney, NSW, Australia: Office of the NSW Chief Scientist & Engineer; Available at: http://www.chiefscientist.nsw.gov.au/__data/assets/pdf_file/0017/51911/060814-FINAL-Initial-Report-Tunnel-Air-Quality-WEB.pdf (accessed 8 May 2015). [Google Scholar]
- Oliveri Conti G., Ledda C., Fiore M., Mauceri C., Sciacca S., Ferrante M. (2011) Allergic rhinitis and asthma in children and indoor pollution. Ig San Pub 67: 467–480. [PubMed] [Google Scholar]
- Olsson A., Gustavsson P., Kromhout H., Peters S., Vermeulen R., Brüske I., et al. (2011) Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am J Respir Crit Care Med 183: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penard-Morand C., Raherison C., Charpin D., Kopferschmitt C., Lavaud F., Caillaud D., et al. (2010) Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J 36: 33–40. [DOI] [PubMed] [Google Scholar]
- Petersen A., Hansen J., Olsen J., Netterstrøm B. (2010) Cancer morbidity among Danish male urban bus drivers: a historical cohort study. Am J Ind Med 53: 757–761. [DOI] [PubMed] [Google Scholar]
- Phalen R. (2008) Inhalation Studies: Foundations and Techniques. New York: Informa Health Care. [Google Scholar]
- Polosa R., Salvi S., Di Maria G. (2002) Allergic susceptibility associated with diesel exhaust particle exposure: clear as mud. Arch Environ Health 57: 188–193 [DOI] [PubMed] [Google Scholar]
- Proietti L., Spicuzza L., Polosa R. (2003) Urban air pollution at the crossroads of the allergic pandemic. Ann Ital Med Int 18: 64–72. [PubMed] [Google Scholar]
- Prüss-Üstün A., Corvalán C. (2006) Preventing disease through healthy environments: towards an estimate of the environmental burden of disease. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Pujades-Rodriguez M., McKeever T., Lewis S., Whyatt D., Britton J., Venn A. (2009) Effect of traffic pollution on respiratory and allergic disease in adults: cross-sectional and longitudinal analyses. BMC Pulm Med 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O., Andersen Z., Beelen R., Samoli E., Stafoggia M., Weinmayr G., et al. (2013) Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 14: 813–822. [DOI] [PubMed] [Google Scholar]
- Salvi S., Barnes P. (2009) Chronic obstructive pulmonary disease in non-smokers. Lancet 374: 733–743. [DOI] [PubMed] [Google Scholar]
- Sauerzapf V., Jones A., Cross J. (2009) Environmental factors and hospitalisation for chronic obstructive pulmonary disease in a rural county of England. J Epidemiol Commun Health 63: 324–328. [DOI] [PubMed] [Google Scholar]
- Sava F., Carlsten C. (2012) Respiratory health effects of ambient air pollution: an update. Clin Chest Med 33: 759–769. [DOI] [PubMed] [Google Scholar]
- Schikowski T., Ranft U., Sugiri D., Vierkötter A., Brüning T., Harth V., et al. (2010) Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir Res 11: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T., Sugiri D., Ranft U., Gehring U., Heinrich J., Wichmann H., et al. (2005) Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res 6: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S., Apsley A., Azmina Ibrahim T., Turner S., Cherrie J. (2014) Fine particulate matter concentrations in smoking households: just how much secondhand smoke do you breathe in if you live with a smoker who smokes indoors? Tob Control. DOI: 10.1136/tobaccocontrol-2014-051635. [DOI] [PubMed] [Google Scholar]
- Spirić V., Janković S., Vraneš A., Maksimović J., Maksimovic N. (2012) The impact of air pollution on chronic respiratory diseases. Pol J Environ Stud 21: 481–490. [Google Scholar]
- Tao Y., Mi S., Zhou S., Wang S., Xie X. (2014) Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ Pollut 185: 196–201. [DOI] [PubMed] [Google Scholar]
- Trupin L., Balmes J., Chen H., Eisner M., Hammond S., Katz P., et al. (2010) An integrated model of environmental factors in adult asthma lung function and disease severity: a cross-sectional study. Environ Health 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency (US EPA) (2004) Final regulatory analysis: control of emissions from nonroad diesel engines. EPA420-R-04-007. Washington DC: US EPA; Available at: http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P10003DE.TXT (accessed 8 May 2015). [Google Scholar]
- US Environmental Protection Agency (US EPA) (2014) Nitrogen dioxide. Washington DC: US EPA; Available at: http://www.epa.gov/oaqps001/nitrogenoxides/health.htm (accessed 8 May 2015). [Google Scholar]
- Villeneuve P., Parent M., Sahni V., Johnson K. Canadian Cancer Registries Epidemiology Research Group (2011) Occupational exposure to diesel and gasoline emissions and lung cancer in Canadian men. Environ Res 111: 727–735. [DOI] [PubMed] [Google Scholar]
- Vineis P., Hoek G., Krzyzanowski M., Vigna-Taglianti F., Veglia F., Airoldi L., et al. (2006) Air pollution and risk of lung cancer in a prospective study in Europe. Int J Cancer 119: 169–174. [DOI] [PubMed] [Google Scholar]
- Vineis P., Hoek G., Krzyzanowski M., Vigna-Taglianti F., Veglia F., Airoldi L., et al. (2007) Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ying Q., Hu J., Zhang H. (2014) Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013–2014. Environ Int 73: 413–422. [DOI] [PubMed] [Google Scholar]
- Weisel C., Cody R., Lioy P. (1995) Relationship between summertime ambient ozone levels and emergency department visits for asthma in central New Jersey. Environ Health Perspect 103(Suppl. 2): 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014) Ambient (outdoor) air quality and health. Fact sheet Np. 313. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Willart M., Lambrecht B. (2009) The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 39: 12–19. [DOI] [PubMed] [Google Scholar]
- World Bank (2007) Cost of pollution in China. Washington, DC: World Bank. [Google Scholar]
- Yamazaki S., Shima M., Nakadate T., Ohara T., Omori T., Ono M., et al. (2014) Association between traffic-related air pollution and development of asthma in school children: cohort study in Japan. J Expo Sci Environ Epidemiol 24: 372–379. [DOI] [PubMed] [Google Scholar]
- Young M., Sandler D., DeRoo L., Vedal S., Kaufman J., London S. (2014) Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med 190: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A., Schwartz J., Dockery D. (2000) Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ Health Perspect 108: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuraimi M., Tham K., Chew F., Ooi P., Koh D. (2011) Home air-conditioning, traffic exposure, and asthma and allergic symptoms among preschool children. Pediatr Allergy Immunol 22: e112–e118. [DOI] [PubMed] [Google Scholar]