Abstract
Ferric citrate is a novel phosphate binder that allows the simultaneous treatment of hyperphosphatemia and iron deficiency in patients being treated for end-stage renal disease with hemodialysis (HD). Multiple clinical trials in HD patients have uniformly and consistently demonstrated the efficacy of the drug in controlling hyperphosphatemia with a good safety profile, leading the US Food and Drug Administration in 2014 to approve its use for that indication. A concurrent beneficial effect, while using ferric citrate as a phosphate binder, is its salutary effect in HD patients with iron deficiency being treated with an erythropoietin-stimulating agent (ESA) in restoring iron that becomes available for reversing chronic kidney disease (CKD)-related anemia. Ferric citrate has also been shown in several studies to diminish the need for intravenous iron treatment and to reduce the requirement for ESA. Ferric citrate is thus a preferred phosphate binder that helps resolve CKD-related mineral bone disease and iron-deficiency anemia.
Keywords: efficacy, ferric citrate, hemodialysis, hyperphosphatemia, iron deficiency, safety
Introduction
End-stage renal disease (ESRD) requiring renal replacement therapy (RRT) in the form of dialysis is a complex clinical condition that affects multiple organ systems and requires a multifaceted therapeutic approach.
Among the organ systems directly affected in ESRD that require therapeutic intervention are the skeletal and hematopoietic systems. It has long been recognized that hyperphosphatemia, due to the reduced clearance of phosphate by the failing kidney, is an important contributor to renal bone disease [Bricker et al. 1969; Molony and Stephens, 2011]. It has more recently been recognized that hyperphosphatemia is also an independent risk factor for cardiovascular disease that is associated with increased mortality in ESRD [Covic et al. 2009; Isakova et al. 2009; Molony and Stephens, 2011]. Iron deficiency, which has a multifactorial etiology in chronic kidney disease (CKD), is an important contributor to anemia of ESRD, particularly in patients concurrently treated with an erythropoietin-stimulating agent (ESA) [Wittwer, 2013].
A large number of patients treated with dialysis exhibit simultaneous hyperphosphatemia and iron deficiency that require therapeutic intervention in the form of phosphate binders and iron supplementation. Hyperphosphatemia and iron deficiency are thus two major foes in the patient with CKD on dialysis, and treatment of both conditions is often challenging. The challenge is due to the limited efficacy of the therapeutic agents currently available, the side effects of the individual drugs, economic constraints, poor patient adherence to treatment, and in particular, a large pill load.
Treatment of hyperphosphatemia
Hyperphosphatemia in patients with CKD is mostly diet dependent, resulting from an imbalance between the amount of phosphate ingested and the amount cleared by residual kidney function and dialysis [Galassi et al. 2014]. The treatment of hyperphosphatemia in patients with CKD has therefore been based on oral ingestion of compounds that absorb phosphate within the gastrointestinal tract and prevent phosphate absorption [Hutchison et al. 2011]. Such compounds are usually composed of two elements, one having a strong affinity for phosphate. Once ingested, these compounds dissociate, with one element binding phosphate, and the other binding to other elements that are present within the gastrointestinal tract.
Historically, the first phosphate binder used in patients with CKD was aluminum hydroxide [Bailey et al. 1971; Morgan and Gabriel, 1979; Bellinghieri et al. 2007]. Aluminum is a potent binder of phosphate, and the hydroxide molecule that dissociates from aluminum binds to protons within the upper gastrointestinal tract, thus acting simultaneously as a phosphate binder and an anti-acid. Unfortunately, however effective aluminum hydroxide has proven to be as a phosphate binder, its use had to be discontinued because of concurrent toxicity. After many years during which aluminum hydroxide served as the mainstay of therapy to prevent hyperphosphatemia, clinicians came to realize that part of the ingested aluminum was absorbed in its elemental form in the gastrointestinal tract. This led to aluminum accumulation in various organ systems and to the unforeseen, yet catastrophic development of complications such as aluminum-induced osteomalacia due to the accumulation of aluminum within the skeletal system, dementia due to accumulation of aluminum in the brain, and anemia due to aluminum accumulation in the bone marrow [Van de Vyver et al. 1987].
Aluminum hydroxide was superseded by calcium carbonate, calcium being an effective binder of phosphate and carbonate acting as an extracellular buffer that is beneficial in the acidic uremic milieu [Emmett, 2004; Bellinghieri et al. 2007]. The efficacy of calcium carbonate as a phosphate binder, however, was lower than that of aluminum, often requiring large doses of the compound [Emmett, 2004], and imposing a large pill burden. Calcium acetate was subsequently introduced as a phosphate binder as it was found to be more effective than calcium carbonate [Mai et al. 1989]. Calcium-based phosphate binders also turned out to be inexpensive and became the preferred mode of treatment for hyperphosphatemia in CKD. Within several years, however, clinicians discovered that a significant part of the ingested calcium was absorbed, leading to an increased calcium load in CKD patients and the development of soft tissue and vascular calcifications and its potential harmful consequences [London et al. 2008].
The realization that the large calcium burden was detrimental to the patients’ health led to the search for other phosphate binders that were neither aluminum nor calcium based. Two compounds emerged almost simultaneously, lanthanum carbonate and sevelamer hydrochloride, the latter being switched eventually to sevelamer carbonate [Bellinghieri et al. 2007]. Both lanthanum and sevelamer are potent phosphate binders commonly used at present and that allow efficient control of hyperphosphatemia in patients with CKD and ESRD [Malberti, 2013].
Treatment of iron deficiency
Iron deficiency in patients with advanced CKD and in those on RRT is often due to multiple causes, and is aggravated when patients are treated simultaneously with ESA, when the demand for iron increases [Eckardt, 2000]. The only effective treatment has been replenishment of the iron stores. Attempts to achieve this goal with oral iron preparations have met with failure, mostly due to the relatively poor iron absorption from the gastrointestinal tract and to poor patient tolerability of available compounds [Macdougall, 1999]. Most patients in predialysis or on dialysis with iron deficiency consequently require intermittent intravenous iron treatment [Bailie et al. 2000]. Although effective, intravenous iron treatment, as opposed to oral treatment, is not without risk [Kovesdy and Kalantar-Zadeh, 2009], as the intravenous route necessarily carries with it an inherent risk of introducing infection. The need for repeated use of veins for intravenous infusions is also undesirable in patients with advanced CKD, when patent and ‘healthy’ veins become a precious commodity to be spared for future use in the construction of arteriovenous shunts for hemodialysis (HD). Finally, intravenous administration of iron is at times most inconvenient to patients, particularly to those not yet on dialysis, as it requires repeated attendance at a medical facility especially for that purpose.
Ferric citrate – a novel iron-delivering phosphate binder
Ferric citrate (Figure 1), also known as KRX0502 and JTT-751, was recently developed, primarily as a novel oral phosphate binder, in response to the need for improved phosphate binding in CKD patients. Based on the ingredients from which ferric citrate is composed, it could theoretically fulfill three beneficial and desirable functions in CKD patients: as a phosphate binder, as a supplier of elemental iron, and as an acid buffer.
Figure 1.
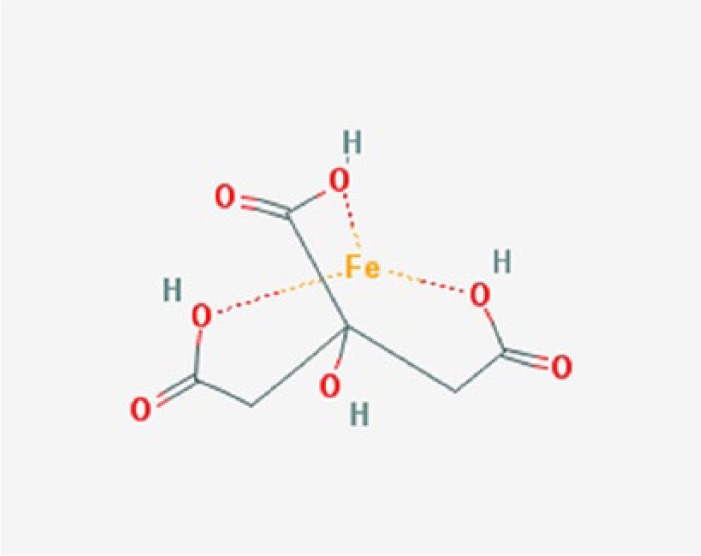
Chemical structure of ferric citrate (http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=4989393&t=l).
As a phosphate binder, the ferric ion in ferric citrate combines with dietary phosphorus, once it dissociates in the gastrointestinal tract from the parent compound. Ferric phosphate is generated, and is insoluble. As this new compound is excreted in the feces, dietary phosphate is disposed of, rather than absorbed. The ability of ferric salts to decrease net intestinal absorption of phosphate was in fact first demonstrated experimentally in a metabolic study in the rat by Hsu and colleagues as early as 1999 [Hsu et al. 1999].
As a supplier of elemental iron, not all the ferric ion that dissociates from ferric citrate binds phosphate. Some of it is reduced by the bowel mucosa to ferrous iron through the action of ferric reductase. Ferrous iron is then trapped in the duodenal mucosa and can be effectively reabsorbed into the systemic circulation, thus replenishing iron stores when required.
Citrate, which dissociates in the gastrointestinal tract from the iron element in ferric citrate, by virtue of its being a weak acid, can act as a buffer. Whether citrate acts locally as a buffer within the upper gastrointestinal tract or is absorbed and contributes to buffering of the extracellular compartment, remains to be established. Both effects, if shown to be effective, would be beneficial to the patient with CKD who has a high incidence of peptic disease [Liang et al. 2014], and who invariably expresses metabolic acidosis [Chen and Abramowitz, 2014].
Thus, ferric citrate appears to provide an ideal solution to at least two, perhaps three common problems encountered in advanced CKD, that is, hyperphosphatemia and iron deficiency, and also possibly to metabolic acidosis, in an all-in-one pill form.
Since the development of ferric citrate, multiple studies have been conducted to determine its efficacy and safety as a phosphate binder and more recently as a means to simultaneously replenish iron stores by the oral route. The principal clinical studies that were published are listed in Table 1.
Table 1.
Principal clinical trials demonstrating the efficacy and safety of ferric citrate.
| Year of publication | Authors | Journal | Title | Duration of treatment | Study design | Phase | Study population | Sample size n |
|---|---|---|---|---|---|---|---|---|
| 2002 | Yang et al. | Nephrol Dial Transplant | An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. | 4 weeks | Open-label, random- order, crossover comparison | HD | 55 | |
| 2012 | Sinsakul et al. | Nephron Clin Pract | The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. | 4 weeks | Open-label | 2 | HD | 55 |
| 2012 | Yokoyama et al. | Am J Nephrol | Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. | 4 weeks | Multicenter, rando-mized, placebo-controlled, double-blind, parallel-group comparative | HD | 192 | |
| 2013 | Dwyer et al. | Am J Kidney Dis | Dose-response and efficacy of ferric citrate to treat hyperphospha-temia in hemodialysis patients: a short-term randomized trial. | 4 weeks | Dose-response and efficacy | 2 | HD | 151 |
| 2013 | Umanath et al. | Hemodial Int | Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. | 58 weeks | Randomized active- control, open-label | 3 | Dialysis | 441 |
| 2014 | Block et al. | Am J Kidney Dis | A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3–5. | 12 weeks | Double-blind, placebo-controlled, randomized | CKD 3–5 | 149 | |
| 2014 | Lewis et al. | J Am Soc Nephrol | Ferric citrate controls phosphorus and delivers iron in patients on dialysis. | 52 weeks | Randomized active- control | HD | 441 | |
| 2014 | Yokoyama et al. | Nephron Clin Pract | JTT-751 for treatment of patients with hyperphosphatemia on peritoneal dialysis. | 12 weeks | Multicenter, open-label, dose-adjusted | 3 | PD | 56 |
| 2014 | Yokoyama et al. | Nephrol Dial Transplant | A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. | 12 weeks | Multicenter, randomized, open-label, parallel-group | 3 | HD | 230 |
| 2014 | Yokoyama et al. | J Ren Nutr | Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. | 52 weeks | Multicenter, open-label, dose- titration, long-term | 3 | HD | 180 |
| 2014 | Yokoyama et al. | Clin J Am Soc Nephrol | Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. | 12 weeks | Multicenter, rando-mized, double- blind, placebo-controlled | 3 | CKD | 90 |
CKD, chronic kidney disease; HD, hemodialysis; PD, peritoneal dialysis.
What is the evidence that ferric citrate is an effective phosphate binder in patients on HD?
There are at least seven published studies of variable duration that provide strong evidence that treatment of hyperphosphatemia with ferric citrate in dialysis patients is safe and efficacious. Four studies were short term (4 weeks), one was medium term (12 weeks), and two were long term (48 weeks and 52 weeks).
The first early report was by Yang and colleagues who extended the observations of Hsu and colleagues from animals to humans. Yang was first to perform an open-label, random-order, crossover study, comparing one dose of ferric citrate (3 g/day) with calcium carbonate (3 g/day) in the treatment of hyperphosphatemia in 23 female and 22 male HD patients over a period of 4 weeks [Yang et al. 2002]. Subjects were from Taiwan and of Chinese descent. The results showed that both drugs induced a significant reduction of serum phosphorus levels, with a somewhat lower efficacy of ferric citrate as a phosphate binder at the given dose, suggesting the need for further studies aimed at finding the effective dose of ferric citrate.
A decade later, Sinsakul and colleagues published the results of a phase II, open-label study in 23 female and 32 male HD patients, evaluating the short-term (4 weeks) safety and tolerability of ferric citrate as a phosphate binder [Sinsakul et al. 2012]. Male and female subjects were recruited to the study in the USA and were racially and ethnically diverse, including African Americans and Hispanics. The use of phosphate binders was discontinued and patients were started on ferric citrate 4.5–6.0 g/day, the dose being titrated over 4 weeks to maintain a phosphorus level of 1.1–1.7 mmol/L. At the end of the study, phosphorus and calcium levels were unchanged from baseline. Adverse effects included stool discoloration (69%), constipation (15%), and bloating (7%). The authors concluded that ferric citrate was well tolerated by patients after 4 weeks of treatment with ferric citrate, with no significant clinical or biochemical adverse events.
At the end of the same year, Yokoyama and colleagues published the results of a larger multicenter, randomized, placebo-controlled, double-blind, parallel-group, comparative phase II study in Japan [Yokoyama et al. 2012]. A total of 72 female and 120 male HD patients with hyperphosphatemia between 1.9 mmol/L and 3.2 mmol/L were randomized to ferric citrate (1.5 g/day, 3 g/day, or 6 g/day), or to placebo treatment for 4 weeks. Changes in serum phosphorus level from baseline were examined. The results demonstrated a dose-response relationship up to 6 g/day. The most common adverse side effects were gastrointestinal, but were of mild nature. The authors concluded that ferric citrate was efficacious and safe with the majority of patients achieving normal phosphorus levels with the 6 g/day dose.
A year later, Dwyer and colleagues reported on a 4-week randomized, open-label, prospective, multicenter, dose-response and efficacy phase III study of oral ferric citrate in the treatment of hyperphosphatemia in 151 HD patients [Dwyer et al. 2013]. Subjects were men and women of mixed race and ethnicity, including African Americans and Hispanics, all recruited in the USA. Ferric citrate was provided at a fixed dose of 1 g/day, 6 g/day, or 8 g/day. Phosphorus levels decreased in a dose-dependent manner (−0.03 ± 0.4 mmol/L, −0.6 ± 0.5 mmol/L, and −0.7 ± 0.6 mmol/L in the 1 g/day, 6 g/day, and 8 g/day group, respectively). There was no statistically significant difference in reduction in phosphorus levels between the 6 g/dose and 8 g/dose groups. The most common adverse event was stool discoloration. The authors concluded that ferric citrate was efficacious as a phosphate binder in a dose-dependent manner.
Yokoyama and colleagues published one year later, a longer 12-week, phase III, multicenter, randomized, open-label, parallel-group study, in which they compared the efficacy and safety of ferric citrate (1.5 g/day and 6.0 g/day) to sevelamer hydrochloride (3.0 g/day and 9.0 g/day) in 230 Japanese patients undergoing HD [Yokoyama et al. 2014b]. The primary outcome, the change in serum phosphorus from baseline to end of treatment, was not different between the groups, establishing noninferiority of ferric citrate to sevelamer. Gastrointestinal disorders were the most common adverse events in both groups; the incidence of diarrhea was higher in the ferric citrate group, while constipation occurred frequently in the sevelamer group. The author concluded that the efficacy and safety of ferric citrate were comparable with sevelamer in patients on HD with hyperphosphatemia.
In 2013, Umanath and colleagues announced the onset of an ongoing long-term phase III study on the use of ferric citrate for the treatment of hyperphosphatemia, describing the rationale and study design [Umanath et al. 2013]. The results of the study were published 1 year later by Lewis and colleagues [Lewis et al. 2014]. The study was a randomized, open-label, active-control design and the subjects consisted of 270 male and 171 female HD and peritoneal dialysis (PD) patients of mixed race and ethnicity recruited in the USA and Israel. The patients were treated with ferric citrate as the study drug (n = 292), or calcium acetate or/and sevelamer carbonate as the active control (n = 141) for 52 weeks. The dosage of the study drugs, as well as that of the active control, was titrated upwards, so as to achieve normalization of phosphorus levels. During this study period, ferric citrate treatment achieved a similar reduction in phosphorus levels as the active control. At the end of the long-term study period, subjects, who completed the 52-week active-control period on ferric citrate, were randomized for an additional 4 weeks to ferric citrate or placebo. During this placebo-control period, ferric citrate reduced serum phosphorus significantly more than placebo (–0.7 + 0.06 mmol/L, p < 0.001). The safety profiles of both study and control groups were not different. The authors concluded that ferric citrate is an efficacious and safe phosphate binder.
Yokoyama and colleagues published recently yet another study on the long-term safety and efficacy of ferric citrate in patients on HD [Yokoyama et al. 2014c]. In this phase III, 52-week, multicenter, open-label, dose-titration, long-term study, 180 Japanese patients on HD were treated with ferric citrate (dose 1.5–6.0 g/day). Dose titration effectively decreased serum phosphorus levels throughout the entire duration of the trial. The most common adverse events were gastrointestinal disorders, which were mild to moderate in intensity. The authors concluded that ferric citrate at a dose of 1.5–6.0 g/day controls serum phosphorus concentrations in patients receiving HD.
Is there evidence that ferric citrate is effective in treating hyperphosphatemia in other forms of dialysis?
Are there data to attest to the efficacy and safety of ferric citrate as a phosphate binder in patients treated with other forms of dialysis, such as hemodiafiltration (HDF) or PD? No data are currently available regarding HDF. One study has been published so far on the use of ferric citrate exclusively in PD [Yokoyama et al. 2014a], and another study included PD patients, although only a few patients were enrolled in that study [Lewis et al. 2014].
Yokoyama and colleagues published most recently the results of a phase III, 12-week, multicenter, open-label, dose-adjusted study investigating the efficacy and safety of ferric citrate in 53 Japanese patients with serum phosphorus between 1.8 mmol/L and 3.2 mmol/L undergoing PD [Yokoyama et al. 2014a]. The dose of ferric citrate was 1.5–6.0 g/day, targeting serum phosphorus 1.1–1.7 mmol/L. Serum phosphorus was reduced by 0.7 mmol/L (p < 0.001), and 76.8% of patients achieved target levels. The most common adverse reactions were diarrhea and constipation, most of them mild. The authors concluded that in PD patients with hyperphosphatemia, 12-week treatment with ferric citrate resulted in significant reductions in serum phosphorus and was well tolerated.
In the study published by Lewis and colleagues, no subanalysis was provided yet for the results of the treatment specifically in the PD-treated population [Lewis et al. 2014]. In a subsequent subanalysis of the data (results of which were presented as a poster at the EDTA-ERA conference in London 2015), ferric citrate proved to be an effective phosphate binder in the small sub-population of PD patients and effectively increased iron stores and reduced iron and ESA utilization, while maintaining hemoglobin levels.
The available data in PD patients are so far scarce, but nonetheless encouraging. More studies are required to ensure the safety and efficacy of ferric citrate in PD.
Is there evidence that ferric citrate is effective in treating hyperphosphatemia in nondialysis CKD patients?
If ferric citrate is safe and effective in treating hyperphosphatemia in patients with CKD on dialysis, it stands to reason that similar efficacy and safety should be attained in the ‘predialysis’ population, patients with CKD 4 or 5a (advanced chronic kidney disease but not yet requiring dialysis) who already exhibit the early signs of CKD-related mineral bone disease. To date, only a few studies have tested the safety and efficacy of ferric citrate in nondialysis CKD patients.
Yokoyama and colleagues recently published the results of a phase III, multicenter, randomized, double-blind, placebo-controlled study investigating the efficacy and safety of ferric citrate hydrate in 90 nondialysis Japanese patients with CKD 4–5a [Yokoyama et al. 2014d]. Patients with a serum phosphorus ⩾ 1.6 mmol/L were randomized 2:1 to ferric citrate or placebo for 12 weeks. The primary endpoint was change in serum phosphate from baseline to end of treatment. The mean change in serum phosphate was −0.41 mmol/L in the ferric citrate group and 0.01 mmol/L in the placebo group (p < 0.001), and 64.9% in the ferric citrate group and 6.9% in the placebo group achieved target levels (p < 0.001). Five patients discontinued treatment because of adverse events with ferric citrate, versus one patient with placebo. Overall, adverse drug reactions were similar in patients receiving ferric citrate or placebo, with gastrointestinal disorders occurring in 30.0% of ferric citrate patients and 26.7% of patients receiving placebo. The authors concluded that in CKD patients not yet on dialysis, a 12-week treatment with ferric citrate was efficacious in reducing serum phosphate.
Block and colleagues reported similar results in another randomized trial in the USA in 149 patients with CKD 3–5a and serum phosphorus levels 1.3–1.9 mmol/L who were treated for 12 weeks with ferric citrate or placebo [Block et al. 2014]. Treatment with ferric citrate reduced serum phosphorus levels from 1.4 ± 0.2 mmol/L to 1.2 ± 0.2 mmol/L, while placebo exerted a lesser effect (1.5 ± 0.2 mmol/L to 1.4 ± 0.2 mmol/L; between-group p < 0.001). The authors concluded that short-term use of ferric citrate reduces levels of serum phosphate in patients with CKD 3–5a.
These preliminary data on the ability of ferric citrate to effectively reduce serum phosphorus levels in this large ‘predialysis’ CKD-patient population are most encouraging. More studies, however, are required before approval can be considered for this segment of the population that is much larger than that already on RRT (http://www.usrds.org/2014/view/Default.aspx).
What is the evidence that ferric citrate is effective in replenishing iron stores and availability in dialysis patients?
Most of the studies that investigated the efficacy and safety of ferric citrate as a phosphate binder simultaneously monitored the effect of the treatment on iron variables. Several of the more recent studies aimed primarily to determine the effect of ferric citrate on iron stores and iron-deficiency anemia. Most of the studies were carried out in patients on RRT with HD. One of the studies was carried out in predialysis patients (CKD 4–5a).
In the early study in the rat by Hsu and colleagues, ferric citrate was shown to increase significantly plasma iron levels (p < 0.005) [Hsu et al. 1999]. In the early short-term human study by Yang and colleagues, 4 weeks of treatment with ferric citrate only tended to increase plasma iron levels but achieved a marginal, yet significant increase in plasma ferritin (p < 0.03), suggesting that a small amount of iron was absorbed [Yang et al. 2002].
A later phase II, short-term, 4-week study tested the efficacy and safety of ferric citrate as a phosphate binder in HD patients [Sinsakul et al. 2012]. The investigators in that study also looked at iron parameters. To avoid recruitment of subjects with hemochromatosis, subjects with serum ferritin ⩾ 1000 µg/L or transferrin iron saturation (TSAT) ⩾ 50% at screening were excluded. Ferritin levels at the beginning of the study of 554 ± 296 µg/L increased to 609 ± 340 µg/L (p = 0.02), iron levels increased from 12 ± 4 µmol/L to 13 ± 5 µmol/L (p = 0.04), and iron saturation from 30 ± 7.8% to 35 ± 13% (p = 0.001). The authors noted these changes, commenting that this amount of iron absorption did not warrant discontinuation of the study drug nor suggest excessive gastrointestinal tract iron absorption.
In a medium-term, 12-week study by Yokoyama and colleagues in HD patients [Yokoyama et al. 2014b], changes in ferritin levels, transferrin saturation, and ESA dosage were looked upon as additional outcomes. Treatment with ferric citrate increased significantly ferritin levels and transferrin saturation.
In the long-term, 52-week, active-control study in dialysis patients published by Lewis and colleagues, ferric citrate was studied specifically also as an alternative source for iron replenishment [Lewis et al. 2014]. One of the inclusion criteria in that study was that the patients have TSAT < 50% and serum ferritin < 1000 µg/L. Treatment with ferric citrate resulted in a rise in plasma iron from 12.9 µmol/L to 14.7 µmol/L, in transferrin saturation from 31.3% to 36.0%, and in serum ferritin levels from 593 ng/ml to 858 ng/ml, all these changes being highly statistically significant. The rise in transferrin saturation was fairly rapid, plateauing at 12 weeks, whereas serum ferritin rate of rise decreased at 24 weeks of treatment. Compared with the active-control groups, ferric citrate also significantly reduced ESA usage by 25% (median epoetin-equivalent units per week, ferric citrate 5306 units/week, active-control group 6951 units/week, p = 0.04), and the need for administration of intravenous iron by about 50% (approximately 13 mg/week ferric citrate, approximately 27 mg/week active-control group, p < 0.001). The authors concluded that ferric citrate is efficacious and safe in increasing iron stores and that this mode of therapy reduces intravenous iron and ESA use, while maintaining hemoglobin.
In their long-term, 52-week study in HD patients, Yokoyama and colleagues reported that treatment with ferric citrate increased ferritin levels to a peak after 28 weeks, stabilizing thereafter, and decreased markedly the need for intravenous iron and, by 25%, the dose of ESA [Yokoyama et al. 2014c]. The authors concluded that ferric citrate reduces the need for intravenous iron and ESA in HD patients.
In patients with ESRD treated with PD, Yokoyama and colleagues reported in their 12-week study that ferric citrate significantly increased transferrin saturation, as well as ferritin levels [Yokoyama et al. 2014a].
In patients with CKD 3–5 not on RRT, Block and colleagues reported that in the course of a 12-week treatment with ferric citrate, they observed a significant increase in transferrin saturation and hemoglobin levels [Block et al. 2014]. In another 12-week study in a CKD 4–5a population, Yokoyama and colleagues also reported a significant increase in plasma iron, transferrin saturation, and ferritin levels, compared with placebo [Yokoyama et al. 2014d].
Abundant data are thus emerging that even though the primary aim of instituting therapy with ferric citrate had been control of serum phosphorus levels in patients with advanced CKD, the byproduct of the treatment with ferric citrate has been a significant increase in transferrin saturation, and therefore of available iron, and in ferritin levels suggesting increased iron storage. This reported byproduct of the treatment has uniformly led to a highly desirable outcome, consisting of a decrease in the requirement for intravenous iron therapy and in the dosage of ESA.
Potential adverse effects of ferric citrate
Along with the desirable outcome of iron replenishment in iron-deficient patients with CKD, the risk of iron overload when treating CKD patients for hyperphosphatemia with ferric citrate cannot be overlooked. A significant percentage of patients with CKD are hyperphosphatemic and yet not iron deficient, and they might be considered at a risk of developing iron overload if treated with ferric citrate. Therefore, treatment of hyperphosphatemia with ferric citrate in that segment of the population is best avoided.
Another potential risk during treatment with ferric citrate is aluminum toxicity, which may occur in CKD patients who ingest aluminum-containing compounds [Molitoris et al. 1989], as citrate markedly enhances gastrointestinal aluminum absorption [Froment et al. 1989]. Since the clinical use of aluminum containing compounds is currently avoided in CKD patients, the risk of aluminum toxicity with ferric citrate is marginal in clinical practise.
Additional biochemical effects of ferric citrate
Serum bicarbonate
The citrate anion that dissociates from ferric citrate can be metabolized to bicarbonate and provide additional extracellular buffering. Several of the studies testing the clinical efficacy and safety of ferric citrate have monitored serum bicarbonate before, during, and after treatment. The resulting data indicate that treatment with ferric citrate has a variable effect on serum bicarbonate levels, ranging from a mild, yet statistically significant, increase in serum bicarbonate levels after 4 weeks of treatment [Sinsakul et al. 2012], to no effect on serum bicarbonate levels during short-term [Yang et al. 2002], and long-term treatment [Lewis et al. 2014; Yokoyama et al. 2014c]. The ferric citrate-induced increase in serum bicarbonate, at least during short-term treatment, may be dose related [Sinsakul et al. 2012; Dwyer et al. 2013]. We conclude that the data available, so far, on the effect of ferric citrate on serum bicarbonate levels appear to show no effect or only a mild effect, which does not appear to be clinically significant.
Serum calcium
During treatment with ferric citrate, the resulting reduction in serum phosphorus levels is expected to result in a rise in serum calcium levels. The data obtained in the clinical studies on the efficacy and safety of ferric citrate indicate a variable response. During short-term treatment with ferric citrate (4 weeks), a mild increase in serum calcium levels was observed, with no dose response and the values remaining within normal levels [Yokoyama et al. 2012; Dwyer et al. 2013]. During mid-term treatment (12 weeks), serum calcium significantly increased from baseline, but the change was of mild magnitude and not considered clinically meaningful [Yokoyama et al. 2014a, 2014b, 2014d]. During long-term treatment with ferric citrate (52 weeks), ferric citrate did not change serum calcium levels [Lewis et al. 2014; Yokoyama et al. 2014c].
Serum intact parathyroid hormone (iPTH) levels
Correction of hyperphosphatemia is anticipated to decrease serum iPTH levels. The clinical studies with ferric citrate have indeed shown a reduction in serum iPTH levels during short-term treatment [Yokoyama et al. 2012], and intermediate therapy [Yokoyama et al. 2014a, 2014b, 2014d]. Following long-term treatment with ferric citrate, the results vary from no change [Yokoyama et al. 2014c], to a significant reduction in iPTH serum levels [Lewis et al. 2014].
Serum fibroblast growth factor 23 (FGF23)
Correction of hyperphosphatemia is expected physiologically to bring about a reduction in the compensatory increase in FGF23 levels that is observed in patients with CKD. Data on the effect of ferric citrate on FGF23 levels are only available, so far, from two clinical studies. Treatment with ferric citrate over 12 weeks resulted in a significant reduction in FGF23 levels in both studies [Block et al. 2014; Yokoyama et al. 2014d].
US Food and Drug Administration (FDA) approval
Based on a large number of reports on the safety and efficacy of ferric citrate as a phosphate binder, the FDA in September 2014 approved the marketing of ferric-citrate tablets as a means of controlling serum phosphorus concentration in patients undergoing dialysis [Thompson, 2014].
According to the product’s FDA-approved labeling, the starting dosage of ferric citrate is two 1 g tablets taken three times daily with meals (amounting to 6 g/day). The dosage can be increased or decreased by one or two tablets at 1-week or longer intervals to control a patient’s serum phosphorus concentration. No more than 12 tablets should be taken in 1 day. The most commonly reported adverse events in patients who received ferric citrate were diarrhea, nausea, constipation, vomiting, and cough.
The FDA approval recognizes that some of the iron in ferric citrate may be absorbed from the gastrointestinal tract, and therefore concludes that the drug is contraindicated in patients when iron overload is present. On the other hand, although not specified in the FDA approval, the use of ferric citrate in conditions with concurrent hyperphosphatemia and iron deficiency, such as patients with advanced CKD, appears to be highly advantageous over other phosphate binders.
Interestingly, the FDA approval states that ferric citrate can be used in patients undergoing dialysis, without stating which mode of dialysis. The vast majority of studies have been in patients undergoing standard HD. Only a few studies have investigated its use in PD, and none in patients on HDF. Should this approval be extrapolated to patients being treated for ESRD with PD and HDF as well? FDA approval for the use of ferric citrate in CKD-patients not receiving dialysis has not yet been granted.
European Medicines Agency approval for ferric citrate as a phosphate binder is pending. In Japan, ferric citrate was approved in January 2014 for patients with all stages of CKD.
Other iron-based phosphate binders
Ferric citrate is not the only iron-based, calcium-free phosphate binder that has been approved by the FDA for the control of hyperphosphatemia. Sucroferric oxyhydroxide (Velphoro, formerly known as PA21) is another such compound that has most recently been approved by the FDA for such indication in patients with CKD on dialysis. The efficacy and safety of the drug has been compared with sevelamer [Floege et al. 2015], but no data are yet available that allow its comparison with ferric citrate. One seemingly important difference between the two compounds, however, is that iron is partially absorbed during administration of ferric citrate, but not during treatment with sucroferric oxyhydroxide, rendering the former advantageous when treating hyperphosphatemic patients who are also iron deficient [Negri and Urena Torres, 2015].
Conclusion
Based on the published data, ferric citrate appears to have a preferential place in clinical practice by virtue of its efficacy in the treatment of CKD-related hyperphosphatemia, and as an efficient mode of simultaneously loading the patient with iron in CKD-related anemia. Its efficacy and safety profile in patients on HD with hyperphosphatemia who require iron replacement has already been established. The major advantage of ferric citrate over other phosphate binders is that it provides simultaneous iron administration. This dual therapy in a one-pill form may render ferric citrate a preferred alternative to the use of sevelamer or lanthanum for hyperphosphatemia by avoiding or reducing the use of intravenous iron therapy for iron deficiency with its attendant risks and costs. The efficacy and safety of ferric citrate in patients with CKD not yet on dialysis and in patients with ESRD treated with PD appear promising but remain to be established with additional clinical trials. The use of ferric citrate in patients treated with HDF still remains to be studied.
Acknowledgments
We confirm that ethical committee approval was sought where necessary and is acknowledged within the text of the submitted manuscript. We confirm that guidelines on patient consent have been met and any details of informed consent obtained are indicated within the text of the submitted manuscript.
Footnotes
Funding: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors have received funds from Keryx Biopharmaceuticals as follows: YY, research; SZF, speaker bureau; KSK, research; UB, research; MS, research and travel; JBL, research, travel, and honoraria; DN, research.
Contributor Information
Yoram Yagil, Department of Nephrology and Hypertension, Barzilai University Medical Center, 2 Hahistadrut St, Ashkelon 78278, Israel.
Stephen Z. Fadem, Division of Nephrology, Baylor College of Medicine, Houston, TX, USA
Kotagal S. Kant, Division of Nephrology and Hypertension, University of Cincinnati, Cincinnati, OH, USA
Udayan Bhatt, Division of Nephrology, Ohio State University Wexner Medical Center, Columbus, OH, USA.
Mohammed Sika, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, USA.
Julia B. Lewis, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, USA
Dana Negoi, Department of Nephrology and Hypertension, University of Vermont Medical Group, Burlington, VT, USA.
References
- Bailey R., Eastwood J., Clarkson E., Luck V., Hynson W., O’Riordan J., et al. (1971) The effect of aluminium hydroxide on calcium, phosphorus and aluminium balances and the plasma parathyroid hormone in patients with chronic renal failure. Clin Sci 41: 5P–6P. [DOI] [PubMed] [Google Scholar]
- Bailie G., Johnson C., Mason N. (2000) Parenteral iron use in the management of anemia in end-stage renal disease patients. Am J Kidney Dis 35: 1–12. [DOI] [PubMed] [Google Scholar]
- Bellinghieri G., Santoro D., Savica V. (2007) Emerging drugs for hyperphosphatemia. Expert Opin Emerg Drugs 12: 355–365. [DOI] [PubMed] [Google Scholar]
- Block G., Fishbane S., Rodriguez M., Smits G., Shemesh S., Pergola P., et al. (2014) A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3–5. Am J Kidney Dis 65: 728–736. [DOI] [PubMed] [Google Scholar]
- Bricker N., Slatopolsky E., Reiss E., Avioli L. (1969) Caclium, phosphorus, and bone in renal disease and transplantation. Arch Intern Med 123: 543–553. [PubMed] [Google Scholar]
- Chen W., Abramowitz M. (2014) Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol 15: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic A., Kothawala P., Bernal M., Robbins S., Chalian A., Goldsmith D. (2009) Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 24: 1506–1523. [DOI] [PubMed] [Google Scholar]
- Dwyer J., Sika M., Schulman G., Chang I., Anger M., Smith M., et al. (2013) Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis 61: 759–766. [DOI] [PubMed] [Google Scholar]
- Eckardt K. (2000) Pathophysiology of renal anemia. Clin Nephrol 53: S2–S8. [PubMed] [Google Scholar]
- Emmett M. (2004) A comparison of clinically useful phosphorus binders for patients with chronic kidney failure. Kidney Int Suppl 66: S25–S32. [DOI] [PubMed] [Google Scholar]
- Floege J., Covic A., Ketteler M., Mann J., Rastogi A., Spinowitz B., et al. (2015) Long-term effects of iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 30: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froment D., Molitoris B., Buddington B., Miller N., Alfrey A. (1989) Site and mechanism of enhanced gastrointestinal absorption of aluminum by citrate. Kidney Int 36: 978–984. [DOI] [PubMed] [Google Scholar]
- Galassi A., Cupisti A., Santoro A., Cozzolino M. (2014) Phosphate balance in ESRD: diet, dialysis and binders against the low evident masked pool. J Nephrol 23 September 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hsu C., Patel S., Young E. (1999) New phosphate binding agents: ferric compounds. J Am Soc Nephrol 10: 1274–1280. [DOI] [PubMed] [Google Scholar]
- Hutchison A., Smith C., Brenchley P. (2011) Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 7: 578–589. [DOI] [PubMed] [Google Scholar]
- Isakova T., Gutierrez O., Chang Y., Shah A., Tamez H., Smith K., et al. (2009) Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdy C., Kalantar-Zadeh K. (2009) Iron therapy in chronic kidney disease: current controversies. J Ren Care 35(Suppl. 2): 14–24. [DOI] [PubMed] [Google Scholar]
- Lewis J., Sika M., Koury M., Chuang P., Schulman G., Smith M., et al. (2014) Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Muo C., Wang I., Chang C., Chou C., Liu J., et al. (2014) Peptic ulcer disease risk in chronic kidney disease: ten-year incidence, ulcer location, and ulcerogenic effect of medications. PLoS One 9: e87952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London G., Marchais S., Guerin A., Boutouyrie P., Metivier F., de Vernejoul M. (2008) Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall I. (1999) Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl 69: S61–S66. [DOI] [PubMed] [Google Scholar]
- Mai M., Emmett M., Sheikh M., Santa Ana C., Schiller L., Fordtran J. (1989) Calcium acetate, an effective phosphorus binder in patients with renal failure. Kidney Int 36: 690–695. [DOI] [PubMed] [Google Scholar]
- Malberti F. (2013) Hyperphosphataemia: treatment options. Drugs 73: 673–688. [DOI] [PubMed] [Google Scholar]
- Molitoris B., Froment D., Mackenzie T., Huffer W., Alfrey A. (1989) Citrate: a major factor in the toxicity of orally administered aluminum compounds. Kidney Int 36: 949–953. [DOI] [PubMed] [Google Scholar]
- Molony D., Stephens B. (2011) Derangements in phosphate metabolism in chronic kidney diseases/endstage renal disease: therapeutic considerations. Adv Chronic Kidney Dis 18: 120–131. [DOI] [PubMed] [Google Scholar]
- Morgan J., Gabriel R. (1979) Aluminium sucrose biscuits in the treatment of hyperphosphataemia. J Hum Nutr 33: 231–232. [PubMed] [Google Scholar]
- Negri A., Urena Torres P. (2015) Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney J 8: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsakul M., Sika M., Koury M., Shapiro W., Greene T., Dwyer J., et al. (2012) The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract 121: c25–c29. [DOI] [PubMed] [Google Scholar]
- Thompson C. (2014) Ferric citrate approved as phosphate binder for patients on dialysis. Am J Health Syst Pharm 71: 1822. [DOI] [PubMed] [Google Scholar]
- Umanath K., Sika M., Niecestro R., Connelly C., Schulman G., Koury M., et al. (2013) Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int 17: 67–74. [DOI] [PubMed] [Google Scholar]
- Van de Vyver F., Silva F., D’Haese P., Verbueken A., De Broe M. (1987) Aluminum toxicity in dialysis patients. Contrib Nephrol 55: 198–220. [DOI] [PubMed] [Google Scholar]
- Wittwer I. (2013) Iron deficiency anaemia in chronic kidney disease. J Ren Care 39: 182–188. [DOI] [PubMed] [Google Scholar]
- Yang W., Yang C., Hou C., Wu T., Young E., Hsu C. (2002) An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant 17: 265–270. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Akiba T., Fukagawa M., Nakayama M., Hirakata H. (2014a) JTT-751 for treatment of patients with hyperphosphatemia on peritoneal dialysis. Nephron Clin Pract 128: 135–140. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Akiba T., Fukagawa M., Nakayama M., Sawada K., Kumagai Y., et al. (2014b) A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant 29: 1053–1060. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Akiba T., Fukagawa M., Nakayama M., Sawada K., Kumagai Y., et al. (2014c) Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr 24: 261–267. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Hirakata H., Akiba T., Fukagawa M., Nakayama M., Sawada K., et al. (2014d) Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Hirakata H., Akiba T., Sawada K., Kumagai Y. (2012) Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36: 478–487. [DOI] [PubMed] [Google Scholar]


