Abstract
Diabetic macular oedema (DMO) is the most common cause of visual loss in the working age population. Intravitreal therapy has superseded macular laser as the first-line treatment for the management of centre-involving DMO in most patients. As well as the proven efficacy of intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents, phase II and III clinical trials of Ozurdex intravitreal dexamethasone implants for DMO have also demonstrated a mean increase in visual acuity and corresponding mean reduction in central macular thickness, particularly in pseudophakic eyes. Because of the risk of visual loss from cataract, glaucoma and intraocular infection with the use of intravitreal steroids, Ozurdex tends to be reserved for use in patients unresponsive to anti-VEGF therapy for centre-involving DMO. Situations where Ozurdex may be considered a first-line treatment option for eyes with centre-involving DMO include pseudophakia, impending cataract surgery, or in the context of a recent arterial thromboembolic event. Because of their stable pharmacokinetics, Ozurdex slow-release implants may also be considered in vitrectomized eyes.
Keywords: dexamethasone implant, diabetic macular oedema, pharmacotherapy
Introduction
The worldwide prevalence of diabetes mellitus was estimated at 366 million in 2011, with projections the prevalence of this chronic disease will reach 552 million people by 2030 [Whiting et al. 2011]. Diabetic macular oedema (DMO), affecting approximately 6.8% of all patients with diabetes, is the most common cause of visual loss in this predominantly working age population [Moss et al. 1998; Bunce and Wormald, 2008; Yau et al. 2012]. The introduction of intravitreal pharmacological therapies in the management of DMO has made it possible to improve rather than just stabilize vision, with the potential to significantly improve patients’ quality of life and economic independence [Hariprasad et al. 2008; Gonder et al. 2014]. This review summarizes treatment options available for DMO, details the main clinical trials of Ozurdex (Allergan, Inc., Irvine, CA) dexamethasone intravitreal implants in DMO, and concludes with our perspective on its place in current management.
Summary of current treatments
Modifying systemic risk factors
Targeting key modifiable systemic risk factors including glycaemic control and blood pressure can prevent or reduce DMO [Diabetes Control and Complications Trial Research Group, 1995; UK Prospective Diabetes Study (UKPDS) Group, 1998; Matthews et al. 2004]. Lipid lowering agents may also have a role in the management of DMO [Kiire et al. 2013]. Fenofibrate has been shown to have a beneficial effect in the prevention of DMO. Although it is primarily a lipid lowering drug, its mechanism of action in DMO appears to be independent of serum lipid concentration and is postulated to be due to an anti-vascular endothelial growth factor (anti-VEGF) effect [Keech et al. 2007; Wong et al. 2012].
Macular laser
Before the advent of anti-VEGF therapy, the standard of care for DMO management was macular laser. The Early Treatment Diabetic Retinopathy Study (ETDRS) trial undertaken in the 1980s confirmed the benefit of macular laser for DMO [Early Treatment Diabetic Retinopathy Study Research Group, 1985]. This trial found the 3 year risk of moderate visual loss, defined as a loss of 15 letters or 3 lines of LogMAR visual acuity, decreased by 50% in laser treated eyes. The incidence of clinically significant macula oedema (CSMO) decreased from 74% at baseline to 24% at 3 years. Of those who had a visual acuity worse than Snellen 6/12, less than 3% of treated eyes experienced 15 letter visual gain. Thus there was a major unmet need for better treatments of DMO. Newer approaches of delivering laser therapy with less collateral damage, such as subthreshold diode micropulse laser, have shown some early promising results but require a stronger evidence base before more widespread use [Romero-Aroca et al. 2014].
Vitrectomy
A recent systematic review identified little evidence to support vitrectomy as an intervention for DMO in the absence of epiretinal membrane or vitreomacular traction [Simunovic et al. 2014]. Furthermore, removal of the vitreous sump can reduce the length of time intravitreal pharmacological agents remain within the eye, making ongoing management of DMO more difficult [Chin et al. 2005].
Triamcinolone
Steroid therapy has anti-inflammatory, antipermeability and angiostatic effects in treating DMO [Wang et al. 2008]. The Triamcinolone for Diabetic Macular Oedema (TDMO) study was the first randomized clinical trial of intravitreal steroid for DMO. It was a 2 year, double-masked, placebo-controlled trial of intravitreal triamcinolone (Kenacort 40, Bristol-Myers Squibb Pharmaceuticals, Australia) in 69 eyes from 43 patients with DMO refractive to macular laser therapy [Gillies et al. 2006]. Repeated intravitreal injections of 4 mg triamcinolone were allowed at a maximum frequency of every 6 months. The primary outcome of improvement in best corrected visual acuity (BCVA) by ⩾5 logMAR letters at 2 years was achieved in 56% of triamcinolone treated eyes and 26% of the placebo group (p = 0.006). Compared with placebo, triamcinolone treated eyes had 5.7 letters mean greater improvement in BCVA and 59 µm mean greater reduction in foveal thickness. However, cataract surgery was required in 54% of subjects in the triamcinolone group compared with none in the placebo group. Elevated intraocular pressure (IOP) >5 mmHg was noted in 68% of triamcinolone treated eyes compared with 10% in the placebo group, and 5.9% of triamcinolone treated eyes required trabeculectomy surgery.
After 2 years, the TDMO study became open label and patients in the original placebo group could also be treated with triamcinolone. By 5 years, modest improvement in BCVA of ⩾5 letters was found in 42% of those eyes initially treated with intravitreal triamcinolone compared with 32% initially treated with placebo, but this finding was not statistically significant (p = 0.4). There was also no difference in the mean central macular thickness (CMT) reduction between the 2 groups after 5 years. The earlier use of triamcinolone did not reduce the need for retreatment between the third and fifth years. By 5 years, 9% of the initial triamcinolone group had required trabeculectomy and 71% had undergone cataract surgery [Gillies et al. 2009].
Kenacort 40 is not licensed for intraocular use, with reports that the preservative it contains may cause sterile endophthalmitis [Otsuka et al. 2013]. This has led to the development of preservative-free triamcinolone preparations including Triescence (Alcon, USA) and Trivaris (Allergan, USA). However, these preparations have different pharmacokinetic properties resulting in a shorter duration of action than Kenacort 40 [Zacharias et al. 2013].
The Diabetic Retinopathy Clinical Research Network (DRCRnet) has undertaken large clinical trials which have compared preservative-free triamcinolone (Trivaris) to other treatments, including macular laser and anti-VEGF agents. Protocol B was a randomized controlled trial comparing modified ETDRS macular laser (n = 330) against triamcinolone at either a dose of 1 mg (n = 256) or 4 mg (n = 254) [Diabetic Retinopathy Clinical Research Network, 2008]. All patients were eligible for re-treatment at 4 monthly intervals if DMO persisted. Although, the mean BCVA was better in the 4 mg triamcinolone group compared with both the 1 mg triamcinolone and the laser group at 4 months, by 2 years, the mean BCVA was better in the laser group than in either of the triamcinolone groups. By 3 years, the cumulative probability of having cataract surgery was 83% in the 4 mg triamcinolone group, 46% in the 1 mg triamcinolone group and 31% in the macular laser group. The IOP increased >10 mmHg from baseline at any visit over the 3 year study in 33% of the 4 mg triamcinolone group, with 5% having undergone glaucoma surgery. This compared with an IOP increase >10 mmHg from baseline in 18% in the 1 mg triamcinolone group and 4% in the macular laser group with no glaucoma surgery required in these groups. A limitation of this study was that only 36% of patients were able to achieve the 3-year follow up. DRCRnet protocol B had different baseline characteristics compared with the TDMO trial, having excluded eyes which investigators considered unlikely to benefit from macular laser treatment and therefore including eyes with on average milder disease. This may account for the contradictory results of the TDMO and DRCRnet trials. A small subgroup analysis of patients in the DRCRnet trial with severe visual impairment at baseline (BCVA 6/60 to 6/96) demonstrated that 77% of the 4 mg triamcinolone group versus 42% of the laser group experienced improvement of ⩾10 letters.
The DRCRnet group has also compared the use of 4 mg intravitreal preservative-free triamcinolone (Trivaris) combined with laser versus laser alone versus ranibizumab with prompt or deferred laser for the management of centre-involving DMO [Diabetic Retinopathy Clinical Research Network et al. 2010]. The trial (Protocol I) showed that BCVA outcomes for ranibizumab treated eyes were better than the triamcinolone treated eyes, except for those eyes which were pseudophakic at baseline where the visual acuity results were similar. However, raised IOP was more common in the triamcinolone treated eyes.
Fluocinolone
Fluocinolone acetonide was initially developed as a nonbiodegradable, surgically implanted device with sustained release over 3 years (Retisert®, Bausch and Lomb, Rochester, NY). A prospective randomized clinical trial of the Retisert implant (n = 127) against standard of care with macular laser or observation (n = 69) was carried out in patients with persistent DMO despite previous macular laser. By 3 years, BCVA had improved by >3 lines in 31% of Retisert treated eyes versus 20% in the standard of care group (p = 0.16). However, as the device required implantation in an operating theatre and there was a very high incidence of IOP elevation requiring incisional glaucoma surgery (Table 1) with 2.4% of patients having their implant removed to relieve IOP, it has not been approved by regulatory authorities for the management of DMO.
Table 1.
Summary of ocular side effects reported in major randomized clinical trials of intravitreal steroid therapy for diabetic macular oedema.
| Clinical trial | Steroid agent | Dose | Duration | Intraocular pressure rise | Cataract | Endophthalmitis | |
|---|---|---|---|---|---|---|---|
| Method for recording IOP rise varied across trials | Incisional glaucoma surgery | Cataract surgery | |||||
| TDMO | Triamcinolone | 4 mg | 2 years | 68% | 5.9% | 54% | 1 case reported |
| Kenacort 40 | (>5 mmHg from baseline) | ||||||
| TDMO extension | Triamcinolone | 4 mg | 5 years | 79% | 9.0% | 71% | Nil additional |
| Kenacort 40 | (>5 mmHg from baseline) | ||||||
| DRCRnet Protocol B | Triamcinolone | 1 and 4 mg | 3 years | 18% | 0% | 46% | Nil |
| Trivaris | 33% | 5% | 86% | Nil | |||
| (>10 mmHg from baseline) | |||||||
| DRCRnet Protocol I | Triamcinolone | 4 mg | 2 years | 42% | 1% | 55% | Nil |
| Trivaris | (>10 mmHg from baseline) | ||||||
| Retisert for DMO | Fluocinolone Retisert | 0.59 mg | 4 years | 61% | 33.8% | 91% | Nil |
| (IOP > 30 mmHg) | |||||||
| FAME | Fluocinolone Iluvien | 0.2 μg per day | 3 years | 38.2% | 4.8% | 80% | Nil |
| (IOP lowering medication) | |||||||
| PLACID | Dexamethasone | 0.7 mg | 1 year | 15.2% | 0% | 3.2% | Nil |
| Ozurdex | (>10 mmHg from baseline) | ||||||
| MEAD | Dexamethasone | 0.35 and 0.7 mg | 3 years | 24.8% | 0.3% | 52.3% | Nil |
| Ozurdex | 27.7% | 0.6% | 59.2% | 1 case reported | |||
| (>10 mmHg from baseline) | |||||||
| BEVORDEX | Dexamethasone | 0.7 mg | 1 year | 46% | 0% | 6.5% | Nil |
| Ozurdex | (>5 mmHg from baseline) | 1 case of syphilitic chorioretinitis | |||||
Note that there are inherent limitations to comparing complications across trials, particularly different entry requirements, baseline characteristics, duration of trials, methods for recording IOP rise and threshold for considering incisional glaucoma surgery.
IOP, intraocular pressure.
Fluocinolone acetonide was later developed into a smaller nonbiodegradable intravitreal insert (Iluvien®, Alimera, Alpharetta, GA) for treatment of DMO with sustained release for up to 3 years. It can be introduced through a 25G introducer as an office procedure. The Fluocinolone Acetonide intravitreal implant for diabetic Macular Edema (FAME) study included over 900 patients randomized to receive a low dose 0.2 μg/day fluocinolone insert (n = 375), a high dose 0.5 μg/day fluocinolone insert (n = 393) or a sham injection (n = 185). The treatment efficacy of both fluocinolone acetonide doses was similar, but fewer adverse events occurred with the lower dose steroid, so the 0.2 μg/day implant was taken to market. At 24 months, FAME demonstrated an improvement in BCVA of ⩾15 letters in 28.7% of the low dose steroid group versus 16.2% of controls, and these results were sustained in the third year [Campochiaro et al. 2012]. For those patients in the low dose steroid group, 80% of eyes phakic at baseline had undergone cataract surgery, 38.4% required IOP lowering medication and 4.8% had undergone incisional glaucoma surgery. Preplanned subgroup analysis showed a particular benefit compared with control in those patients with duration of DMO >3 years, although there has been some question as to how disease duration was calculated [Cunha-Vaz et al. 2014].
Anti-VEGF therapy
A Cochrane review of anti-angiogenic therapy for DMO identified strong evidence of a clinical benefit in maintaining and improving vision using anti-VEGF drugs versus laser photocoagulation [Querques et al. 2009; Mitchell et al. 2011; Do et al. 2012; Rajendram et al. 2012; Virgili et al. 2014]. These clinical trials had inclusion criteria of macular oedema involving the foveal centre – termed ‘centre-involving’ DMO. The systematic review reported that further data are needed to assess differences between drugs, effectiveness under real-world monitoring and treatment conditions, and safety in high-risk populations, particularly regarding cardiovascular risk [Virgili et al. 2014].
Protocol T recently published 1 year data on the relative efficacy of aflibercept 2 mg, bevacizumab 1.25 mg and ranibizumab 0.3 mg in the management of centre-involving DMO [Diabetic Retinopathy Clinical Research Network, 2015]. In patients with visual acuity better than 6/15, no difference was identified between the 3 drugs. In a subgroup of patients with visual acuity of approximately 6/15 or worse, aflibercept led to a statistically greater improvement in visual acuity and reduction in CMT than bevacizumab or ranibizumab. Baseline visual acuity was not even between groups, but this was adjusted for in the analysis. No assessment of baseline macular ischaemia using fluorescein angiography was made – a potential source of bias. There was no significant difference in serious adverse events reported between the drugs. The 2 year results of the trial and further supportive studies are awaited.
Combined data from the RISE and RIDE studies showed a dose-related increase in mortality rate of 4.4% for participants with DMO treated with ranibizumab 0.5 mg every month for 2 years compared with 1.2% for controls [Nguyen et al. 2012]. Real-world registry outcomes data will provide a mechanism for collecting systemic safety data in high-risk populations excluded from clinical trials. Furthermore, after 2 years of ranibizumab injections every month in the RISE and RIDE studies, macular oedema persisted in 23% of patients [Nguyen et al. 2012]. Therefore, other treatment options are required for patients with centre-involving DMO not suitable or not responsive to anti-VEGF therapy.
Clinical trials of Ozurdex in DMO
PLACID 12 month data
Ozurdex is a biodegradable intravitreal implant that slowly releases the corticosteroid, dexamethasone. The PLACID study randomized patients with diffuse DMO to 0.7 mg Ozurdex implant followed by laser photocoagulation at 1 month (n = 126) or to sham injection followed by laser photocoagulation at 1 month (n = 127). Subjects were eligible for an additional Ozurdex implant or sham injection 6 months after the first injection. Macular laser could be applied to both groups as needed every 3 months. There was no significant difference in BCVA between the groups at month 12. However, there was a significantly greater improvement in BCVA from baseline to various time points up to 9 months in the group receiving the Ozurdex implant. Additionally, area under the curve analysis for BCVA over the 12 months showed a significant benefit for patients treated with the Ozurdex implant in combination with macular laser compared with macular laser alone. In eyes that received Ozurdex, increase in IOP of ⩾10 mmHg occurred in 15.2% of eyes, with no incisional glaucoma surgery such as trabeculectomy required. Cataract surgery was performed in 3.2% of eyes over the 12 months of this study. The therapeutic effect of Ozurdex is around 4 months. However, the mistaken belief that it lasts as long as 6 months in all patients has led to some clinical trials having difficulty meeting their originally specified endpoints.
MEAD 3 year data
The MEAD study [Boyer et al. 2014] examined the safety and efficacy of the Ozurdex dexamethasone intravitreal implant in DMO over 3 years. It comprised two parallel randomized, multi-centre, masked phase III clinical trials. A total of 1048 patients with DMO were recruited, with BCVA of 20/50 to 20/200 and optical coherence tomography (OCT) measured CMT of ⩾300 µm. The patients were randomized 1:1:1 to dexamethasone implant 0.7 mg, dexamethasone implant 0.35 mg or sham, and they were followed for 3 years. Patients could be retreated no more often than every 6 months. The main outcome measurement was improvement in BCVA ⩾15 logMAR letters from baseline to study exit. Significantly more participants who received steroid treatment achieved a 15 letter gain: 22.2% in the 0.7 mg group and 18.4% of the 0.35 mg group, compared with 12.0% of the sham group (p = 0.018). A secondary outcome, the mean reduction in CMT from baseline, was greater in the groups receiving dexamethasone implants at a dose of 0.7 mg (−111.6 µm) and 0.35 mg (−107.9 µm) versus sham (−41.9 µm; p < 0.001). The rate of IOP rise >10 mmHg from baseline at any study visit was 27.7%, 24.8% and 3.7% in the 0.7 mg, 0.35 mg and sham groups, respectively. A total of 2 patients (0.6%) in the 0.7 mg group and 1 (0.3%) in the 0.35 mg group required trabeculectomy surgery. The rate of cataract surgery during the 3 year study was 59.2%, 52.3% and 7.2% in the 0.7 mg, 0.35 mg and sham groups, respectively.
While mean CMT decreased significantly in eyes receiving the dexamethasone implant, loss of efficacy appeared to occur before the minimum of 6 month interval for retreatment prospectively chosen in this trial (Figure 1). There were protocol amendments during the trial relating to OCT CMT retreatment criteria and when exit BCVA was recorded relative to the last Ozurdex implant. There was significant loss to follow up, with over a third of patients in the Ozurdex treatment groups and over half of patients in the sham group exiting the study early.
Figure 1.
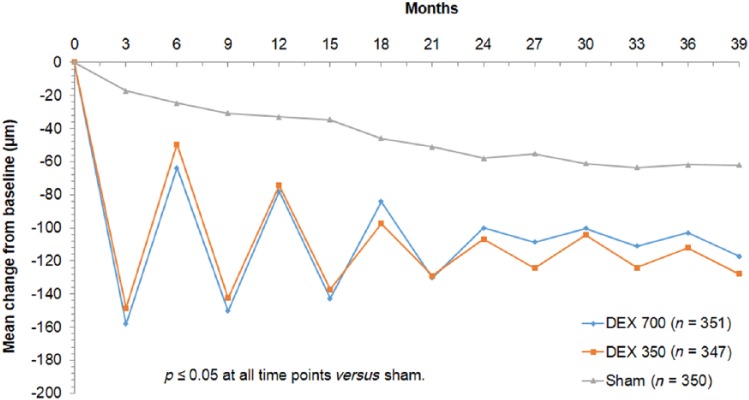
MEAD study: mean change in central macular thickness as measured by OCT.
The mean reduction in central macular thickness from baseline was greater in the groups receiving intravitreal dexamethasone implants at a dose of 0.7 mg (−111.6 µm) and 0.35 mg (−107.9 µm) versus sham (−41.9 µm; p < 0.001). Significant fluctuation in central macular thickness was noted with dosing of Ozurdex at a maximum frequency of every 6 months in the MEAD study.
DEX, dexamethasone; OCT, optical coherence tomography.
BEVORDEX 12 month data
The BEVORDEX study [Gillies et al. 2014] reported the 12 month results of a randomized head-to-head clinical trial that compared bevacizumab (Avastin, Genentech) with the Ozurdex 0.7 mg dexamethasone implant. There were 88 eyes of 61 patients with centre-involving DMO enrolled; 42 eyes receiving bevacizumab up to every 4 weeks and 46 eyes receiving an Ozurdex implant up to every 16 weeks, both as necessary (PRN). The primary outcome was the proportion of eyes with improvement in BCVA by at least 10 logMAR letters. This was achieved in 40% of bevacizumab treated eyes and 41% of Ozurdex treated eyes (p = 0.83). Bevacizumab treated eyes received a mean of 8.6 injections over 12 months, compared with 2.7 injections for the Ozurdex group.
A total of 26 of the 88 eyes (29.5%) were pseudophakic at baseline, 10 of which were treated with bevacizumab (24% of the bevacizumab treated eyes), whereas 16 were treated with the dexamethasone implant (35% of the dexamethasone implant eyes). There was no significant effect based on treatment received for the change in BCVA for the pseudophakic eyes, with a mean increase in BCVA for bevacizumab eyes of 7.7 letters and a mean increase in BCVA for dexamethasone treated eyes of 10.4 letters (p = 0.47)
None of the 42 eyes treated with bevacizumab lost 10 or more letters, but 11% (5/46) of the eyes given the Ozurdex implant did. Of these, 4 cases were due to increase in cataract density, but 1 patient with undiagnosed secondary syphilis developed the rare complication of syphilitic chorioretinitis 1 week after administration of the dexamethasone implant with significant loss of vision. Increase in cataract density by ⩾2 grades from baseline was reported in 13% (6/46) of eyes in the dexamethasone implant group and in 4.8% (2/42) of eyes in the bevacizumab group. Most cataract progression is anticipated in the second year of steroid use and the effect on visual outcomes on the 24 month outcomes will be of interest.
Over the initial year of the study, 12 eyes demonstrated an IOP of > 25 mmHg at least once during follow-up visits, all in the dexamethasone implant treatment group. These eyes were managed successfully with either observation or topical IOP lowering medications. No eye required incisional glaucoma surgery in the first year of the BEVORDEX trial.
Subgroup analysis of 25 of 34 patients who had one eye only enrolled in the BEVORDEX study and who had completed the Impact of Vision Impairment questionnaire at baseline and 12 months found no significant difference between the average improvement in scores between the dexamethasone implant and bevacizumab groups (p > 0.1). The 27 patients who had both eyes enrolled in the study were asked which treatment they preferred. A total of 25 responses were collected: 8 (33%) preferred bevacizumab, 11 (46%) preferred the dexamethasone implant, and 5 (21%) had no preference (p > 0.1). No significant difference in patient reported outcomes for the 2 drugs was noted at 12 months.
The BEVORDEX trial was the first head-to-head clinical trial of bevacizumab versus Ozurdex for centre-involving DMO either unresponsive or unlikely to benefit from macular laser. The baseline bevacizumab and Ozurdex groups were well matched. Also, the prospectively defined minimal time interval between Ozurdex implants was 16 weeks rather than the 6 months chosen in MEAD. Consequently, there was less recurrence of DMO during the BEVORDEX trial (Figure 2). The authors would not recommend regular retreatment intervals less than 4 months because of the potential risk of steroid-induced IOP rise. The trial continues to 24 months for safety outcomes.
Figure 2.
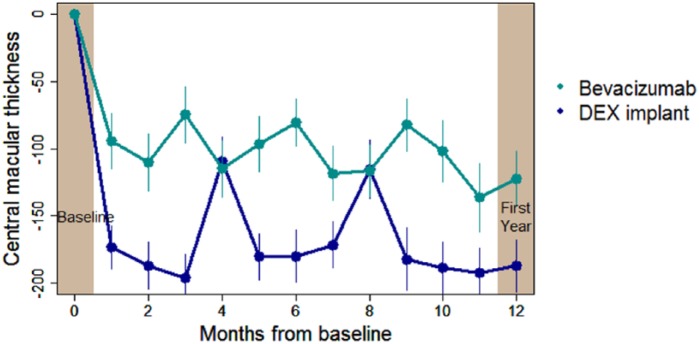
BEVORDEX study: mean change in central macular thickness as measured by OCT.
The mean reduction in central macular thickness was 122 µm in the bevacizumab group and 189 µm in the dexamethasone intravitreal implant group (p = 0.015). Note that in the BEVORDEX study, fluctuation in central macular thickness was less significant with dosing of Ozurdex at a maximum frequency of every 4 months.
DEX, dexamethasone; OCT, optical coherence tomography.
NCT01492400 clinical trial
There is a question as to how the dexamethasone implant compares to licensed anti-VEGF agents in head-to-head trials for DMO. Allergan has recently completed a randomized clinical trial comparing the safety and efficacy of dexamethasone implant versus ranibizumab in patients with DMO [ClinicalTrials.gov identifier: NCT01492400], with publication of results expected soon. In light of the results of the recent DRCRnet Protocol T trial, a head to head trial of the dexamethasone implant versus aflibercept might be warranted.
Perspective on the role of Ozurdex in the management of DMO
Control of systemic risk factors such as blood pressure, blood sugars and lipid profile is important to emphasize in patients diagnosed with diabetic maculopathy. A primary care physician or endocrinologist should be actively involved in the patient’s care and fenofibrate therapy considered. Our approach is to offer macular laser for non centre-involving CSMO. However, with the risk of extension of macular laser scars and the availability of pharmacologic therapies, we do not apply macular laser within 1000 µm of the foveal centre. Anti-VEGF intravitreal therapy is the first-line therapy offered for centre-involving DMO in the majority of patients, with an understanding of the relatively high number of intravitreal injections required in the first year of treatment.
Data from the MEAD and BEVORDEX clinical trials suggest that Ozurdex can be considered as a first-line treatment option in pseudophakic eyes. The injection frequency is lower than with intravitreal anti-VEGF therapy, but there is an increased risk of raised IOP. In an ideal scenario, clinicians will have controlled DMO prior to cataract surgery because of the risk of severely exacerbating macular oedema after surgery. Sometimes this is not possible, and in that scenario the use of Ozurdex can be considered prior to cataract surgery so as to ameliorate the pro-inflammatory status of the eye postoperatively [Cordero-Coma et al. 2013]. The Ozurdex implant interferes with vision less than triamcinolone but is more expensive. In the MEAD study, Ozurdex markedly reduced macular oedema after cataract surgery compared with sham. A prospective trial is underway to validate this [ClinicalTrials.gov identifier: NCT01546402].
Patients with centre-involving DMO in the RISE and RIDE studies received ranibizumab every month for 2 years and showed an increase in mortality rate of 4.4% compared with 1.2% for controls. However in a recent Cochrane review of randomized clinical trials of anti-angiogenic agents in DMO, many of which did not have continuous retreatment regimens, the safety profile was good, with adverse outcomes such as death and systemic arterial thromboembolic events unlikely to be increased in the short to medium term [Virgili et al. 2014]. Further evidence is required to determine whether cardiovascular adverse events are increased in high-risk populations usually excluded from randomized clinical trials [Virgili et al. 2014]. Real-world registries with long-term follow up may provide us with this information. In the interim, Ozurdex can be considered for the management of DMO in patients with recent stroke or heart attack.
In vitrectomized eyes, the loss of the vitreous sump means the length of time agents remain in the vitreous cavity and active is reduced, potentially limiting the role of anti-VEGF therapy [Yanyali et al. 2007]. Therefore, insertion of an Ozurdex slow-release dexamethasone implant may be considered. CHAMPLAIN was a prospective noncontrolled study of 55 vitrectomized eyes with treatment resistant DMO that received the Ozurdex implant. They identified a statistically and clinically significant improvement in both vision and CMT lasting approximately 3 months in these difficult-to-treat eyes, with an acceptable safety profile in this short-term study [Boyer et al. 2011]. Ozurdex is contraindicated if the anterior segment is not compartmentalized from the posterior segment, because of case reports of implant migration to the anterior chamber in cases with disruption of the posterior lens capsule or zonular dehiscence [Bansal et al. 2012; Malcles et al. 2013].
Foveal-threatening hard exudation is one situation where the Ozurdex intravitreal implant can be considered in the management of DMO. The consequences of foveal plaquing are visually devastating. Greater regression of hard exudates was identified in Ozurdex compared with bevacizumab treated eyes in the BEVORDEX trial at 12 months, although no study eye developed plaques of lipid at the fovea (data presented at the 2014 Annual Meeting of the American Academy of Ophthalmology). This is consistent with data from a randomized, placebo-controlled clinical trial that showed rapid reduction of hard exudates in eyes with diabetic maculopathy 3 months after treatment with intravitreal triamcinolone acetonide [Larsson et al. 2009]. Analysis of RISE and RIDE data, looking at the effect of continuous intravitreal ranibizumab therapy on retinal hard exudate resolution, reported that gradual clearance occurred from month 6 onwards [Domalpally et al. 2015]. Natural history studies are required to determine how distance of hard exudates from the foveal centre correlates with time to progression to foveal plaquing so as to guide clinicians when rapid intervention is required.
Retrospective studies, including ARTES (a collaborative retrospective trial on the efficacy and safety of intravitreal dexamethasone implant in patients with DMO), are currently underway assessing whether Ozurdex is more effective in eyes with chronic compared with acute DMO as fluocinolone appeared to be in the FAME study. Prospective controlled studies with strict definitions of chronic DMO are required to validate this. Identifying baseline features which predict response to treatment would also be helpful. Factors like thicker CMT at baseline, cystic changes, presence of epiretinal membrane and loss of ellipsoid layer or external limiting membrane integrity may represent more chronic disease that is more likely to benefit from treatment with intravitreal steroids.
Ozurdex intravitreal implants can be considered second-line treatment for DMO in eyes unresponsive to anti-VEGF therapy. Defining nonresponse can be challenging. In a prospective study of Ozurdex for the treatment of persistent DMO in eyes previously treated with anti-VEGF, nonresponse was defined as a lack of anatomical and visual improvement after three intravitreal anti-VEGF injections [Lazic et al. 2014]. The SwitchDMO trial (switching to Ozurdex for DMO unresponsive to bevacizumab) [ClinicalTrials.gov identifier: NCT01787669] being carried out at our centre has defined nonresponse as 6 consecutive months of anti-VEGF therapy without anatomical improvement (personal communication, S.F.B.).
In patients with DMO and concomitant proliferative or severe nonproliferative diabetic retinopathy, there is interest whether intravitreal anti-VEGF or steroid therapy for DMO also slows progression or improves diabetic retinopathy. Several clinical trials of intravitreal anti-VEGF therapy for DMO have demonstrated significant improvement in diabetic retinopathy grade over the course of the study [Adamis et al. 2006; Bressler et al. 2013; Brown et al. 2013]. The US Food and Drug Administration (FDA) recently approved ranibizumab and aflibercept to treat diabetic retinopathy (nonproliferative or proliferative) in patients with DMO. There are also clinical trials that have shown some benefit of intravitreal steroid agents on progression of diabetic retinopathy [Bressler et al. 2013; Cunha-Vaz et al. 2014]. Analysis of 24 month BEVORDEX data will allow assessment of the relative effect of PRN bevacizumab versus PRN Ozurdex on grade of diabetic retinopathy.
Absolute contraindications for the insertion of Ozurdex implants include advanced or uncontrolled glaucoma, local infection or communication with the anterior chamber. Patients considered to be at risk for syphilis, tuberculosis or other potentially infective chorioretinopathies should have these conditions excluded prior to considering intravitreal steroid therapy.
Insertion of the 22G Ozurdex intravitreal implant is technically more challenging than intravitreal injections, but can be safely carried out in an office environment. Cases of vitreous haemorrhage, intraocular lens damage and fracture of the dexamethasone implant during implantation have been described [Agrawal et al. 2014; Coca-Robinot et al. 2014; Guigou et al. 2014]. Recent improvements in the design of the introducer have made insertion easier with less depression of the globe required than previously [Eaton et al. 2014; Meyer et al. 2014]. We apply subconjunctival lidocaine to the site of entry at least 5 minutes before insertion of the Ozurdex implant to improve patient comfort. We offer Ozurdex intravitreal implants at a maximum frequency of 4 months, informed by CMT data from the BEVORDEX trial rather than the 6 month maximum frequency employed in the PLACID and MEAD trials. Patients will sometimes report the presence of a ‘hair’ fixed in their vision that gradually clears as the implant degrades. We arrange IOP checks 1–3 weeks after each Ozurdex implant. Long-term follow up with either an ophthalmologist or optician should be in place to check for a late rise in IOP even after the diabetic retinopathy has been stabilized.
Triamcinolone, fluocinolone and dexamethasone activate different patterns of gene expression in human trabecular meshwork cell lines [Nehme et al. 2009]. Dexamethasone is less lipophilic than triamcinolone or fluocinolone and therefore may not accumulate in the trabecular meshwork and lens to the same extent [Thakur et al. 2011]. Studies directly comparing these different steroid agents in the management of DMO are lacking. Because of the likely reduced requirement for incisional glaucoma surgery (Table 1) with the Ozurdex implant compared with 4 mg intravitreal triamcinolone injections or fluocinolone acetonide implants, we prefer to use the dexamethasone implant when intravitreal steroid is indicated. However, it should be noted that follow up of patients treated with Ozurdex for DMO in clinical trials is limited to 3 years with longer term safety data likely to be collected from real-world registries. Additionally, the cost of the Ozurdex implant is significantly higher than preservative-free intravitreal triamcinolone.
US and European regulatory authorities have recently extended their licensed indications for Ozurdex in the management of DMO. In September 2014, the FDA expanded the indication for Ozurdex to the ‘general patient population being treated for DMO’. In the same month, the European Medicines Agency (EMA) granted marketing authorization for the use of Ozurdex in ‘adult patients with visual impairment due to DMO who are pseudophakic or who are considered insufficiently responsive to or unsuitable for noncorticosteroid therapy’.
Conclusion
There is evidence from randomized clinical trials demonstrating the benefit of Ozurdex in improving visual acuity and reducing CMT in patients with DMO. This is achievable with a reduced intravitreal injection frequency compared with anti-VEGF therapy. The risks of cataract, glaucoma and infection with the use of these slow-release dexamethasone intravitreal implants must be considered, although the side effect profile especially in terms of requirement for incisional glaucoma surgery appears to be more favourable with this steroid formulation compared with others. Because of these steroid-related side effects, Ozurdex slow-release intravitreal implants are mainly used second-line in patients unresponsive to anti-VEGF therapy for centre-involving DMO. Situations where the Ozurdex intravitreal implant may be considered a first-line treatment option for centre-involving DMO are in pseudophakic eyes or in those eyes about to undergo cataract surgery, in vitrectomized eyes, or in the context of a recent arterial thromboembolic event.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Hemal Mehta, The Save Sight Institute, University of Sydney, Australia.
Mark Gillies, The Save Sight Institute, University of Sydney, Australia.
Samantha Fraser-Bell, Macula Research Group, Save Sight and Eye Health Institute, University of Sydney, 8 Macquarie Street, Sydney, NSW 2000, Australia.
References
- Adamis A., Altaweel M., Bressler N., Cunningham E., Jr., Davis M., Goldbaum M., et al. (2006) Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology 113: 23–28. [DOI] [PubMed] [Google Scholar]
- Agrawal R., Fernandez-Sanz G., Bala S., Addison P. (2014) Desegmentation of Ozurdex implant in vitreous cavity: report of two cases. Br J Ophthalmol 98: 961–963. [DOI] [PubMed] [Google Scholar]
- Bansal R., Bansal P., Kulkarni P., Gupta V., Sharma A., Gupta A. (2012) Wandering Ozurdex(®) implant. J Ophthalmic Inflamm Infect 2: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer D., Faber D., Gupta S., Patel S., Tabandeh H., Li X., et al. (2011) Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 31: 915–923. [DOI] [PubMed] [Google Scholar]
- Boyer D., Yoon Y., Belfort R., Jr., Bandello F., Maturi R., Augustin A., et al. (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121: 1904–1914. [DOI] [PubMed] [Google Scholar]
- Bressler S., Qin H., Melia M., Bressler N., Beck R., Chan C., et al. (2013) Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. J Am Med Assoc Ophthalmol 131: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Nguyen Q., Marcus D., Boyer D., Patel S., Feiner L., et al. (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
- Bunce C., Wormald R. (2008) Causes of blind certifications in England and Wales: April 1999-March 2000. Eye (Lond) 22: 905–911. [DOI] [PubMed] [Google Scholar]
- Campochiaro P., Brown D., Pearson A., Chen S., Boyer D., Ruiz-Moreno J., et al. (2012) Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 119: 2125–2132. [DOI] [PubMed] [Google Scholar]
- Chin H., Park T., Moon Y., Oh J. (2005) Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 25: 556–560. [DOI] [PubMed] [Google Scholar]
- Coca-Robinot J., Casco-Silva B., Armada-Maresca F., Garcia-Martinez J. (2014) Accidental injections of dexamethasone intravitreal implant (Ozurdex) into the crystalline lens. Eur J Ophthalmol 24: 633–636. [DOI] [PubMed] [Google Scholar]
- Cordero-Coma M., Garzo I., Calleja S., Galan E., Franco M., Ruiz De Morales J. (2013) Preoperative cataract surgery use of an intravitreal dexamethasone implant (Ozurdex) in a patient with juvenile idiopathic arthritis and chronic anterior uveitis. J AAPOS 17: 632–634. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J., Ashton P., Iezzi R., Campochiaro P., Dugel P., Holz F., et al. (2014) Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 121: 1892–1903. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group (1995) The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44: 968–983. [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network (2008) A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 115: 1447–1449, 1449.e1–1449.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network, Elman M., Aiello L., Beck R., Bressler N., Bressler S., et al. (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117: 1064–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do D., Nguyen Q., Boyer D., Schmidt-Erfurth U., Brown D., Vitti R., et al. (2012) One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology 119: 1658–1665. [DOI] [PubMed] [Google Scholar]
- Domalpally A., Ip M., Ehrlich J. (2015) Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology 122: 779–786. [DOI] [PubMed] [Google Scholar]
- Eaton A., Gordon G., Booth D., Wafapoor H., Avery R. (2014) Injection force comparison of the old and new dexamethasone implant insertion needles in porcine eyes and synthetic sclera. Ophthalmic Surg Lasers Imaging Retina 45: 232–238. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol 103: 1796–1806. [PubMed] [Google Scholar]
- Gillies M., Lim L., Campain A., Quin G., Salem W., Li J., et al. (2014) A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121: 2473–2481. [DOI] [PubMed] [Google Scholar]
- Gillies M., Simpson J., Gaston C., Hunt G., Ali H., Zhu M., et al. (2009) Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology 116: 2182–2187. [DOI] [PubMed] [Google Scholar]
- Gillies M., Sutter F., Simpson J., Larsson J., Ali H., Zhu M. (2006) Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 113: 1533–1538. [DOI] [PubMed] [Google Scholar]
- Gonder J., Walker V., Barbeau M., Zaour N., Zachau B., Hartje J., et al. (2014) Costs and quality of life in diabetic macular edema: Canadian burden of diabetic macular edema observational study (C-REALITY). J Ophthalmol 2014: 939315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigou S., Hajjar C., Parrat E., Merite P., Pommier S., Matonti F., et al. (2014) [Multicenter Ozurdex® assessment for diabetic macular edema: MOZART study]. J Fr Ophtalmol 37: 480–485. [DOI] [PubMed] [Google Scholar]
- Hariprasad S., Mieler W., Grassi M., Green J., Jager R., Miller L. (2008) Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol 92: 89–92. [DOI] [PubMed] [Google Scholar]
- Keech A., Mitchell P., Summanen P., O’day J., Davis T., Moffitt M., et al. (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370: 1687–1697. [DOI] [PubMed] [Google Scholar]
- Kiire C., Porta M., Chong V. (2013) Medical management for the prevention and treatment of diabetic macular edema. Surv Ophthalmol 58: 459–465. [DOI] [PubMed] [Google Scholar]
- Larsson J., Kifley A., Zhu M., Wang J., Mitchell P., Sutter F., et al. (2009) Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol 87: 275–280. [DOI] [PubMed] [Google Scholar]
- Lazic R., Lukic M., Boras I., Draca N., Vlasic M., Gabric N., et al. (2014) Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina 34: 719–724. [DOI] [PubMed] [Google Scholar]
- Malcles A., Janin-Manificat H., Yhuel Y., Russo A., Agard E., El Chehab H., et al. (2013) [Anterior chamber migration of intravitreal dexamethasone implant (Ozurdex®) in pseudophakic eyes: report of three cases]. J Fr Ophtalmol 36: 362–367. [DOI] [PubMed] [Google Scholar]
- Matthews D., Stratton I., Aldington S., Holman R., Kohner E. UK Prospective Diabetes Study Group. (2004) Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 122: 1631–1640. [DOI] [PubMed] [Google Scholar]
- Meyer C., Liu Z., Brinkmann C., Rodrigues E., Bertelmann T. German Retinal Vein Occlusion, Group. (2014) Penetration force, geometry, and cutting profile of the novel and old Ozurdex needle: the MONO study. J Ocul Pharmacol Ther 30: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Bandello F., Schmidt-Erfurth U., Lang G., Massin P., Schlingemann R., et al. (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118: 615–625. [DOI] [PubMed] [Google Scholar]
- Moss S., Klein R., Klein B. (1998) The 14-year incidence of visual loss in a diabetic population. Ophthalmology 105: 998–1003. [DOI] [PubMed] [Google Scholar]
- Nehme A., Lobenhofer E., Stamer W., Edelman J. (2009) Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Brown D., Marcus D., Boyer D., Patel S., Feiner L., et al. (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119: 789–801. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Kawano H., Sonoda S., Nakamura M., Sakamoto T. (2013) Particle-induced endophthalmitis: possible mechanisms of sterile endophthalmitis after intravitreal triamcinolone. Invest Ophthalmol Vis Sci 54: 1758–1766. [DOI] [PubMed] [Google Scholar]
- Querques G., Bux A., Martinelli D., Iaculli C., Noci N. (2009) Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol 87: 623–630. [DOI] [PubMed] [Google Scholar]
- Rajendram R., Fraser-Bell S., Kaines A., Michaelides M., Hamilton R., Esposti S., et al. (2012) A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 130: 972–979. [DOI] [PubMed] [Google Scholar]
- Romero-Aroca P., Reyes-Torres J., Baget-Bernaldiz M., Blasco-Sune C. (2014) Laser treatment for diabetic macular edema in the 21st century. Curr Diabetes Rev 10: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M., Hunyor A., Ho I. (2014) Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol 49: 188–195. [DOI] [PubMed] [Google Scholar]
- Thakur A., Kadam R., Kompella U. (2011) Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol 129: 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853. [PubMed] [Google Scholar]
- Virgili G., Parravano M., Menchini F., Evans J. (2014) Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 10: CD007419. [DOI] [PubMed] [Google Scholar]
- Wang K., Wang Y., Gao L., Li X., Li M., Guo J. (2008) Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull 31: 1541–1546. [DOI] [PubMed] [Google Scholar]
- Whiting D., Guariguata L., Weil C., Shaw J. (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94: 311–321. [DOI] [PubMed] [Google Scholar]
- Wong T., Simo R., Mitchell P. (2012) Fenofibrate - a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol 154: 6–12. [DOI] [PubMed] [Google Scholar]
- Yanyali A., Aytug B., Horozoglu F., Nohutcu A. (2007) Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol 144: 124–126. [DOI] [PubMed] [Google Scholar]
- Yau J., Rogers S., Kawasaki R., Lamoureux E., Kowalski J., Bek T., et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias L., Lin T., Migon R., Ghosn C., Orilla W., Feldmann B., et al. (2013) Assessment of the differences in pharmacokinetics and pharmacodynamics between four distinct formulations of triamcinolone acetonide. Retina 33: 522–531. [DOI] [PubMed] [Google Scholar]


