Abstract
Two cDNA sequences of Kazal-type serine protease inhibitors (KSPIs) in Nasonia vitripennis, NvKSPI-1 and NvKSPI-2, were characterized and their open reading frames (ORFs) were 198 and 264 bp, respectively. Both NvKSPI-1 and NvKSPI-2 contained a typical Kazal-type domain. Real-time quantitative PCR (RT-qPCR) results revealed that NvKSPI-1 and NvKSPI-2 mRNAs were mostly detected specifically in the venom apparatus, while they were expressed at lower levels in the ovary and much lower levels in other tissues tested. In the venom apparatus, both NvKSPI-1 and NvKSPI-2 transcripts were highly expressed on the fourth day post eclosion and then declined gradually. The NvKSPI-1 and NvKSPI-2 genes were recombinantly expressed utilizing a pGEX-4T-2 vector, and the recombinant products fused with glutathione S-transferase were purified. Inhibition of recombinant GST-NvKSPI-1 and GST-NvKSPI-2 to three serine protease inhibitors (trypsin, chymotrypsin, and proteinase K) were tested and results showed that only NvKSPI-1 could inhibit the activity of trypsin. Meanwhile, we evaluated the influence of the recombinant GST-NvKSPI-1 and GST-NvKSPI-2 on the phenoloxidase (PO) activity and prophenoloxidase (PPO) activation of hemolymph from a host pupa, Musca domestica. Results showed PPO activation in host hemolymph was inhibited by both recombinant proteins; however, there was no significant inhibition on the PO activity. Our results suggested that NvKSPI-1 and NvKSPI-2 could inhibit PPO activation in host hemolymph and trypsin activity in vitro.
Keywords: Nasonia vitripennis, Kazal-type, serine protease inhibitors, humoral immunity
1. Introduction
The Kazal-type serine protease inhibitors (KSPIs) comprise a large family of protease inhibitors. They are present widely in mammals, birds, crayfish, and insects and are named in reference to the work on the pancreatic secretory trypsin inhibitor first isolated by Kazal et al. [1]. During the 1950s–1980s, KSPIs were explosively studied in vertebrates, particularly mammals and birds [2]. Studies on KSPIs from invertebrates began in the 1990s when Friedrich et al. first reported a double-headed Kazal-type thrombin inhibitor, rhodniin, from Rhodnius prolixus (Hemiptera: Reduviidae) [3]. In 1994, a four-domain Kazal protease inhibitor from the blood cells of crayfish Pacifastacus leniusculus (Crustacea: Decapoda) [4] and a leech-derived tryptase inhibitor from the medicinal leech Hirudo medicinalis (Hirudinea: Hirudinidae) [5] were documented, respectively. After that, studies on invertebrate KSPIs have extended to other invertebrate species including shrimp, blood-sucking insects, silk moths, locusts, and so on [6,7,8,9,10,11,12].
KSPIs have the conserved structures of one or more Kazal domains (KDs). A typical KD is composed of 40–60 amino acids including six cysteine residues and the following conservative motif: C1-X(1-7)-C2-X(5)-PVC3-X(4)-TYXNXC4-X(2-6)-C5-X(9-16)-C6. These six cysteine residues formed three intra-domain disulfide bridges between cysteine numbers 1–5, 2–4, and 3–6, resulting in a characteristic three-dimensional structure [13]. So far, hundreds of KSPIs with various functions have been reported [14]. KSPIs are involved in many important physiological processes, such as embryogenesis, development, excessive autophagy, microbial invasion, inflammation, and immune responses [15,16,17,18,19]. Native KSPIs from blood-feeding arthropods can inhibit trypsin, thrombin, elastase, chymotrypsin, plasmin, subtilisin A, and factor XIIa [3,20,21,22,23].
Parasitic wasps are natural resources that play an important role in biological control. They lay eggs into hosts or on the surface of hosts along with maternal and embryonic factors such as venom, polydnavirus (PDV), virus-like particles (VLP), ovarian proteins, teratocytes, and proteins secreted from larvae to interfere with the host immune responses for successful parasitization [24,25,26]. Unlike ichneumonid and braconid parasitoids, Nasonia vitripennis (Hymenoptera: Pteromalidae) injects venom, but not PDVs, into its host after oviposition. N. vitripennis venom is responsible for multiple functions in regulating the physiological processes of its host including induction of pathological and ultrastructural changes in cultured cells, interfering with the cellular immunity of host hemocytes, causing cell death, stimulation of intracellular calcium release in cultured cells, and disruption of the pupariation and eclosion behavior of the host [27,28,29]. Using proteomics methods, De Graaf et al. [30] previously identified 79 venom proteins from N. vitripennis, two of which are KSPIs (NCBI accession numbers NM_001161523 and NM_001170879). Recently, our group determined the transcriptome and proteome of venom gland and other residual tissues in N. vitripennis by RNA-seq (RNA sequencing) and LC-MS/MS (liquid chromatography–tandem mass spectrometry) methods [31], and we also detected two KSPIs in venom, as described by de Graaf et al. [30].
N. vitripennis is not only an advantageous ectoparasitoid to flies but an ideal perfect model insect for genetic and developmental biology studies [32,33]. In addition, genome sequences and comparative analyses have been reported for N. vitripennis and two other closely related parasitoid wasps, N. giraulti, and N. longicornis (Hymenoptera: Pteromalidae) [34]. While these reports document progress in understanding the composition of N. vitripennis venom, information on the activity and functional molecular mechanisms of venom actions are still lacking.
Here, we molecularly characterized two KSPIs (NvKSPI-1 and NvKSPI-2) in N. vitripennis and determined their tissue and developmental expression patterns. We also tested the inhibition of recombinant NvKSPI-1 and NvKSPI-2 on three serine protease inhibitors’ in vitro and PO activity and PPO activation of host hemocytes. These results will provide further insight into the role of KSPIs in insects, especially in parasitoid wasps.
2. Results
2.1. Molecular Characterization of NvKSPI-1 and NvKSPI-2
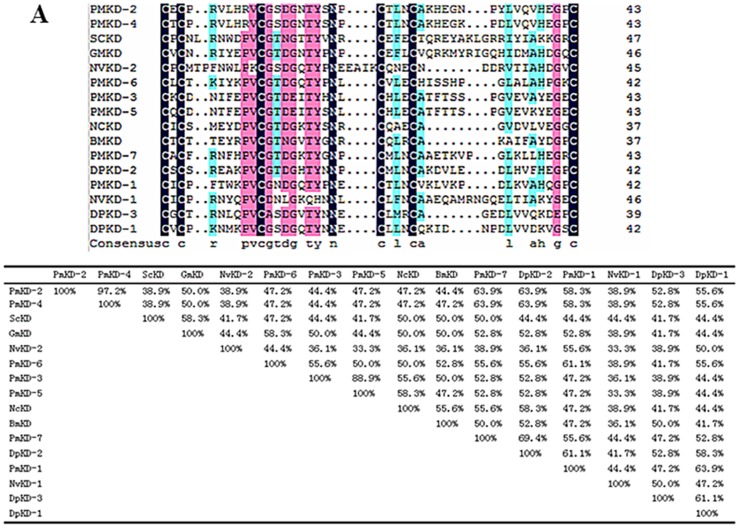
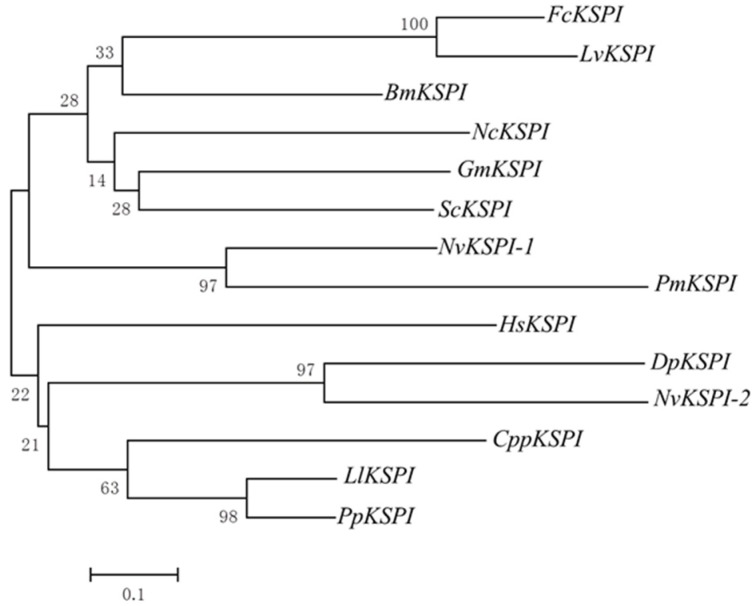
Fragments of NvKSPI-1 and NvKSPI-2 containing 198 and 264 nucleotides were amplified and sequenced; they respectively encoded 65 and 87 amino acids with predicted secretory N-terminal signal peptides (Figure 1). BLASTn results showed that these two cDNA sequences were completely consistent with N. vitripennis KSPIs in the NCBI database (NM_001161523 and NM_001170879). A multiple sequence alignment of KDs from NvKSPI-1, NvKSPI-2, and other KSPIs showed that although the numbers of KDs in these species varied from one to seven, typical KD motifs were highly similar, including the six cysteine residues that formed disulfide bonds between cysteine numbers 1–5, 2–4, and 3–6 (Figure 2). Phylogenetic analyses of NvKSPI-1 and NvKSPI-2 with homologs in 12 other species using their mature peptide region sequences indicated that NvKSPI-1 and NvKSPI-2 were not classified into the same cluster and showed close genetic distances with Danaus plexippus (Lepidoptera: Danaidae) and Panstrongylus megistus (Hemiptera: Reduviidae), respectively (Figure 3). In Diptera insects, Lutzomyia longipalpis (Diptera: Psychodidae) and Phlebotomus papatasi (Diptera: Psychodidae) are classified into one cluster, while Glossina morsitans (Diptera, Glossinidae) and Stomoxys calcitrans (Diptera: Muscidae) are classified into another cluster. In general, NvKSPI-1 and NvKSPI-2 were genetically closer to insects, and farther from crustaceans and vertebrates.
Figure 1.
Nucleotide and deduced amino sequences of NvKSPI-1 (A) and NvKSPI-2 (B) cDNAs from N. vitripennis. Signal peptides are underlined; the initiator codon “ATG” and terminator codon “TAA” are bolded and highlighted.
Figure 2.
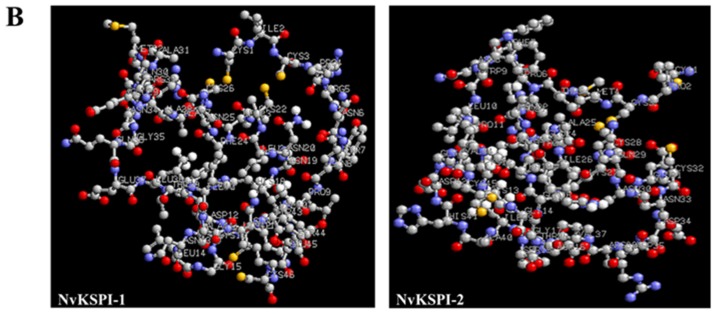
Sequence analysis of NvKSPI-1 and NvKSPI-2. (A) Multiple sequences alignment and amino acid identity analysis of Kazal domains (KDs) between NvKSPI-1, NvKSPI-2, and other KSPIs. (B) Predicted tertiary structure of NvKSPI-1 and NvKSPI-2 KDs by phyre2 on line. Yellow spheres represent cysteines forming three intra-domain disulfide bridges between cysteine numbers 1–5, 2–4, and 3–6. The corresponding species of abbreviations and their GenBank accession numbers are as follows: PMKD-1,2,3,4,5,6,7: Panstrongylus megistuss (ADF97836); SCKD: Stomoxys calcitrans (AAY98015); GMKD: Glossina morsitans (AFG28187); NCKD: Neospora caninum (AAM29188); BMKD: Bombyx mori (NP_001037047); DPKD-1,2,3: Danaus plexippus (EHJ76238); NVKVD1 and NVKVD2: Nasonia vitripennis (NM_001161523 and NM_001170879).
Figure 3.
Phylogenetic analysis of NvKSPI-1, NvKSPI-2, and other KSPIs’ amino acid sequences based on the neighbor-joining method. The origin of amino acid sequences and their GenBank accession numbers are as follows: FcKSPI: Fenneropenaeus chinensis (ABC33915); LvKSPI: Litopenaeus vannamei (AAT09421); BmKSPI: Bombyx mori (NP_001037047); NcKSPI: Neospora caninum (AAM29188); GmKSPI: Glossina morsitans (AFG28187); ScKSPI: Stomoxys calcitrans (AAY98015); PmKSPI: Panstrongylus megistus (ADF97836); HsKSPI: Homo sapiens (NP_115955); DpKSPI: Danaus plexippus (EHJ76238); CppKSPI: Culex pipiens pallens (AFN41343); LlKSPI: Lutzomyia longipalpis (ABV60319); PpKSPI: Phlebotomus papatasi (ABV44739); NvKSPI-1 and NvKSPI-2: Nasonia vitripennis (NM_001161523 and NM_001170879).
2.2. Expression and Purification of Recombinant NvKSPI-1 and NvKSPI-2
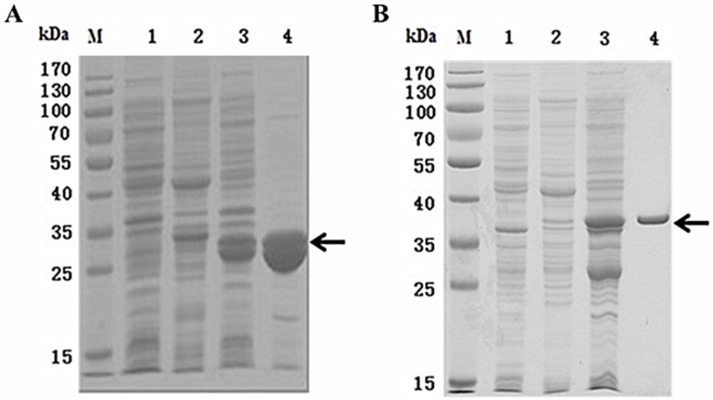
Two recombinant proteins fused with glutathione S-transferase at their N-terminuses, namely GST-NvKSPI-1 and GST-NvKSPI-2, were successfully detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with molecular weights of about 33 kDa and 36 kDa, respectively. The recombinant proteins were mainly detected in the supernatant and not in the precipitate. As soluble fusion proteins, GST-NvKSPI-1 and GST-NvKSPI-2 were purified with the GST•Bind™ Resin Kit (Novagen, Hilden, Germany) and the purified proteins were analyzed by SDS-PAGE (Figure 4). The protein concentrations of purified GST-NvKSPI-1 and GST-NvKSPI-2 were 1.8 mg/mL and 2.1mg/mL, respectively, and the concentration of the GST fusion proteins was 2.4 mg/mL.
Figure 4.
SDS-PAGE analysis of NvKSPI-1 (A) and NvKSPI-2 (B) recombinant proteins. M: Protein molecular weight marker; 1: Not induced by IPTG; 2: Precipitation after IPTG induction. 3: Supernatant after IPTG induction; 4: Purified proteins.
2.3. Transcriptional Profiles of NvKSPI-1 and NvKSPI-2 in Different Tissues and Developmental Stages
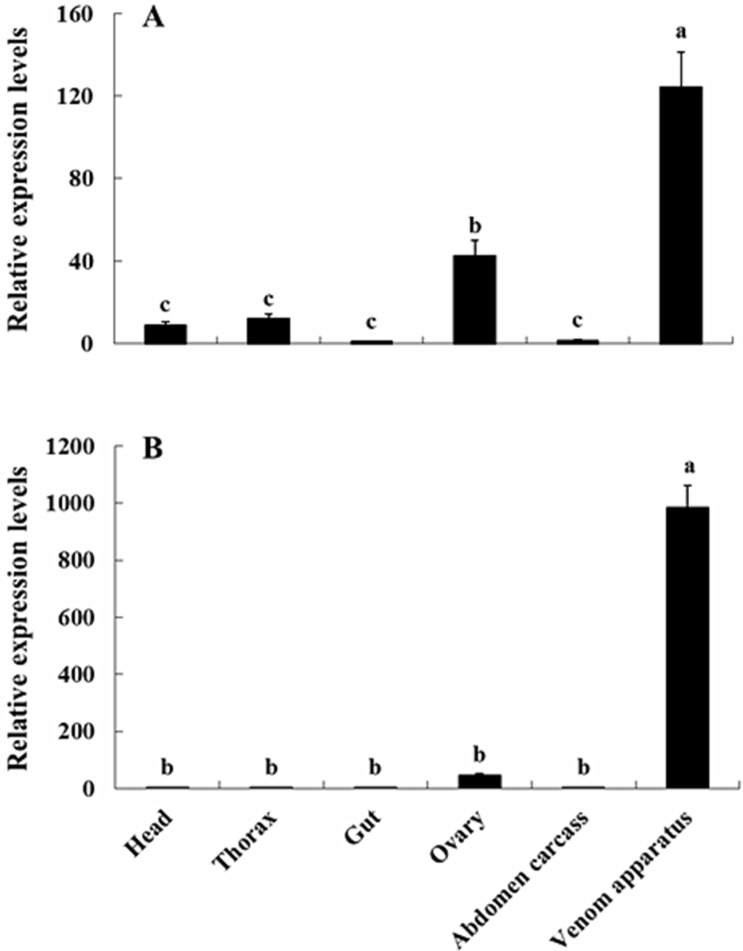
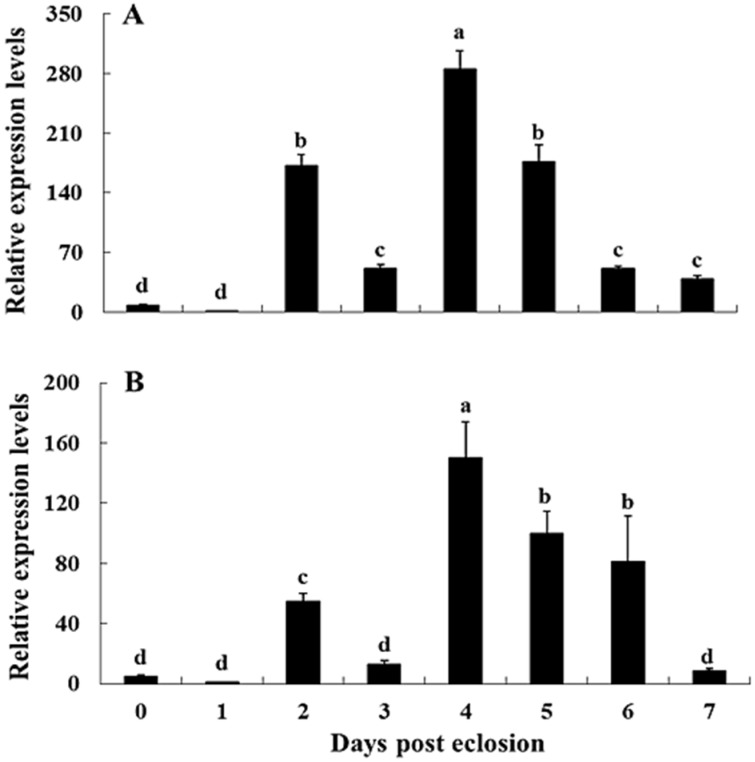
Levels of NvKSPI-1 and NvKSPI-2, confirmed by genes by RT-qPCR using 18S rRNA as the reference gene, showed similar transcript profiles (Figure 5 and Figure 6). In tissues, NvKSPI-1 and NvKSPI-2 mRNAs were both expressed specifically in the venom apparatus, while they were detected at lower levels in the ovary and much lower amounts in other tissues tested. In detecting the transcript levels of NvKSPI-1 and NvKSPI-2 at different developmental stages (0–7 days post eclosion) in the venom apparatus of female adults, they were found to be lower at 0, 1 and 3 days post eclosion, but higher on the second day and highest on the fourth day post eclosion, after which they declined gradually. NvKSPI-1 and NvKSPI-2 had similar transcript profiles.
Figure 5.
Tissue distribution of transcript levels of NvKSPI-1 (A) and NvKSPI-2 (B) in N. vitripennis. Abdomen carcass represents the rest of abdomen post dissection. Venom apparatus contains venom reservoir and gland, and venom released. All values in the figure are represented as mean ± standard deviation. Bars labeled with different letters are significantly different (one-way ANOVA followed by LSD test, p < 0.05).
Figure 6.
Developmental stages of transcript levels of NvKSPI-1 (A) and NvKSPI-2 (B) in venom apparatus of N. vitripennis. Female adults were sampled on days 0 to 7 post eclosion. All values are presented as mean ± standard deviation. Bars labeled with different letters are significantly different (one-way ANOVA followed by LSD test, p < 0.05).
2.4. Serine Protease Inhibition Activity of NvKSPIs
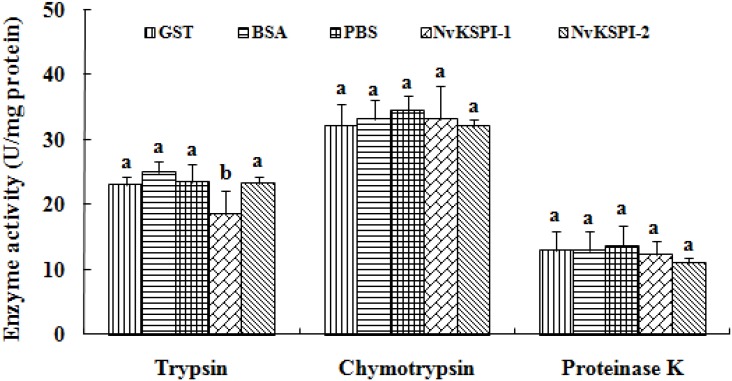
To define whether NvKSPI-1 and NvKSPI-2 can affect serine protease activity, we determined their enzyme activity inhibition spectrum to trypsin, chymotrypsin, and proteinase K with recombinant GST-NvKSPI-1 and GST-NvKSPI-2. Results showed that only NvKSPI-1 could inhibit the activity of trypsin, while NvKSPI-2 did not inhibit the activity of any of the three serine proteases tested (F = 4.159, p = 0.04; F = 0.247, p = 0.932; F = 0.552, p = 0.714, respectively) (Figure 7).
Figure 7.
Effects of recombinant NvKSPI-1 and NvKSPI-2 on the activity of serine proteases. GST: GST-tag protein expressed by pGEX-4T-2; BSA and Buffer: treated by BSA and reaction buffer respectively; NvKSPI-1 and NvKSPI-2: treated by recombination proteins respectively. All values in the figure are represented as mean ± standard deviation. Bars labeled with different letters are significantly different (one-way ANOVA followed by LSD test, p < 0.05).
2.5. Effects of NvKSPIs on Prophenoloxidase (PPO) Activation and Phenoloxidase (PO) Activity
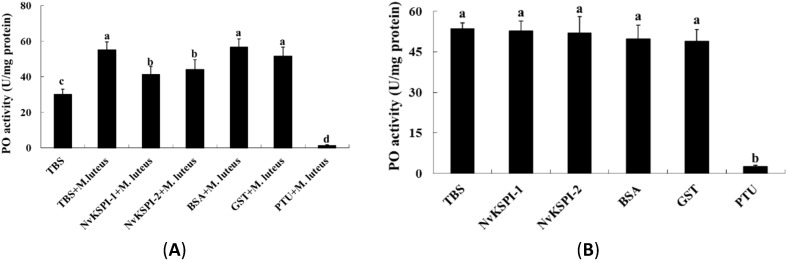
To test whether NvKSPI-1 and NvKSPI-2 played a role in PPO activation and PO of hemolymph of host pupae, inhibition of PPO activation and PO activity were tested with recombinant GST-NvKSPI-1 and GST-NvKSPI-2 and compared with positive and negative controls. The PO activity between samples incubated with NvKSPI-1/M. luteus and NvKSPI-2/M. luteus were significantly different compared with the BSA and GST controls (F = 73.255, p < 0.001) (Figure 8A). The results indicated that recombinant GST-NvKSPI-1 and GST-NvKSPI-2 could inhibit PPO activation in the hemolymph of the host. When PPO in the hemolymph was pre-activated with M. luteus, recombinant GST-NvKSPI-1 and GST-NvKSPI-2 had no effect on PO activity (F = 27.26, p < 0.001) (Figure 8B). These results suggested that NvKSPI-1 and NvKSPI-2 could inhibit PPO activation in the host hemolymph but could not inhibit PO activity after PPO was activated.
Figure 8.
Effects of recombinant NvKSPI-1 and NvKSPI-2 on the PPO activation (A) and PO activity (B) of hemolymph of M. domestica pupae. (A) Screened hemolymph was incubated with TBS alone, or TBS/M. luteus, NvKSPI-1/M. luteus, NvKSPI-2/M. luteus, BSA/M. luteus, GST/M. luteus, PTU/M. luteus in TBS for 20 min at 25 °C. PPO activation was assayed using l-dopamine as a substrate, as described in Materials and Methods. (B) Screened hemolymph was incubated with M. luteus in TBS for 10 min at 25 °C, and then incubated with TBS, NvKSPI-1, NvKSPI-2, BSA, GST, or PTU for 10 min at 25 °C. PO activity was assayed using l-dopamine as a substrate, as described in Materials and Methods. All values in the figure are represented as mean ± standard deviation. Bars labeled with different letters are significantly different (one-way ANOVA followed by LSD test, p < 0.05).
3. Discussion
Structural studies of KSPIs have shown that they usually possess one to several conserved KDs consisting of six cysteine residues, but active sites of KSPIs have become highly variable due to long-term evolution [15]. Studies on P. leniusculus and Penaeus monodon (Crustacea: Decapoda) revealed that there were at least 26 and 20 different KDs from the hemocyte KSPIs of P. leniusculus and P. monodon, respectively. The position of the P1 site (usually the second amino acid residue after the second cysteine), a determinant for substrate specificity, varied highly [2,35]. In general, the domain with the P1 site of Arg or Lys inhibits trypsin; the P1 site of Tyr, Leu, Pro, Phe, or Met inhibits chymotrypsin; the P1 site of Ala or Ser inhibits elastase; and the P1 site of Thr or Asp inhibits proteinase K or subtilisin A.
Here, we characterized two KSPIs in N. vitripennis (NvKSPI-1 and NvKSPI-2); both have a typical KD consisting of six cysteine residues. For NvKSPI-1, the P1 site is Arg, and it was supposed to inhibit the activity of trypsin but not chymotrypsin (or proteinase K). On the other hand, the P1 site of NvKSPI-2 is Thr, and it was supposed not to inhibit the activity of our tested serine proteases (trypsin, chymotrypsin, and proteinase K). Our results with recombinant NvKSPI-1 and NvKSPI-2 were consistent with the general principle. As one of the most important families of protease inhibitors, KSPIs not on played a role in protecting the pancreas of vertebrates, but are also involved in many physiological processes in arthropods, such as dissolution, food digestion, blood coagulation, embryogenesis, ontogenesis, and inflammation and immune responses [16,36]. In two common shrimps, Litopenaeus vannamei (Crustacea: Decapoda) and Fenneropenaeus chinensis (Crustacea: Decapoda), KSPIs were cloned from their hemocytes’ cDNA libraries, and the mRNA levels of the shrimp KSPIs were upregulated after injection of a Gram-negative marine bacterium, Vibrio alginolyticus, suggesting a probable role of KSPIs in the immune response [7,9]. In the black tiger shrimp, P. monodon, a specific KSPI, SPIPm2, did not function as a protease inhibitor but inhibited the regulatory function of WSV477. Interaction between SPIPm2 and viral protein WSV477 reduced the replication of the white spot syndrome virus [10]. In bivalves, a five-domain KSPI was identified from the pearl oyster Pinctada fucata (Mollusca: Bivalvia) that could inhibit chymotrypsin and trypsin activities [37]. Two KSPIs (MdSPI-1 and MdSPI-2) that are important humoral factors of innate immunity were identified from the surf clam Mesodesma donacium (Mollusca: Bivalvia). They were significantly upregulated at 2 and 8 h post infection with V. anguillarum [38]. In the coconut rhinoceros beetle, Oryctes rhinoceros (Coleoptera: Scarabaeidae), a single-domain KSPI was shown to play a key role in protection against bacterial infections [39]. In blood-sucking bugs Rhodnius prolixus (Hemiptera: Reduviidae) and Dipetalogaster maximus (Hemiptera: Reduviidae), synthesized double-headed KSPIs, which are highly specific for thrombin, could prevent the host blood coagulation [3,40]. In Triatoma infestans (Hemiptera: Reduviidae), one KSPI, factor XIIa, was found to participate in its meal acquisition and digestion, while another KSPI, Infestin 1R, was also demonstrated to be able to impair mammalian cell invasion by Trypanosoma cruzi [21]. In the mosquito, Aedes aegypti (Diptera: Culicidae), KSPIs not only acted as anticoagulants during blood feeding and digestive processes, but served other functions during the development of the mosquito [41]. In silk moths, Bombyx mori (Lepidoptera: Bombycidae), one three-domain KSPI was identified from the cDNA library of the pupae, and was speculated to inhibit the invasion of pathogenic microorganisms [11]. In Asian honey bees, Apis cerana (Hymenoptera: Apidae), a venom KSPI acting as a microbial serine protease inhibitor was identified and demonstrated to have inhibitory activity against subtilisin A and proteinase K [42]. In the desert locust, Schistocerca gregaria (Orthoptera: Acrididae), one KSPI specific for elastase, subtilisin, and chymotrypsin were identified from its ovary gland [12]. Therefore, KSPIs have diverse functions in insects, particularly related to insect immune responses.
Compared to other insects, few studies on KSPIs have been reported in parasitoid wasps. Only de Graaf et al. [30] and our group identified two cDNA sequences of KSPIs (NvKSPI-1 and NvKSPI-2) from N. vitripennis, and our previous proteome determination of venom of N. vitripennis indicated that NvKSPI-1 and NvKSPI-2 could be secreted into the venom (unpublished), but no further studies were performed. Parasitoid wasps are important potential resources for biological control. N. vitripennis is an ectoparasitoid of flies and deposits its eggs along with the venom to ensure the development of the offspring by inhibiting the immune response or growth and development of the host [43,44,45]. Here, we presented a detailed study on the functions of NvKSPI-1 and NvKSPI-2 to host immunity. Our results showed that NvKSPI-1 and NvKSPI-2 were both expressed in N. vitripennis venom apparatus, in which their transcribed levels were hundreds of times higher than in other tissues tested, suggesting that NvKSPI-1 and NvKSPI-2 were possibly involved in inhibition of the host immune response. For the expression profiles of NvKSPIs in venom apparatus, the expression level of NvKSPIs declined at 3 days post eclosion compared to that of the second day; this might be due to the biological rhythm of female wasps. The first three days post eclosion were the main mating and oviposition periods; after oviposition peak, female wasps would re-express NvKSPIs for the subsequent parasitism. Of course, NvKSPIs might be involved in other biological functions during the eclosion period. PPO and PO activation assays of hemolymph from M. domestica (host) pupae showed that NvKSPI-1 and NvKSPI-2 were inhibitors of PPO. The PPO activation system is an important component of the immune system in arthropods, and activation of zymogen PPO to active PO involves a serine protease cascade [46,47] and is tightly regulated by serine proteases and serpins [48,49,50]. N. vitripennis, as an important ectoparasitoid, had two KSPIs (NvKSPI-1 and NvKSPI-2) that were expressed specifically in venom apparatus and secreted into venom, suggesting that NvKSPI-1 and NvKSPI-2 played a role in repressing host melaninization through inhibition of trypsin activity and PPO activation. However, more studies are needed to investigate how NvKSPI-1 and NvKSPI-2 inhibit PPO activation and screen for their potential interaction factors.
4. Materials and Methods
4.1. Insect Rearing
Cultures of Musca domestica (Diptera: Muscidae) and N. vitripennis were collected from the experimental field of Zhejiang University, Hangzhou, China. The laboratory colonies of N. vitripennis and its host M. domestica were maintained as described previously [51] and used in all experiments. In brief, the host larvae were fed an artificial diet composed of 15% milk powder, 35% wheat bran, and 50% water, in 500 mL glass canning jars (at 25 ± 1 °C, light:dark = 10h:14h, relative humidity (R. H.) = 75%) until eclosion. M. domestica adults were maintained on a mixture of sugar and milk powder (10:1) and water, within a stainless steel-mesh cage (55 cm × 55 cm × 55 cm) under the same conditions just described. Freshly pupated hosts were exposed to mated female wasps (pupae:wasps = 1:10) in a 500 mL glass canning jar for 24 h. The parasitized pupae were maintained under the conditions just described. After emerging, the female wasps were collected and held in glass containers (also under the conditions just described) and fed ad lib on 20% (v/v) honey solution to lengthen the life span for 3–4 days until dissection of the venom reservoir and gland.
4.2. Sample Preparation
Female N. vitripennis from 0 to 7 days post eclosion were collected and paralyzed for 5 min at −70 °C. The head, thorax, gut, ovary, remaining abdomen carcass (the rest of the abdomen after dissection), and venom apparatus (containing venom reservoir and gland) were then collected on ice under a stereoscope (Lecia, Frankfurt, Germany). Samples were stored at −70 °C.
4.3. RNA Extraction and cDNA Cloning
The collected tissues were homogenized in liquid nitrogen, and total RNA were extracted using the TRizol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. RNA integrity was confirmed by ethidium bromide gel staining and RNA quantity was determined spectrophotometrically at A260/280. Single-stranded cDNAs were synthesized by using PrimeScript™ One Step RT-PCR Kit (Takara, Dalian, China). Oligonucleotide primers (Table 1) were designed based on cDNA sequences of N. vitripennis KSPIs (GenBank accession numbers are NM_001161523 and NM_001170879). PCR conditions were as follows: an initial delay at 94 °C for 5 min; 34 cycles of denaturation at 94 °C for 30 min; annealing at 55 °C for 30s; and extending at 72 °C for 1 min. PCR products were fractionated in a 1% agarose gel by electrophoresis and purified with the DNA gel extraction kit (Aidlab, Beijing, China), and then cloned into the pGEM®-T easy cloning vector (Promega, Madison, Wisconsin, USA). Positive clones were selected by PCR and confirmed by sequencing at Invitrogen, Shanghai, China.
Table 1.
Primers used in this study.
| Primers Function | Primer Name | Primer Sequence(5ʹ-3ʹ) |
|---|---|---|
| Gene cloning | NvKSPI-1-F | TTGAGACGTGTCACCGAACA |
| NvKSPI -1-R | ACTTGGCAATGGTAAGTTCTTG | |
| NvKSPI -2-F | ATGAAGTTAACCGTATTCCTCTTC | |
| NvKSPI -2-R | TTAAACAAGTCCGAAGTTTGCATC | |
| RT-qPCR | RT-NvKSPI-1-F | ACTACCAACCAGTATGTGAC |
| RT-NvKSPI-1-R | TCACTGTACTTGGCAATGGT | |
| RT-NvKSPI-2-F | CGGACGATTATGAAGAAGAAG | |
| RT-NvKSPI-2-R | ACTTGATAGCTTCCTCGTTAG | |
| RT-18S-F | TGGGCCGGTACGTTTACTTT | |
| RT-18S-R | CACCTCTAACGTCGCAATAC | |
| Recombinant expression | NvKSPI-1-F-B | CGCGGATCCTGCATTTGTCCAAGAAACTACC |
| NvKSPI-1-R-E | CCGGAATTCTTAACATTCACTGTACTTGGCAATG | |
| NvKSPI-2-F-B | CGCGGATCCCAACTTGAATCGGACGATTATGAAG | |
| NvKSPI-2-R-E | CCGGAATTCTTAAACAAGTCCGAAGTTTGCATCG |
4.4. Sequence Analysis
The cDNAs and deduced amino acid sequences were analyzed by DNAStar software (version 5.02, DNAStar, Madison, Wisconsin, USA) and online Blast [52]. The signal peptides were analyzed by Signal P [53]. Multiple sequence alignments were carried out with DNAman software (Lynnon Biosoft, Quebec, QC, Canada) and Clustal W2 [54]. The phylogenetic tree was constructed by using MEGA 5.1 (Tokyo Metropolitan University, Tokyo, Japan) with the neighbor-joining (NJ) method.
4.5. Protein Expression, Purification, and Antibody Preparation
The primers (Table 1) containing BamH I and Xho I restriction enzyme sites were designed to amplify the ORF cDNA sequences of NvKSPI-1 and NvKSPI-2 by PCR. PCR products and the pGEX-4T-2 vector were ligated after they were double digested by BamH I and Xho I (Takara, Dalian, China). The recombinant plasmids were confirmed by sequencing, transformed into Escherichia coli BL21 (DE3) cells (AxyGen, Shanghai, China), and induced by 1 mM isopropyl thiogalactoside (IPTG) for protein expression. The recombinant proteins were analyzed by 12% SDS-PAGE and then purified using GST•Bind™ Resin Kit (Novagen, Hilden, Germany) according to the protocols. Protein concentrations were determined by using the BCA Protein Assay Kit (Novagen, Hilden, Germany).
4.6. Real-Time Quantitative PCR (RT-qPCR)
RT-qPCR was used to determine the transcription profiles of NvKSPI-1 and NvKSPI-2 in different tissues and ages of N. vitripennis females. The 18S rRNA gene (accession number GQ410677) was used as an internal control. Ten microliters of each first-strand cDNA product were diluted with 90 µL of sterilized water before use. The RT-qPCR system was performed with each 20 µL reaction containing 10 μL SsoFast EvaGreen SuperMix (Bio-Rad, Hercules, California, USA), 1 µL forward primer (200 nM), 1 µL reverse primer (200 nM), 1 µL diluted cDNA, and 7 μL sterile water. The thermal cycling conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. Amplification was monitored on the iCycleriQ TM Real-Time PCR Detection System (Bio-Rad, Hercules, California, USA). The specificity of the SYBR-Green PCR signal was further confirmed by melting curve analysis. The experiments were repeated three times as independent biological replicates. The mRNA expression was quantified using the 2−ΔΔCt method [55].
4.7. Serine Protease Inhibition Assays
Protease inhibition assays were performed with purified recombinant NvKSPI-1 and NvKSPI-2 proteins using the method described by Ling et al. [56]. Three typical serine proteases (bovine pancreatic trypsin, bovine pancreatic chymotrypsin, and proteinase K, 200 ng/mL) (Sigma, Taufkirchen, Germany) and their corresponding substrates (N-benzoyl-Val-Gly-Arg-p-nitroanilide, N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilid, and N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilid) (Sigma, Taufkirchen, Germany) were selected for determination of the spectrum of enzyme inhibition by recombinant NvKSPI-1 and NvKSPI-2 as follows. The recombinant NvKSPI-1 and NvKSPI-2 (1 µg each) were pre-incubated with a reaction buffer (100 mM Tris-HCl, 100 mM NaCl, 1 mM CaCl2, pH 7.5) containing 200 ng/mL serine protease for 30 min at room temperature, and then 200 μL substrate was added (0.1 mM, 100 mM Tris-HCl, 100 mM NaCl, 1 mM CaCl2, pH 7.5) before measuring the absorbance at 405 nm every min for 30 min. Substrates alone were used as blanks. Buffer and buffer with bovine serum albumin (BSA) were used as controls. One unit of enzyme activity was defined as an increase of absorbance by 0.001 per min. The experiments were repeated three times as independent biological replicates.
4.8. Prophenoloxidase (PPO) Activation and Phenoloxidase (PO) Activity Assays
PPO activation and PO activity assays were performed with purified recombinant NvKSPI-1 and NvKSPI-2 according to the method described by Ling et al. [48]. M. domestica in the white pupal stage were sterilized by 75% alcohol, rinsed with sterilized water and finally air-dried at room temperature before use. Hemolymph was collected from pupae by puncturing the pupal cuticle with a sterilized dissecting pin and rapidly transferred into a sterilized 1.5 mL Eppendorf tube. The collected hemolymph was then centrifuged at 3300 g for 5 min at 4 °C, and the supernatant was transferred into another 1.5 mL Eppendorf tube. Two microliters of each hemolymph sample were incubated with or without Micrococcus luteus (0.5 μg) in 10 μL of Tris buffered saline (TBS) at pH 7.4 in wells of a 96-well plate for 60 min at room temperature. Then 200 μL of l-dopamine (2 mM in TBS, pH 6.5) was added and absorbance at 470 nm was monitored. One unit of PO activity was defined as an increase of absorbance at 470 nm by 0.001 per min. Plasma samples with low PO activity when incubated alone but high PO activity after incubation with M. luteus were selected for subsequent PPO and PO activation assays.
For PPO activation assays, each 2 μL of prepared hemolymph was incubated with 10 μL TBS, 10 μL TBS/M. luteus (0.5 μg)/NvKSPI-1 (1 μg) mixture, 10 μL TBS/M. luteus (0.5 μg)/NvKSPI-2 (1 μg) mixture, 10 μL TBS/M. luteus (0.5 μg)/BSA (1 μg) mixture, 10 μL TBS/M. luteus (0.5 μg)/GST (1 μg) mixture, and 10 μL TBS/PTU (phenyl thiourea, saturated), respectively, for 20 min at 25 °C. Finally, 200 μL of l-dopamine substrate (2 mM) was added to each sample, and the PO activity was measured at 470 nm in a plate reader for 30 min. One unit of PO activity was defined as an increase of absorbance at 470 nm by 0.001 per minute.
For PO activity assays, each 2 μL of prepared hemolymph was incubated with 10 μL TBS/M. luteus (0.5 μg) mixture for 10 min at 25 °C. Then 2 μL TBS, 2 μL TBS, 2 μL NvKSPI-1 (1 μg), 2 μL NvKSPI-2 (1 μg), 2 μL BSA (1 μg), 2 μL GST (1 μg), and 2 μL PTU was added, respectively, and incubated for 10 min at 25 °C. Finally, 200 μL of l-dopamine substrate (2 mM) were added to each sample, and the PO activity was measured at 470 nm in a plate reader for 30 min. One unit of PO activity was defined as an increase of absorbance at 470 nm by 0.001 per min. All of the above experiments were repeated three times as independent biological replicates.
4.9. Statistical Analysis
All data were calculated as the mean ± standard deviation. Differences between samples were analyzed by one-way analysis of variance (ANOVA). Means were compared by least significant difference (LSD) tests. All statistical calculations were run using DPS software (version 8.01) [35] and statistical significance was set at p < 0.05.
5. Conclusions
In summary, we executed the molecular cloning and functional studies of KSPI-1 and KSPI-2 in N. vitripennis. We found that KSPI-1 and KSPI-2 are specifically expressed by N. vitripennis venom apparatus. The recombinant GST-NvKSPI-1 and GST-NvKSPI-2 can inhibit the PPO activation in host hemolymph and NvKSPI-1 can inhibit the trypsin activity. Those findings suggest that NvKSPI-1 and NvKSPI-2 play a role in repressing host melaninization through inhibition of trypsin activity and PPO activation. However, more studies are needed to investigate the immune mechanism of KSPIs in insects.
Acknowledgments
This research was supported by grants from the National Program on Key Basic Research Projects (973 Program, 2013CB127600), the National Natural Science Foundation of China (Grant No. 31272098, 31472038), the Research Fund for the Doctoral Program of Higher Education of China (Grant Number: 2012010113004), the National Science Fund for Innovative Research Groups of Biological Control (Grant No. 31321063), the Zhejiang Provincial Natural Science Foundation of China (Grant Number: Y14C140006), and the Fundamental Research Funds for the Central Universities (Grant Number: 2014FZA6014).
Author Contributions
Gong-Yin Ye and Qi Fang conceived and designed the experiments; Cen Qian performed the experiments; Lei Wang analyzed the data; Cen Qian and Gong-Yin Ye wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
References
- 1.Kazal L.A., Spicer D.S., Brahinsky R.A. Isolation of a crystalline trypsin inhibitor-anticoagulant protein from pancreas. J. Am. Chem. Soc. 1948;70:3034–3040. doi: 10.1021/ja01189a060. [DOI] [PubMed] [Google Scholar]
- 2.Laskowski M., Jr., Kato I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich T., Kroger B., Bialojan S., Lemaire H.G., Hoffken H.W., Reuschenbach P., Otte M., Dodt J. A Kazal-type inhibitor with thrombin specificity from Rhodnius prolixus. J. Biol. Chem. 1993;268:16216–16222. [PubMed] [Google Scholar]
- 4.Johansson M.W., Keyser P., Soderhall K. Purification and cDNA cloning of a four-domain Kazal proteinase inhibitor from crayfish blood cells. Eur. J. Biochem. 1994;223:389–394. doi: 10.1111/j.1432-1033.1994.tb19005.x. [DOI] [PubMed] [Google Scholar]
- 5.Sommerhoff C.P., Sollner C., Mentele R., Piechottka G.P., Auerswald E.A., Fritz H. A Kazal-type inhibitor of human mast cell tryptase: Isolation from the medical leech Hirudo medicinalis, characterization, and sequence analysis. Biol. Chem. Hoppe. Seyler. 1994;375:685–694. doi: 10.1515/bchm3.1994.375.10.685. [DOI] [PubMed] [Google Scholar]
- 6.Vargas-Albores F., Villalpando E. A new type of Kazal proteinase inhibitor related to shrimp Penaeus (Litopenaeus) vannamei immunity. Fish Shellfish Immunol. 2012;33:134–137. doi: 10.1016/j.fsi.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez-Vega F., Vargas-Albores F. A four-Kazal domain protein in Litopenaeus vannamei hemocytes. Dev. Comp. Immunol. 2005;29:385–391. doi: 10.1016/j.dci.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.González Y., Pons T., Gil J., Besada V., Alonso-del-Rivero M., Tanaka A.S., Araujo M.S., Chávez M.A. Characterization and comparative 3D modeling of CmPI-II, a novel “non-classical” Kazal-type inhibitor from the marine snail Cenchritis muricatus (Mollusca) Biol. Chem. 2007;388:1183–1194. doi: 10.1515/BC.2007.129. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z.H., Zhao X.F., Wang J.X. Characterization, kinetics, and possible function of Kazal-type proteinase inhibitors of Chinese white shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2009;26:885–897. doi: 10.1016/j.fsi.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Visetnan S., Donpudsa S., Supungul P., Tassanakajon A., Rimphanitchayakit V. Domain 2 of a Kazal serine proteinase inhibitor SPIPm2 from Penaeus monodon possesses antiviral activity against WSSV. Fish Shellfish Immunol. 2014;41:526–530. doi: 10.1016/j.fsi.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Q.L., Chen J., Nie Z.M., Lv Z.B., Wang D., Zhang Y.Z. Expression, purification and characterization of a three-domain Kazal-type inhibitor from silkworm pupae (Bombyx mori) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:234–240. doi: 10.1016/j.cbpb.2006.10.106. [DOI] [PubMed] [Google Scholar]
- 12.Brillard-Bourdet M., Hamdaoui A., Hajjar E., Boudier C., Reuter N., Ehret-Sabatier L., Bieth J.G., Gauthier F. A novel locust (Schistocerca gregaria) serine protease inhibitor with a high affinity for neutrophil elastase. Biochem. J. 2006;400:467–476. doi: 10.1042/BJ20060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krowarsch D., Cierpicki T., Jelen F., Otlewski J. Canonical protein inhibitors of serine proteases. Cell Mol. Life Sci. 2003;60:2427–2444. doi: 10.1007/s00018-003-3120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christeller J.T. Evolutionary mechanisms acting on proteinase inhibitor variability. FEBS. J. 2005;272:5710–5722. doi: 10.1111/j.1742-4658.2005.04975.x. [DOI] [PubMed] [Google Scholar]
- 15.Rimphanitchayakit V., Tassanakajon A. Structure and function of invertebrate Kazal-type serine proteinase inhibitors. Dev. Comp. Immunol. 2010;34:377–386. doi: 10.1016/j.dci.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhu L., Song L., Chang Y., Xu W., Wu L. Molecular cloning, characterization and expression of a novel serine proteinase inhibitor gene in bay scallops (Argopecten irradians, Lamarck 1819) Fish Shellfish Immunol. 2006;20:320–331. doi: 10.1016/j.fsi.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Kanost M.R. Serine proteinase inhibitors in arthropod immunity. Dev. Comp. Immunol. 1999;23:291–301. doi: 10.1016/S0145-305X(99)00012-9. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H., Kanost M.R. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 2000;30:95–105. doi: 10.1016/S0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 19.Di Cera E. Serine proteases. IUBMB Life. 2009;61:510–515. doi: 10.1002/iub.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos I.T., Amino R., Sampaio C.A., Auerswald E.A., Friedrich T., Lemaire H.G., Schenkman S., Tanaka A.S. Infestin, a thrombin inhibitor presents in Triatoma infestans midgut, a Chagas disease vector: Gene cloning, expression and characterization of the inhibitor. Insect Biochem. Mol. Biol. 2002;32:991–997. doi: 10.1016/S0965-1748(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 21.Campos I.T., Tanaka-Azevedo A.M., Tanaka A.S. Identification and characterization of a novel factor XIIa inhibitor in the hematophagous insect, Triatoma infestans (Hemiptera: Reduviidae) FEBS Lett. 2004;577:512–516. doi: 10.1016/j.febslet.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Lovato D.V., de Campos I.T.N., Amino R., Tanaka A.S. The full-length cDNA of anticoagulant protein infestin revealed a novel releasable Kazal domain, a neutrophil elastase inhibitor lacking anticoagulant activity. Biochimie. 2006;88:673–681. doi: 10.1016/j.biochi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Meiser C.K., Piechura H., Werner T., Dittmeyer-Schäfer S., Meyer H.E., Warscheid B., Schaub G.A., Balczun C. Kazal-type inhibitors in the stomach of Panstrongylus megistus (Triatominae, Reduviidae) Insect Biochem. Mol. Biol. 2010;40:345–353. doi: 10.1016/j.ibmb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J.Y., Ye G.Y., Fang Q., Wu M.L., Hu C. A pathogenic picorna-like from the endoparasitoid wasp, Pteromalus puparum: Initial discovery and partial genomic characterization. Virus Res. 2008;138:144–149. doi: 10.1016/j.virusres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Dong S.Z., Ye G.Y., Zhu J.Y., Chen Z.X., Hu C., Liu S. Vitellin of Pteromalus puparum (Hymenoptera: Pteromalidae), a pupal endoparasitoid of Pieris rapae (Lepidoptera: Pieridae): Biochemical characterization, temporal patterns of production and degradation. J. Insect Physiol. 2007;53:468–477. doi: 10.1016/j.jinsphys.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Dong S.Z., Ye G.Y., Hu C. Roles of Ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae) Gen. Comp. Endoc. 2009;160:102–108. doi: 10.1016/j.ygcen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Rivers D.B., Uçkan F., Ergin E., Keefer D.A. Pathological and ultrastructural changes in cultured cells induced by venom from the ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) J. Insect Physiol. 2010;56:1935–1948. doi: 10.1016/j.jinsphys.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Ye G.Y., Cai J., Hu C. Comparative venom toxicity between Pteromalus puparum and Nasonia vitripennis (Hymenoptera: Pteromalidae) toward the hemocytes of their natural hosts, non-target insects and cultured insect cells. Toxicon. 2005;46:337–349. doi: 10.1016/j.toxicon.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Dong S.Z., Ye G.Y., Yao P.C., Huang Y.L., Chen X.X., Shen Z.C., Hu C. Effects of starvation on the vitellogenesis, ovarian development and fecundity in the ectoparasitoid, Nasonia vitripennis (Hymenoptera: Pteromalidae) Insect Sci. 2008;15:429–440. doi: 10.1111/j.1744-7917.2008.00230.x. [DOI] [Google Scholar]
- 30.De Graaf D.C., Aerts M., Brunain M., Desjardins C.A., Jacobs F.J., Werren J.H., Devreese B. Insight into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatics and proteomic studies. Insect Mol. Biol. 2010;19:11–26. doi: 10.1111/j.1365-2583.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian C. Ph. D. Dissertation. Zhejiang University; Hangzhou, China: 2013. Identification of Venom Proteins from Nasonia vitripennis and Functional Analysis of Pacifastin and Kazal Type Genes. [Google Scholar]
- 32.Shuker D., Lynch J., Peire Morais A. Moving from model to non-model organisms? Lessons from Nasonia wasps. Bioessays. 2003;25:1247–1248. doi: 10.1002/bies.10367. [DOI] [PubMed] [Google Scholar]
- 33.Wurm Y., Keller L. Parasitoid wasps: from natural history to genomic studies. Curr. Biol. 2010;20:R242–R244. doi: 10.1016/j.cub.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Werren J.H., Richards S., Desjardins C.A., Niehuis O., Gadau J., Colbourne J.K., Desplan C., Beukeboom L.W., Elsik C.G., Grimmelikhuijzen C.J., et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu W., Apostol I., Qasim M.A., Warne N., Wynn R., Zhang W.L., Anderson S., Chiang Y.W., Ogin E., Rothberg I., et al. Binding of amino acid side-chains to S1 cavities of serine proteinases. J. Mol. Biol. 1997;266:441–461. doi: 10.1006/jmbi.1996.0781. [DOI] [PubMed] [Google Scholar]
- 36.Van Hoef V., Breugelmans B., Spit J., Simonet G., Zels S., Vanden B.J. Phylogenetic distribution of protease inhibitors of the Kazal-family within the Arthropoda. Peptides. 2013;41:59–65. doi: 10.1016/j.peptides.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D., Ma J., Jiang S. Molecular characterization, expression and function analysis of a five-domain Kazal-type serine proteinase inhibitor from pearl oyster Pinctada fucata. Fish Shellfish Immunol. 2014;37:115–121. doi: 10.1016/j.fsi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado-Aguayo W., Núñez-Acuña G., Valenzuela-Muñoz V., Chávez-Mardones J., Gallardo-Escárate C. Molecular characterization of two Kazal-type serine proteinase inhibitor genes in the surf clam Mesodesma donacium exposed to Vibrio anguillarum. Fish Shellfish Immunol. 2013;34:1448–1454. doi: 10.1016/j.fsi.2013.03.356. [DOI] [PubMed] [Google Scholar]
- 39.Horita S., Ishibashi J., Nagata K., Miyakawa T., Yamakawa M., Tanokura M. Isolation, cDNA cloning, and structure-based functional characterization of oryctin, a hemolymph protein from the coconut rhinoceros beetle, Oryctes rhinoceros, as a novel serine protease inhibitor. J. Biol. Chem. 2010;285:30150–30158. doi: 10.1074/jbc.M110.124735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mende K., Petoukhova O., Koulitchkova V., Schaub G.A., Lange U., Kaufmann R., Nowak G. Dipetalogastin, a potent thrombin inhibitor from the blood-sucking insect. Dipetalogaster maximus cDNA cloning, expression and characterization. Eur. J. Biochem. 1999;266:583–590. doi: 10.1046/j.1432-1327.1999.00895.x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe R.M., Soares T.S., Morais-Zani K., Tanaka-Azevedo A.M., Maciel C., Capurro M.L., Torquato R.J., Tanaka A.S. A novel trypsin Kazal-type inhibitor from Aedes aegypti with thrombin coagulant inhibitory activity. Biochimie. 2010;92:933–939. doi: 10.1016/j.biochi.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Kim B.Y., Lee K.S., Zou F.M., Wan H., Choi Y.S., Yoon H.J., Kwon H.W., Je Y.H., Jin B.R. Antimicrobial activity of a honeybee (Apis cerana) venom Kazal-type serine protease inhibitor. Toxicon. 2013;76:110–117. doi: 10.1016/j.toxicon.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Beckage N.E., Gelman D.B. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 2004;49:299–330. doi: 10.1146/annurev.ento.49.061802.123324. [DOI] [PubMed] [Google Scholar]
- 44.Asgari S. Venom proteins from polydnavirus-producing endoparasitoids: Their role in host-parasite interactions. Arch. Insect Biochem. Physiol. 2006;61:146–156. doi: 10.1002/arch.20109. [DOI] [PubMed] [Google Scholar]
- 45.Abt M., Rivers D.B. Characterization of phenoloxidase activity in venom from the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) J. Invertebr. Pathol. 2007;94:108–118. doi: 10.1016/j.jip.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Cerenius L., Lee B.L., Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Cerenius L., Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 48.Tong Y., Jiang H., Kanost M.R. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin-5 and their association with components of the prophenol oxidase activation pathway. J. Biol. Chem. 2005;280:14932–14942. doi: 10.1074/jbc.M500532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong Y., Kanost M.R. Manduca sexta serpin-4 and serpin-5 inhibit the prophenol oxidase activation pathway: cDNA cloning, protein expression, and characterization. J. Biol. Chem. 2005;280:14923–14931. doi: 10.1074/jbc.M500531200. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Wang Y., Gorman M.J., Jiang H., Kanost M.R. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J. Biol. Chem. 2003;278:46556–46564. doi: 10.1074/jbc.M309682200. [DOI] [PubMed] [Google Scholar]
- 51.Ye G.Y., Dong S.Z., Dong H., Hu C., Shen Z.C., Cheng J.A. Effects of host (Boettcherisca peregrina) copper exposure on development, reproduction and vitellogenesis of the ectoparasitic wasp, Nasonia vitripennis. Insect Sci. 2009;16:43–50. doi: 10.1111/j.1744-7917.2009.00252.x. [DOI] [Google Scholar]
- 52.BLAST: Basic Local Alignment Search Tool. [(accessed on 30 July 2015)]; Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
- 53.CBS: Center of Biological Sequence Analysis. [(accessed on 30 July 2015)]. Available online: http://www.cbs.dtu.dk/services/SignalP/
- 54.ClustalW2. [(accessed on 30 July 2015)]. Available online: http://www.ebi.ac.uk/Tools/clustalw2/
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Ling E.J., Rao X.J., Ao J.Q., Yu X.Q. Purification and characterization of a small cationic protein from the tobacco hornworm Manduca sexta. Insect Biochem. Mol. Biol. 2009;39:263–271. doi: 10.1016/j.ibmb.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]