Abstract
Ruminant diets include cereals, protein feeds, their by-products as well as hay and grass, grass/legume, whole-crop maize, small grain or sorghum silages. Furthermore, ruminants are annually or seasonally fed with grazed forage in many parts of the World. All these forages could be contaminated by several exometabolites of mycotoxigenic fungi that increase and diversify the risk of mycotoxin exposure in ruminants compared to swine and poultry that have less varied diets. Evidence suggests the greatest exposure for ruminants to some regulated mycotoxins (aflatoxins, trichothecenes, ochratoxin A, fumonisins and zearalenone) and to many other secondary metabolites produced by different species of Alternaria spp. (e.g., AAL toxins, alternariols, tenuazonic acid or 4Z-infectopyrone), Aspergillus flavus (e.g., kojic acid, cyclopiazonic acid or β-nitropropionic acid), Aspergillus fuminatus (e.g., gliotoxin, agroclavine, festuclavines or fumagillin), Penicillium roqueforti and P. paneum (e.g., mycophenolic acid, roquefortines, PR toxin or marcfortines) or Monascus ruber (citrinin and monacolins) could be mainly related to forage contamination. This review includes the knowledge of mycotoxin occurrence reported in the last 15 years, with special emphasis on mycotoxins detected in forages, and animal toxicological issues due to their ingestion. Strategies for preventing the problem of mycotoxin feed contamination under farm conditions are discussed.
Keywords: mycotoxins, silage, hay, dairy cow, heifers, ruminants
1. Introduction
Mycotoxins are defined as molecules of low molecular weight produced by fungi that elicit a toxic response through a natural route of exposure both in humans and other vertebrate animals [1,2,3]. They are often very stable molecules and all are secondary metabolites of molds belonging to several genera, in particular Aspergillus, Fusarium, and Penicillium spp. [4,5,6]. Furthermore, other genera such as Alternaria, Chaetomium, Cladosporium, Claviceps, Diplodia, Myrothecium, Monascus, Phoma, Phomopsis, Pithomyces, Trichoderma and Stachybotrys include mycotoxigenic species [7,8,9,10,11]. Mycotoxin contamination represents a worldwide problem for various agricultural commodities both pre and post-harvest [7,12,13]. To date, there are about 18,000 fungal secondary metabolites described in Antibase2014, but only a restricted number [4,14] has received scientific interest from the 1960s and onwards (Table 1). As expected, the most studied are regulated mycotoxins (i.e., aflatoxins (AFs), citrinin, trichothecenes such as deoxynivalenol (DON), patulin, ochratoxin A (OTA), fumonisins (FBs) and zearalenone (ZEA)) and some major toxins of endophytic fungi (ergot toxins and ergotamine).
Table 1.
Number of Scopus database citations for several secondary metabolites produced by mycotoxigenic fungi and their scientific interests.
| Secondary Metabolites | Scopus Citation | Scientific Interest a | Secondary Metabolites | Scopus Citation | Scientific Interest a |
|---|---|---|---|---|---|
| AAL toxin | 100 | ** | Infectopyrones | 3 | * |
| Aflatoxins | 16,939 | ***** | Islanditoxin | 10 | * |
| Aflavinine | 12 | * | Luteoskyrin | 135 | ** |
| Agroclavine | 214 | *** | Marcfortine A, B and C | 38 | * |
| Alternariol | 396 | **** | Monacolins | 242 | *** |
| Andrastins | 30 | * | Moniliformin | 399 | **** |
| Aspergillic acid | 66 | * | Monoacetoxyscirpenol | 64 | * |
| Aurofusarin | 55 | * | Mycophenolic acid | 241 | *** |
| Beauvericin | 441 | **** | Neosolaniol | 242 | *** |
| β-nitropropionic acids | 4 | * | Nivalenol | 1014 | ***** |
| Botryodiploidin | 36 | * | Novae-zelandins | 1 | * |
| Butenolide | 1337 | ***** | Ochratoxins | 5162 | ***** |
| Byssochlamic acid | 31 | * | Oosporein | 45 | * |
| Chlamydosporol | 21 | * | Orsellinic acid | 205 | *** |
| Chrysogine | 18 | * | Paspalitrems | 7 | * |
| Citreoviridin | 124 | ** | Patulin | 1606 | ***** |
| Citrinin | 1994 | ***** | Penicillic acid | 437 | **** |
| Citreoisocoumarin | 9 | * | Penitrem | 202 | *** |
| Clavine alkaloids | 146 | ** | Phomopsin | 123 | ** |
| Culmorin | 33 | * | PR toxin | 320 | **** |
| Cyclopiazonic Acid | 2307 | ***** | PR-amide | 6 | * |
| Deoxynivalenol | 3720 | ***** | PR-imine | 5 | * |
| Diacetoxyscirpenol | 759 | **** | Pseurotins | 56 | * |
| Dicoumarol | 3811 | ***** | Roquefortines | 213 | *** |
| Diketopioperazines | 1 | * | Roridins | 32 | * |
| Eremofortin C | 10 | * | Rubratoxin | 191 | ** |
| Ergot toxins | 7567 | ***** | Rubrofusarin | 75 | * |
| Ergotamine | 7298 | ***** | Scirpentriol | 69 | * |
| Festuclavine | 74 | * | Slaframine | 103 | ** |
| Fumagillin | 939 | **** | Sphingofungin | 47 | * |
| Fumigatins | 23 | * | Sporidesmin | 207 | *** |
| Fumiquinazolines | 56 | * | Stachbotryotoxins | 1 | * |
| Fumitremorgen | 11 | * | Sterigmatocystin | 1000 | **** |
| Fumitremorgines | 357 | **** | T-2 & HT-2 toxin | 470 | **** |
| Fumonisins | 3542 | ***** | Tentoxin | 208 | *** |
| Fusarenone-X | 54 | * | Tenuazonic acid | 256 | *** |
| Fusaric Acid | 675 | **** | Tremorgens | 37 | * |
| Fusarins | 100 | ** | Tremorgens | 46 | * |
| Fusariocin | 2 | * | Trypacidin | 20 | * |
| Gliotoxin | 996 | **** | Verruculogen | 112 | ** |
| Helvolic acid | 89 | ** | Zearalenone | 3443 | ***** |
a: The scientific interest associated to each secondary metabolite was assigned on the basis of number of Scopus citations obtained by using “Article title, Abstract, Keywords” document search criterion; *: for 1–99 citations; **: for 100–199 citations; ***: 200–299 citations; ****: 300–999 citations; *****: >1000 citations.
Generally, the term mycotoxicosis refers to the syndromes resulting from ingestion, skin contact or inhalation of these fungal metabolites [1,7,15,16,17,18,19]. When livestock ingest one or more mycotoxins, the effect on health could be acute, meaning evident signs of disease are present or even causing death. However, acute manifestation of mycotoxicosis is rare under farm conditions, e.g., mainly seen in South America from Baccharis plants that have endophyte infection [20,21]. The effects of mycotoxin ingestion are mainly chronic, implying hidden disorders with reduced ingestion, productivity and fertility [3,8,22]. Such effects cause severe economic losses through clinically ambiguous changes in animal growth, feed intake reduction or feed refusal, alteration in nutrient absorption and metabolism, effects on the endocrine system as well as suppression of the immune system [2,3,23,24,25].
Ruminants are less susceptible to mycotoxins than monogastrics, because of the rumen microbiota and the feed particles contained in the rumen compartment may be effective in the degradation, deactivation and binding of these toxic molecules, hence protecting animals [3,5,26,27,28,29,30].
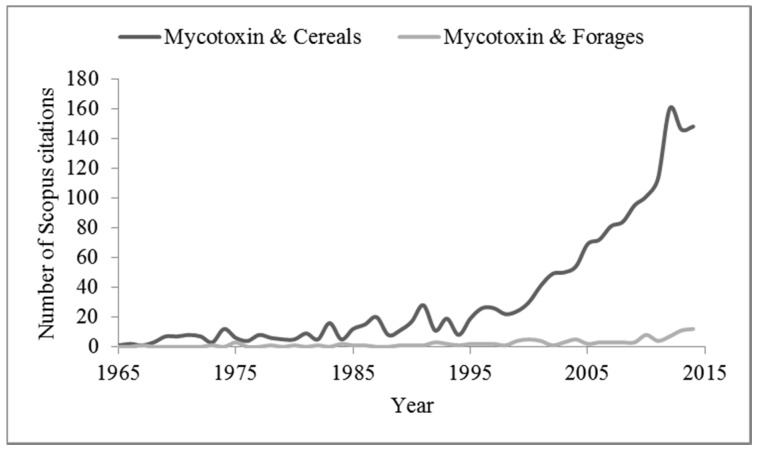
A summary of main toxic products from rumen metabolism and entity of reduction of mycotoxin biological potency were provided by Fink-Gremmels [31]. However, ruminant diets include starch (mainly cereals) and protein feeds, their by-products as well as grazed forage, hay or grass (GS), grass/legume (GLS), whole-crop forage maize (MS), small grain (SMS) and sorghum (SS) silages [32,33], which increase the risk of mycotoxin exposure compared to swine and poultry that have less varied diets. Some recent evidence suggests the greatest exposure to some regulated mycotoxins in cows could be related to forage contamination [10,34,35,36,37,38], even if this aspect remains poorly investigated. In particular, published articles where researchers investigated the presence of mycotoxins in hay and silages are very limited when compared to those analyzing the problem of mycotoxin contaminations in cereals (Figure 1). Furthermore, many other secondary metabolites different from regulated mycotoxins could be detected in forages, even if knowledge of their occurrence in forages is currently limited [10,28,37,39,40].
Figure 1.
Number of Scopus database citations obtained by searching the keywords “Mycotoxins & Cereals” or “Mycotoxins & Forages”.
This review includes the knowledge of mycotoxins in cow feeds obtained in the last 15 years, with special emphasis on mycotoxins detected in forages, and animal toxicological issues due to their ingestion. In addition, the main strategies for preventing the problem of mycotoxin presence under farm conditions are presented and discussed.
2. Mycotoxin Occurrence in Animal Feeds, with Special Emphasis on Their Presence in Forages
From the 1970s, several reviews have been published in which occurrence data as well contamination levels of some mycotoxins in cereals and cereal by-products for animal nutrition have been reported [7,12,41,42,43,44,45,46,47,48,49,50,51] and nowadays more than 100 Countries have issued specific regulated or recommended limits or detailed guidelines for mycotoxin control in products intended for animal feeds [52,53,54,55,56]. In the last 15 years, an emerging issue related to mycotoxin contaminations of forages and factors affecting their occurrence at pre-harvest in the field or during ensiling and storage of forage crops has progressed. These aspects have been the basis of different review papers recently published [5,8,28,37,39,57,58,59,60].
Filamentous fungi can grow on forages and their presence is frequently observed in silage or hay [8,24,61,62]. Usually, the three most important toxigenic genera occurring pre-harvest are Aspergillus, Fusarium and perhaps Alternaria spp. [8]. In particular, the latter two are often categorized as field fungi whereas some species of Aspergillus can occur both pre- and post-harvest. The occurrence of these fungi in the field is related to several factors, including agricultural practices and climatic conditions [63,64]. During ensiling, most fungi can be eliminated [65,66]. However, there are other species, such as Aspergillus fumigatus, Penicillium roqueforti, P. paneum, F. oxysporum and Monascus ruber that are able to tolerate both high levels of organic acids and carbon dioxide in addition to low availability of oxygen [8,28,37,67,68]. In particular, presence of oxygen in some parts of silage during storage or oxygen penetration during feed-out and aerobic spoilage phases could allow mold growth and mycotoxin production. In high quality silage, lactic acid bacteria are effective in hindering any mold growth, but just a small raise in the oxygen concentration could provide the right growth conditions for fungi such as P. roqueforti and P. paneum. Indeed, if most of acetic and lactic acids as well as carbon dioxide evaporate and more oxygen is present, nearly all cereal-associated filamentous fungi may grow [8,69]. Considerable variability in the mycotoxin occurrences and concentration levels has been reported in forages and this could be probably due to a multitude of environment-related (i.e., meteorological conditions, agronomical practices, ensiling procedures, management of forage, types of forage, etc.) or lab-related (sampling procedures, storage and preparation of samples, adopted analytical methods, etc.) factors. Results about occurrence and concentration levels of main mycotoxins detected in hay and silages are presented in Table 2. In Table 3, we also report mycotoxins analyzed but not detected in forages, to improve occurrence data discussion.
Table 2.
Survey of mycotoxins detected in forages and other fibrous feeds from the literature.
| Forage Products | Mycotoxins a | Number of Samples | Incidence (%) | Mean (Excluding not Detectable Data when Possible) | Range or Maximal Detected Value | Nation | References | Notes |
|---|---|---|---|---|---|---|---|---|
| Alternaria spp. derived toxin | ||||||||
| Different feeds | AAL TA toxin | 63 | 97% | 560 μg/kg | 90–1470 μg/kg | WI, US | [75] | |
| MS | AAL TA toxin | 60 | ~30% | 170 μg/kg | 200–2010 μg/kg | PA, US | [76] | |
| Hay and hay silage | AAL TA toxin | 25 | 100% | 720 μg/kg | 290–1160 μg/kg | WI, US | [75] | |
| MS | AAL TB toxin | 60 | ~15% | 50 μg/kg | 30–900 μg/kg | PA, US | [76] | |
| MS | Alternariol | 82 | 2% | 18 μg/kg | max 24 μg/kg | Denmark | [24] | |
| MS | Alternariol ME | 82 | 2% | 8 μg/kg | max 8.8 μg/kg | Denmark | [24] | |
| Aspergillus flavus and A. parasiticus derived toxin | ||||||||
| MS | AFB1 | 1 | - | 28 μg/kg | France | [77] | ||
| MS | AFB1 | 100 | 92% | - | 0.6– > 4 μg/kg | Italy | [78] | only core samples |
| MS | AFB1 | 116 | 13% | 33 μg/kg | 2–54 μg/kg | Brazil | [62] | core samples |
| MS | AFB1 | 9 | - | - | 4–34 μg/kg | France | [79] | from 1 farm |
| Silages | β-nitropropionic acid | 3 | 33% | 1360 μg/kg | - | Netherlands | [16] | |
| Various Aspergillus and Penicillium spp. derived toxin | ||||||||
| Different feeds | Cyclopiazonic acid | 63 | 87% | 340 μg/kg | 120–1820 μg/kg | WI, US | [75] | |
| Hay and hay silage | Cyclopiazonic acid | 25 | 80% | 390 μg/kg | 120–1820 μg/kg | WI, US | [75] | |
| MS | Cyclopiazonic acid | 120 | 37% | 120 μg/kg | 20–1430 μg/kg | PA, US | [80] | 4 samples from 30 bunkers |
| Silages | Cyclopiazonic acid | 3 | 33% | 55 μg/kg | - | Netherlands | [16] | |
| Aspergillus fumigatus derived toxin | ||||||||
| MS | Gliotoxin | 1 | - | 4 μg/kg | France | [77] | ||
| MS | Gliotoxin | 90 | - | 5130 μg/kg | 5100–6500 μg/kg | Argentina | [81] | |
| Silages | Gliotoxin | 3 | 33% | 1870 μg/kg | - | Netherlands | [16] | |
| MS | Gliotoxin | 196 | <1% | 140 μg/kg | max 600 μg/kg | Italy | [69] | 3 samples from 68 silos |
| Fusarium spp. derived toxin Trichothecenes type A | ||||||||
| MS | 15-acetyl DON | 140 | <1% | 901 μg/kg | max 1013 μg/kg | Netherlands | [82] | over three years |
| MS | 15-acetyl DON | 5 | 100% | 59 μg/kg | max 127 μg/kg | Germany | [83] | |
| MS | 3-acetyl DON | 20 | 0% | - | - | Denmark | [84] | |
| Hays | 3-acetyl DON | 28 | 4% | 20 μg/kg | - | Germany | [83] | |
| MS | 3-&5-acetyl DON | 19 | 21% | 217 μg/kg | 135–300 μg/kg | Switzerland | [85] | |
| Different feeds | DON | 63 | 100% | 730 μg/kg | 340–6020 μg/kg | WI, US | [75] | |
| Compound feed | DON | 72 | 54% | 433 μg/kg | max 2408 μg/kg | Netherlands | [36] | |
| MS | DON | 20 | 100% | 1056 μg/kg | 160–5094 μg/kg | Denmark | [84] | |
| MS | DON | 140 | 72% | 854 μg/kg | max 3142 μg/kg | Netherlands | [82] | over three years |
| MS | DON | 82 | 6% | 1629 μg/kg | max 2974 μg/kg | Denmark | [24] | Quantitative analysis |
| MS | DON | 1 | - | 146 μg/kg | - | France | [77] | |
| MS | DON | 196 | 8% | 280 μg/kg | max 560 μg/kg | Italy | [69] | 3 samples from 68 silos |
| MS | DON | 9 | - | - | 100–213 μg/kg | France | [79] | from 1 farm |
| MS | DON | 5 | 100% | 2919 μg/kg | max 3944 μg/kg | Germany | [83] | |
| MS | DON | 19 | 100% | 1356 μg/kg | 780–2990 μg/kg | Switzerland | [85] | |
| MS | DON | 116 | 24% | 1610 μg/kg | 150–3420 μg/kg | Brazil | [62] | core samples |
| Silages | DON | 3 | 100% | 396 μg/kg | max 761 μg/kg | Netherlands | [16] | |
| Ensiled by-products | DON | 29 | 0% | - | - | Netherlands | [36] | |
| Feed commodities | DON | 8 | 38% | 1019 μg/kg | max 1811 μg/kg | Netherlands | [36] | |
| Forage products | DON | 13 | 15% | 348 μg/kg | max 489 μg/kg | Netherlands | [36] | |
| Hay and hay silage | DON | 25 | 100% | 610 μg/kg | 510–720 μg/kg | WI, US | [75] | |
| Hays | DON | 28 | 14% | 41 μg/kg | max 69 μg/kg | Germany | [83] | |
| Silage | DON | 47 | 53% | 550 μg/kg | max 1250 μg/kg | Netherlands | [36] | |
| SGS (Wheat) | DON | 30 | 10% | 621 μg/kg | max 1165 μg/kg | Netherlands | [36] | over two years |
| MS | DON 2000 | 196 | 59% | 1290 μg/kg DM | 240–12,890 μg/kg DM | Germany | [86] | ELISA method |
| MS | DON 2002 | 182 | 89% | 2100 μg/kg DM | 260–14,290 μg/kg DM | Germany | [86] | ELISA method |
| MS | DON 2001 | 32 | 86% | 800 μg/kg | max 3700 μg/kg | PA, US | [87] | over two years |
| MS | DON 2002 | 39 | 66% | 1100 μg/kg | max 5100 μg/kg | PA, US | [87] | over two years |
| MS | Fusarenon X | 20 | 20% | 4 μg/kg | 8–14 μg/kg | Denmark | [84] | |
| MS | Nivalenol | 5 | 100% | 1612 μg/kg | max 2809 μg/kg | Germany | [83] | |
| MS | Nivalenol | 19 | 42% | 521 μg/kg | 190–760 μg/kg | Switzerland | [85] | |
| Hays | Nivalenol | 28 | 4% | 131 μg/kg | max 222 μg/kg | Germany | [83] | |
| MS | Nivalenol | 82 | 13% | 266 μg/kg | max 758 μg/kg | Denmark | [24] | Quantitative analysis |
| Fusarium spp. derived toxin: Trichothecenes type B | ||||||||
| MS | 15monoacetoxyscirpenol | 5 | 60% | 30 μg/kg | max 49 μg/kg | Germany | [83] | |
| MS | HT-2 toxin | 20 | 60% | 104 μg/kg | 2–327 μg/kg | Denmark | [84] | |
| MS | HT-2 toxin | 5 | 100% | 18 μg/kg | max 26 μg/kg | Germany | [83] | |
| MS | HT-2 toxin | 19 | 26% | 95 μg/kg | 76–120 μg/kg | Switzerland | [85] | |
| MS | T-2 toxin | 20 | 5% | 2 μg/kg | - | Denmark | [84] | |
| MS | T-2 toxin | 19 | 42% | 36 μg/kg | 14–84 μg/kg | Switzerland | [85] | |
| Fusarium spp. derived toxin: Fumonisins | ||||||||
| Different feeds | FB1 | 63 | 37% | 280 μg/kg | 20–2120 μg/kg | WI, US | [75] | |
| MS | FB1 | 140 | 1% | 17,000 μg/kg | max 26,200 μg/kg | Netherlands | [82] | over three years |
| MS | FB1 | 86 | 97% | 615 μg/kg | 21–1824 μg/kg | IL, US | [88] | |
| MS | FB1 | 60 | ~75% | 2020 μg/kg | 200–10,100 μg/kg | PA, US | [76] | |
| MS | FB1 | 116 | 15% | 5440 μg/kg | 300–3400 μg/kg | Brazil | [62] | core samples |
| MS | FB1 | 100 | 88% | - | 900– > 10,000 μg/kg | Italy | [78] | only core samples |
| Hay and hay silage | FB1 | 25 | 52% | 120 μg/kg | 20–450 μg/kg | WI, US | [75] | |
| Silages | FB1 | 3 | 33% | 21 μg/kg | - | Netherlands | [16] | |
| MS | FB2 | 64 | 72% | 93 μg/kg | 21–276 μg/kg | IL, US | [88] | |
| MS | FB2 | 60 | ~40% | 980 μg/kg | 200–20,300 μg/kg | PA, US | [76] | |
| MS | FB3 | 51 | 57% | 51 μg/kg | 16–161 μg/kg | IL, US | [88] | |
| Fusarium spp. derived toxin: other Fusarium toxins | ||||||||
| GS (bunkers) | Beauvericin | 88 | - | ~30 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Beauvericin | 56 | - | ~30 μg/kg DM | - | Ireland | [89] | |
| MS | Enniatin A1 | 6 | - | ~120 μg/kg DM | - | Ireland | [89] | |
| GS (bunkers) | Enniatin A1 | 88 | - | ~40 μg/kg DM | - | Ireland | [89] | |
| GS (bunkers) | Enniatin A1 | 88 | - | ~20 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Enniatin A1 | 56 | - | ~25 μg/kg DM | - | Ireland | [89] | |
| MS | Enniatin B | 82 | 24% | 53 μg/kg | max 152 μg/kg | Denmark | [24] | |
| GS (bunkers) | Enniatin B | 88 | - | ~60 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Enniatin B | 56 | - | ~250 μg/kg DM | - | Ireland | [89] | |
| MS | Enniatin B1 | 6 | - | ~160 μg/kg DM | - | Ireland | [89] | |
| GS (bunkers) | Enniatin B1 | 88 | - | ~180 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Enniatin B1 | 56 | - | ~80 μg/kg DM | - | Ireland | [89] | |
| Fusarium spp. derived toxin: Zearalenone | ||||||||
| MS | α-ZOL | 5 | 20% | 15 μg/kg | - | Germany | [83] | |
| MS | β-ZOL | 5 | 20% | 116 μg/kg | - | Germany | [83] | |
| Different feeds | ZEA | 63 | 32% | 220 μg/kg | 120–310 μg/kg | WI, US | [75] | |
| Compound feed | ZEA | 28% | 80 μg/kg | max 363 μg/kg | Netherlands | [36] | ||
| MS | ZEA | 140 | 49% | 174 μg/kg | max 943 μg/kg | Netherlands | [82] | over three years |
| MS | ZEA | 82 | 28% | 66 μg/kg | max 311 μg/kg | Denmark | [24] | Quantitative analysis |
| MS | ZEA | 9 | - | - | 23–41 μg/kg | France | [79] | from 1 farm |
| MS | ZEA | 5 | 100% | 432 μg/kg | max 1790 μg/kg | Germany | [83] | |
| MS | ZEA | 19 | 79% | 180 μg/kg | 83–430 μg/kg | Switzerland | [85] | |
| MS | ZEA | 85 | 15% | - | >50 μg/kg | Italy | [90] | |
| MS | ZEA | 100 | 60% | - | 30–>300 μg/kg | Italy | [78] | only core samples |
| Silages | ZEA | 3 | 100% | 145 μg/kg | max 240 μg/kg | Netherlands | [16] | |
| Ensiled by-products | ZEA | - | - | - | Netherlands | [36] | ||
| Feed commodities | ZEA | 38% | 80 μg/kg | max 108 μg/kg | Netherlands | [36] | ||
| Forage products | ZEA | 8% | 82 μg/kg | - | Netherlands | [36] | ||
| GS | ZEA | 120 | 6% | 936 μg/kg | max 308 μg/kg | Netherlands | [82] | over three years |
| Hay and hay silage | ZEA | 25 | 0% | - | - | WI, US | [75] | |
| Hays | ZEA | 28 | 43% | 24 μg/kg | max 115 μg/kg | Germany | [83] | |
| Hays | ZEA | 44 | 21% | - | - | Ireland | [91] | |
| Haylages | ZEA | 40 | 8% | - | - | Ireland | [91] | |
| Hays | ZEA | 65 | 8% | - | - | Canada | [91] | |
| Silage | ZEA | 17% | 125 μg/kg | max 273 μg/kg | Netherlands | [36] | ||
| Penicillium spp. derived toxin | ||||||||
| Different feeds | PR toxin | 63 | 76% | 130 μg/kg | 50–260 μg/kg | WI, US | [75] | |
| Hay and hay silage | PR toxin | 25 | 80% | 15 μg/kg | 50–260 μg/kg | WI, US | [75] | |
| GS (round bale) | 16-OH-roquefortine C | 5 | 20% | - | range 100–1000 μg/kg | Ireland | [10] | |
| MS | Andrastin A | 82 | 18% | 169 μg/kg | max 691 μg/kg | Denmark | [24] | Quantitative analysis |
| GS (round bale) | Andrastin A | 56 | - | ~500 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Andrastin A | 5 | 100% | - | range trace-20,000 μg/kg | Ireland | [10] | |
| MS | Citreoisocoumarin | 82 | 8% | - | - | Denmark | [24] | Qualitative analysis |
| GS (round bale) | Citreoisocuomarin | 5 | 40% | - | trace | Ireland | [10] | |
| MS | Marcfortine A | 82 | 7% | - | - | Denmark | [24] | Qualitative analysis |
| GS (round bale) | Marcfortine A | 5 | 60% | - | range 100–1000 μg/kg | Ireland | [10] | |
| MS | Marcfortine B | 82 | 1% | Denmark | [24] | Qualitative analysis | ||
| GS (round bale) | Agroclavine | 5 | 40% | - | range 100–1000 μg/kg | Ireland | [10] | from A. fumigatus too [92] |
| GS (round bale) | Festuclavine | 5 | 40% | - | range 100–1000 μg/kg | Ireland | [10] | from A. fumigatus too [92] |
| MS | Mycophenolic Acid | 135 | 28% | 690 μg/kg | 20–23,000 μg/kg | Germany | [93] | |
| MS | Mycophenolic acid | 120 | 42% | 160 μg/kg | 20–1300 μg/kg | PA, US | [80] | 4 samples from 30 bunkers |
| MS | Mycophenolic acid | 82 | 2% | 8 μg/kg | max 8.8 μg/kg | Denmark | [24] | Quantitative analysis |
| MS | Mycophenolic Acid | 196 | 8% | 1760 μg/kg | max 48,000 μg/kg | Italy | [69] | Three samples from 68 silos |
| Silages | Mycophenolic Acid | 3 | 100% | 4244 μg/kg | max 7565 μg/kg | Netherlands | [16] | |
| Ensiled by-products | Mycophenolic acid | 10% | 66 μg/kg | max 83 μg/kg | Netherlands | [36] | ||
| GS (bunkers) | Mycophenolic Acid | 88 | - | ~250 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Mycophenolic Acid | 56 | - | ~1250 μg/kg DM | - | Ireland | [89] | |
| GS | Mycophenolic Acid | 98 | 37% | 2200 μg/kg | 20–35,000 μg/kg | Germany | [93] | |
| GS (round bale) | Mycophenolic acid | 5 | 100% | - | range trace-20,000 μg/kg | Ireland | [10] | |
| Silage | Mycophenolic acid | 13% | 524 μg/kg | max 2630 μg/kg | Netherlands | [36] | ||
| MS | Roquefortine C | 12 | 8% | 200 μg/kg DM | - | Germany | [94] | molded silages |
| MS | Roquefortine C | 12 | 100% | 17,000 μg/kg DM | 700–36,000 μg/kg DM | Germany | [94] | unmolded samples |
| MS | Roquefortine C | 60 | 30% | 5470 μg/kg | 50–28,000 μg/kg DM | Germany | [95] | data of Armbruster, 1994 |
| MS | Roquefortine C | 120 | 60% | 380 μg/kg | 10–5710 μg/kg | PA, US | [80] | 4 samples from 30 bunkers |
| MS | Roquefortine C | 82 | 2% | 173 μg/kg | max 189 μg/kg | Denmark | [24] | Quantitative analysis |
| MS | Roquefortine C | 196 | 5% | 740 μg/kg | max 32,000 μg/kg | Italy | [69] | 3 samples from 68 silos |
| Ensiled by-products | Roquefortine C | 7% | 123 μg/kg | max 170 μg/kg | Netherlands | [36] | ||
| GS (bunkers) | Roquefortine C | 88 | - | ~500 μg/kg DM | - | Ireland | [89] | |
| GS (round bale) | Roquefortine C | 56 | - | ~280 μg/kg DM | - | Ireland | [89] | |
| GS | Roquefortine C | 24 | 13% | - | range 10–580 μg/kg | Germany | [10] | From Ambruster, 2008 PhD thesis |
| GS | Roquefortine C | 20 | 15% | 280 μg/kg | range 10–580 μg/kg | Germany | [95] | From Ambruster, 2008 PhD thesis |
| GS | Roquefortine C | 120 | <1% | 81 μg/kg | - | Netherlands | [82] | over three years |
| GS (round bale) | Roquefortine C | 5 | 40% | - | range 1000–20,000 μg/kg | Ireland | [10] | |
| Silage | Roquefortine C | 19% | 778 μg/kg | max 3160 μg/kg | Netherlands | [36] | ||
| GS (wilted) | Roquefortine C | 12 | 75% | 200 μg/kg DM | 100–300 μg/kg DM | Germany | [94] | molded silages |
| GS (wilted) | Roquefortine C | 12 | 42% | 600 μg/kg DM | 200–15,000 μg/kg DM | Germany | [94] | unmolded samples |
| MS | Roquefortine A | 82 | 11% | - | - | Denmark | [24] | Qualitative analysis |
| GS (round bale) | Roquefortine A | 5 | 40% | - | range 100–1000 μg/kg | Ireland | [10] | |
| GS (round bale) | Roquefortine B | 5 | 40% | - | range 100–1000 μg/kg | Ireland | [10] | |
| GS (round bale) | Roquefortine D | 5 | 40% | - | range 100–1000 μg/kg | Ireland | [10] | |
| MS | Patulin | 120 | 23% | 80 μg/kg | 10–1210 μg/kg | PA, US | [80] | 4 samples from 30 bunkers |
| Silages | Patulin | 3 | 100% | 153 μg/kg | max 211 μg/kg | Netherlands | [16] | |
| Monascusruber derived toxin | ||||||||
| Silages | Monacolin KB | 233 | 21% | 6161 μg/kg | 28–65,400 μg/kg | Germany | [96] | |
| Silages | Monacolin KL | 233 | 19% | 1767 μg/kg | 25–15,600 μg/kg | Germany | [96] | |
| MS | Citrinin | 1 | - | 12 μg/kg | France | [77] | ||
| MS | Citrinin | 9 | 4–25 μg/kg | France | [79] | from 1 farm | ||
| Silages | Citrinin | 233 | 6% | 9 μg/kg | 2–64 μg/kg | Germany | [96] | |
a: AAL TA toxin, Alternaria alternata toxins type A; AAL TB toxin, Alternaria alternata toxins type A; aflatoxin B1, AFB1; Alternariol ME, alternariol monomethyl ether; deoxynivalenol, DON; fumonisin B1, FB1; fumonisin B2, FB2; fumonisin B3, FB3; grass silage, GS; whole-crop forage maize silage, MS; ochratoxin A, OTA; whole-crop small grain cereal silage, SGS; α-zearalenol, α-ZOL; β-Zearalenol, β-ZOL; zearalenone, ZEA.
Table 3.
Survey of mycotoxins not detected in forages from the literature.
| Forage Products | Mycotoxins a not Detected | References |
|---|---|---|
| MS | AFB1, AFB2, AFG1, AFG2, 3-acetyl-DON, DAS, ergotamin, FB2, fusarenon-X, OTA, mycophenolic acid, penicillic acid, roquefortin C, sterigmatocystin, T-2 toxin, HT-2 toxin | [82] |
| MS | AFB1, AFB2, AFG1, AFG2, OTA, T-2 toxin, HT-2 toxin, 3-acetyl-DON, 15-acetyl-DON, DAS, sterigmatocystin, fusarenon-X, ergotamine, penicillic acid | [36] |
| MS | Cyclopiazonic acid, fumitremorgin A, gliotoxin, OTA, patulin, penitrem A, sterigmatocystin, T-2 toxin, tenuazonic acid, altersetin, fumigaclavine A, fumigaclavine C, PR toxin | [24] |
| MS | ZEA, PR toxin | [69] |
| MS | 3-acetyldeoxynivalenol, DAS, fusarenon-X, T-2 toxin, HT-2 toxin, neosolaniol, scirpentriol | [83] |
| Hays | 15-monoacetoxyscirpenol, 15-acetyldeoxynivealenol, DAS, fusarenon-X, T-2 toxin, neosolaniol, scirpentriol, α-ZOL, β-ZOL | [83] |
| MS | FB1, OTA, ZEA | [77] |
| MS | Gliotoxin, OTA | [79] |
| Hays and haylages | FBs, AFs, T-2 toxin, OTA | [91] |
a: aflatoxin B1, AFB1; aflatoxin B2, AFB2; aflatoxin G1, AFG1; aflatoxin G2, AFG2; deoxynivalenol, DON; diacetoxyscirpenol, DAS; fumonisin B1, FB1; fumonisin B2, FB2; fumonisins, FBs; whole-crop forage maize silage, MS; ochratoxin A, OTA; α-zearalenol, α-ZOL; β-Zearalenol, β-ZOL; zearalenone, ZEA.
2.1. Alternaria Toxins in Forages
Different Alternaria species, such as A. alternata, A. arborescens and A. tenuissima, have been isolated from hay and silages [8,65]. However, Andersen et al. [70] recently suggested that A. alternata is a rare species and most strains originally identified as such in reality belong to A. tenuissima species-group, A. arborescens species-group or other Alternaria species-groups. These fungi produce a wide range of compounds, such as alternariols, altertoxins, altenuene, tentoxin and tenuazonic acid, with suspected but still unconfirmed toxic properties [71,72]. However, A. infectoria produces several other secondary metabolites, such as 4Z-infectopyrone, phomapyrones, novae-zelandins, dehydrocurvularin, pyrenochaetic acid or alternarienonic acid [70,73].
Only few reports on the natural occurrence of these compounds in feeds have been reported [74]. Among these, Yu et al. [75] reported high incidence of AAL type A toxins in different feeds, such as hay, hay silages and MS, with concentrations sometimes exciding 1000 μg/kg. These authors analyzed these mycotoxins by using an unspecific screening method, consisting in a direct competitive enzyme-linked immunosorbent assays, and as representative of mycotoxins produced by A. alternata. However, Andersen et al. [70] verified that only one strain of A. arborescens was associated with the production of AAL toxins and none of the other 98 strains of identified A. arborescens or other Alternaria species-groups produced these toxins. Storm et al. [24] reported low occurrences and low concentrations of alternariol and alternariol momomethyl in forages sampled in Denmark. No occurrence data were reported for other Alternaria secondary metabolites.
2.2. Aspergillus Toxins in Forages
Presence of Aspergillus flavus and A. parasiticus has been reported in ensiled products, such as MS and high moisture maize, and the most important mycotoxins produced by these organisms are AFs (AFB1, AFB2, AFG1 and AFG2). Sporadically, these toxins were detected at low levels in forages thus contributing to increase AFB1 intake level in lactating dairy cows [77,78,79]. Otherwise, AFB1 was not quantified in silages such as MS, GS or SS [36,82,97]. However, AFs produced on growing crops may not be uniformly distributed across the field and when samples are collected, they could or could not be representative of the location in which the AFs are present and, consequently, of the AFs distribution in ensiled mass [28]. Therefore, reliability of measurements is strongly affected by protocols adopted to collect representative samples, to prepare samples for analysis or to extract and quantify mycotoxins [98,99,100]. Because of the heterogeneous distribution of AFs [101] and more generally of all mycotoxins [102], the variability associated with mycotoxin test procedures usually depends mainly by sampling plan. For these aspects, the European Commission set the methods of sampling and analysis for official control of the levels of mycotoxins in foodstuff [103] or in cereals, cereal products and compound feeds for animal feeding [53,104]. Nothing is currently done by authorities to set specific sampling procedures for hay or silages.
Additional mycotoxins produced by A. flavus and other Aspergillus species are kojic, cyclopiazonic and β-nitropropionic acids [105], but their presence is sporadically reported in silages [24]. In particular, Santos and Fink-Gremmels [16] reported β-nitropropionic acid in one of three sampled silages in Netherlands, at a concentration of 1360 μg/kg. However, no documentation on the analytical detection of this compound was provided.
Aspergillus fumigatus is one of the main mycotoxigenic fungus infecting forages under warm conditions [8,92,106]. Risk of presence of its related toxins has been reported particularly in silages and it is capable of producing more than 226 potentially bioactive secondary metabolites [92]. Among these, gliotoxin is clearly the most toxic metabolite and it has most often been analyzed to indicate presence of A. fumigatus toxin metabolites in silages [69,77,81]. However, it is mainly believed to be produced during infections of mammalians [107]. Storm et al. [8] reported that gliotoxin is mainly produced on substrates characterized by a low C to N ratio, therefore it does not represent a good marker of A. fumigatus presence. The low incidence of gliotoxin reported for MS sampled in Italy could be presumably related to this aspect [69]. Unfortunately, most other compounds from this fungus have not been assayed in silages and we are unable to report occurrence data. Storm et al. [24] recently discussed the absence of several A. fumigatus derived mycotoxins, such as gliotoxin, fumitremorgin A, fumigaclavines A and C in MS sampled in Denmark. Boundra and Morgavi [108] reported that gliotoxin, helvolic acid and verruculogen are stable during forage storage, whereas fumagillin was unstable under ensiling conditions. Agroclavine and festuclavine are other mycotoxins potentially produced by A. fumigatus [92].
Cyclopiazonic acid is a toxic indole tetramic acid, first isolated from Penicillium griseofulvum and subsequently from other Penicillium species, A. flavus and A. oryzae. Because this toxin can be produced by A. flavus too, co-occurrence with AFs and β-nitropropionic acid has been suspected. Only limited studies were published on cyclopiazonic acid occurrence in forages and it was detected in 37% of MS [80] and 80% of hays and hay silages [75] sampled in US, with contaminations exceeding 1000 μg/kg.
Several fungi of the genera Aspergillus and Penicillium spp. can produce OTA, including A. westerdijkiae, A. niger, A. fresenii, A. carbonarius, P. verrucosum and P. nordicum [109,110,111,112]. Many authors did not detect OTA in forages [24,67,82,91], first at all because these fungi do not tolerate high concentrations of acetic acid and CO2 [109,110,113]. Lastly, maltoryzine is produced by A. clavatus. However, data of its occurrence in forages are not available.
2.3. Fusarium Toxins in Forages
Among Fusarium derived mycotoxins, trichothecenes type A and B are produced by several species [24,85,114]. Among trichothecenes type B, the most studied mycotoxins are DON and to less extent nivalenol and fusarenon-X as well as their acetylated and deacetylated analogues (3-acetyl-DON, 15-acetyl-DON and others). They are primarily produced by F. culmorum and F. graminearum [115]. DON is considered the most prevailing mycotoxin in silages and other forages [8,116] and it can be present at different incidence rates and at different concentration levels. In particular, incidences of DON in forages higher than 80% were reported in North America [75,117] and North Europe [24,36,83,84,86] with average contamination levels highly variable, but in some cases exceeding 2000 μg/kg [83,86]. The distribution of DON could differ in silos, even if this aspect has not been yet clarified [28]. For instance, Richard et al. [15] measured higher DON concentrations in upper than bottom parts of silos, whereas the same authors reported opposite data two years later [77]. Furthermore, other authors [36,69] did not describe any sampling zone effects for neither DON nor ZEA. The incidences of nivalenol could range from 100% [86] to 13% [24] of collected MS, whereas Schollenberger et al. [83] reported an incidence of 4% with average nivalenol concentration level of 131 μg/kg in hays. Lastly, Storm et al. [24] reported about 20% of collected MS were contaminated by fusarenon X at a level lower than 5 μg/kg.
Mycotoxins such as diacetoxyscirpenol (DAS), T-2 and HT-2 toxins and their de-acetylated analogues belong to type A trichothecenes and they are mainly produced by F. poae, F. sporotrichioides and F. langsethiae [115]. Despite some authors [24,83,85] reported these trichothecenes were often detected in MS, the average concentration levels should be normally considered very low. T-2 toxin was not detected in hay and MS collected in Germany, as well as DAS and its acetylated compounds [83].
FBs are primarily produced by F. proliferatum and F. verticillioides [115] and their contamination in pre-harvest crops is often reported [8,118]. Among FBs, FB1 is the predominant and most studied one. For FB1, incidences higher than 30% were reported in MS sampled in North America [75,76,119], whereas in the Netherlands and France the occurrence was low [15,82]. About 50% of hay and hay silages sampled in Wisconsin were contaminated by FB1 [75], with average concentration of 120 μg FB1/kg. Other FBs, such as FB2 and FB3 were also detected in MS, but at very low contamination levels [65,119].
Several authors reported ZEA incidence data in MS, GS, hay or other feeds. On average, the 52% of collected MS resulted contaminated by ZEA with average contamination levels lower than 500 μg/kg [24,78,82,83,85]. ZEA was detected in the 43% of hay collected in Germany [83]. Contrarily, Richard et al. [77] and Gallo et al. [69,120] did not detect ZEA in MS. Other Fusarium derived toxins, such as beauvericins, enniatins and moniliformin were detected in silages (GS round bale, GS in bunkers or MS) both in Ireland and Denmark, but at very low contamination levels [24,89,121]. A degradation process occurring during ensiling was suspected but not still proved [8].
Concerning stability of Fusarium toxins, Boudra and Morgavi [122] reported that the concentrations of DON, FBs and ZEA decreased during ensiling in MS. Depending on DM content of silages, length of ensiling and temperature, toxin disappearances could range from 50% for ZEA to 100% for DON [28]. Furthermore, plants are able to modified mycotoxins by conjugation to polar substances [123,124,125,126]. Different Fusarium toxins, such as ZEA, nivalenol, T-2 and HT-2 toxins or FBs could contaminate feeds in their modified forms [118]. However, no data on presence of modified mycotoxins were reported for forages [126,127].
2.4. Penicillium Toxins in Forages
Species belonging to Penicillium section Roquefortorum such as P. roqueforti and P. paneum [11,110] are considered some of the most prevailing post-harvest fungi found in silages [15,37,81,84,128]. Different critical factors, such as unfavorable weather or storage conditions, could promote fungal growth and mycotoxin production [15,108]. A list of main mycotoxins produced by Penicillium strains was reported by Nielsen et al. [11]. Surely, these mycotoxins are the most researched and detected in forages. As reported by Auerbach et al. [94], P. roqueforti was isolated from 89% of visibly-moldy and from 85% of visibly-unmoldy silages. Similarly, P. roqueforti and P. paneum were isolated from 96% of MS stored in bunker silos or as silage stacks laid on soil [84]. In a survey conducted in the Netherlands [36], incidences of mycotoxins produced by P. roqueforti were reported both in MS and GS, being respectively 50% and 19% of collected samples. PR toxin, a mycotoxin produced by P. roqueforti [11], was detected in several feeds collected in North America. In particular, Yu et al. [75] reported an incidence of 76% of PR toxin in 63 feed samples (i.e., 25 hays and 38 silages and mixed feeds), with an average contamination of 130 μg/kg. However, the employed methodology was a low specific immunochemical screening method and successive studies have not been able to verify the detected levels of this mycotoxin. A survey was carried out in Italy where 68 MS were sampled and authors did not detect presence of PR toxin [69].
Mycophenolic acid and roquefortines could be considered the most studied Penicillium derived compounds in ensiled products. The first, produced by P. roqueforti and B. nivae [8], was detected in MS with variable incidences, being about 40% [80], 30% [93], 10% [69] or lower than 3% [24,84]. Furthermore, mycophenolic acid concentrations higher than 20,000 μg/kg were reported [69,93,94]. Recently, Santos and Fink-Gremmels [16], sampling three GS in different herds in the Netherlands with visible aerobic instability and mold visible in all parts of silo bunkers, detected mycophenolic acid in all samples at levels ranging from 588 to 7565 μg/kg. Among roquefortines, produced by different strains of P. section Roquefortorum, the most studied was roquefortine C and this toxin was detected in more than 40% of sampled silages [10,80,94]. Otherwise, low incidence of roquefortine C was reported in MS [24] and GS [82]. High concentration values (> 20,000 μg/kg) were sporadically reported [69,94]. In particular, Driehuis et al. [36] reported average contaminations of 778 μg/kg for roquefortine C and 524 μg/kg for mycophenolic acid in silages, with maximum levels up to 3160 and 2630 μg/kg, respectively. Other roquefortines exclusively produced by P. roqueforti [11] were detected in 2 of 5 analyzed GS by O’Brien et al. [10] with average concentrations of 100–1000 μg/kg. For these mycotoxins, different authors [36,69] reported higher incidences as well as higher concentrations in MS collected from peripheral than core zones of silos.
Other Penicillium derived exometabolites have been detected in silages, such as andrastin A, citreoisocumarin, agroclavine, festuclavine and the P. paneum biomarker marcfortine A [10,24]. In particular, O’Brien et al. [10] reported 2 of 5 collected MS were contaminated by agroclavine and festuclavine produced by Penicillium strains, with concentrations ranging from 100 to 1000 μg/kg. Storm et al. [24] reported incidences of andrastin A, citreoisocumarin and marcfortine A lower than 20% in 82 collected MS from Denmark. Patulin, produced by P. paneum and B. nivae [8], was detected in 23% of MS with concentrations ranging from 10 to 1210 μg/kg. No information is currently available for other Penicillium derived toxin such as botryodiploidin [11].
2.5. Monascus Ruber Toxins in Forages
In Monascus ruber infected silage, citrinin has been detected [77,79,96]. Among other exometabolites produced by M. ruber, Schneweis et al. [96] reported that monacolin (statin family, cholesterol lowering) was detected in about 20% of collected silages.
2.6. Zygomycetes Fungi in Forages
Some Zygomycetes can, via endophytic bacteria, produce secondary metabolites and toxic rhizonins [9] and rhizonines, but these have not been found in silages. Jensen et al. [129] reported these fungi could cause zygomycosis in immunosuppressed animals.
3. Effect of Mycotoxins Ingestion on Ruminants: In Vitro and in Vivo Experiences
As introduced above, ruminants are considered to be less susceptible to negative effects of mycotoxins than monogastrics, rumen microflora and feed particles contained in rumen being effective in the degradation, deactivation or binding of these toxic molecules [3,29,130,131,132] and rumen microorganisms being able to reduce development of pathogens [133,134]. The mechanisms of action and toxic properties of several mycotoxins frequently detected in concentrates and forages have been studied. We refer to Table 11.1 of the CAST report [4] and the review of Hussein [135] for details. Furthermore, the cytotoxicity of several mycotoxins detectable in forages and produced by Aspergillus fumigatus, Alternaria tenuissima, F. avenaceum, F. graminarum, P. roqueforti, P. paneum, M. ruber or B. nivea, as well as cytotoxic effects of fungal agar or silage extracts were tested in vitro by Rasmussen et al. [136].
Actually, there are limited scientific evidences regarding the negative effects of mycotoxin ingestion on the health status and performance of cattle and the evaluation of the real economic impact of mycotoxins on ruminant livestock production system still represents a main issue that deserves further investigation [2,3,137,138,139,140]. We present in Table 4 the majority of in vitro published works where researchers investigated the effects of mycotoxin presence on rumen microbiota, whereas in Table 5 are summarized results from several in vivo studies designed to investigate effect of mycotoxin ingestion in ruminants.
Table 4.
Survey on the effects of mycotoxins on rumen microbiota tested by in vitro approaches from literatures.
| Mycotoxins a | Media | Tested Dosages | Effects | References |
|---|---|---|---|---|
| AFB1 | rumen fluid | 0, 300, 600, 900 ng AFB1/mL buffered rumen fluid | ↓ gas production, ↓ dry matter digestibility, ↓ NH3-N concentrations | [141] |
| AFB1 | rumen fluid | 1, 10 μg AFB1/mL buffered rumen fluid | ↓ dry matter digestibility | [142] |
| AFB1 | rumen fluid | 9.5 ng AFB1/mL buffered rumen fluid | no effects | [143] |
| AFB1 | rumen fluid | 0, 320, 640, 960 ng AFB1/mL buffered rumen fluid | ↓ final gas production, ↓ rate of degradation, ↓ NH3-N concentrations, ↑ isobutyrate, valerate and isovalerate molar proportions | [144] |
| DON | rumen fluid | 0.36/0.46 or 5.76/6.90 mg of DON/kg diet | None, expect ↓ NDF digestibility | [145] |
| DON | rumen fluid | 0.3 or 3.4/4.4 mg of DON/kg diet | None, expect ↓ NDF digestibility | [146] |
| DON | rumen fluid | 40 μg DON/mL of rumen fluid | ↓ gas production, ↓ VFA and NH3-N concentrations | [147] |
| DON and fusaric acid | culture media | antimicrobial activity of fusaric acid against Ruminococcus albus and Methanobrevibacter ruminantium. No effect of DON | [148] | |
| Gliotoxin | rumen fluid | 0, 1, 2, 5, 10, 20, 40, 80 μg/mL buffered rumen fluid | < 80 μg/mL no effects. At 80 μg/mL ↓ DM degradation, gas and VFA productions | [149] |
| FB1 | rumen fluid | 0, 50 or 100 mg/kg rumen fluid | none | [150] |
| OTA | rumen fluid | 200 μg of OTA/l of rumen fluid | none | [151] |
| Patulin | rumen fluid | 20, 100 and 300 μg of Patulin/mL rumen liquid | ↓ Acetic acid production within 4 h and Inhibition of protein synthesis | [152] |
| Patulin | rumen fluid | 0, 10, 20 and 40 mg of Patulin/mL rumen fluid | ↓ dDM, VFA production, dNDF, dADF, dCHO, dCP and bacterial N flows ↑ NH3-N | [153] |
| Mycopenolic acid, Roquefortine C and PR toxin | rumen fluid | 0.01, 0.30, 1.01, 1.71 and 2.00 μg of each mycotoxin/mL buffered rumen fluid | Mychopenolic acid and roquefortine C ↓ gas production, VFA production. No effect of PR toxin | [130] |
| Citrinin, Monacolin K, Pravastatin and Mevastatin | rumen fluid | 5 or 20 μg of monacolin/mL rumen fluid; 5 or 20 μg of citrinin/mL rumen fluid; Monascus spp. contaminated rice | none, ↓Methane production | [154] |
a: aflatoxin B1, AFB1; ammonia nitrogen, NH3-N; dADF, digestible ADF; dCHO; digestible carbohydrates; dDM, digestible dry matter; deoxynivalenol, DON; DM, dry matter; dNDF, digestible NDF; fumonisin B1, FB1; ochratoxin A, OTA; VFA, Volatile fatty acids.
Table 5.
Survey on the effects of mycotoxins ingestion in ruminants from literatures (Field trial or FT and Experimental trial or ET).
| Mycotoxins a | Study | Animals | Tested Dosages | Reported Effects | References | Notes |
|---|---|---|---|---|---|---|
| AFB1 | FT | Beef | 0.2, 0.4, 0.6 or 0.8 mg of AFB1/kg of BW | ↓ rumen mobility | [155] | |
| AFB1 | FT | Beef | 0, 100, 300, 700 and 1000 μg AFB1/kg diet | For levels 700 and 1000 μg/kg: Growth inhibition, ↓ feed efficiencies, ↑ liver and kidney weights | [156] | |
| AFB1 | FT | Lactating dairy cows | 20 μg AFB1/kg diet | ↓ feed consumption, ↓ milk production | [157] | |
| AFB1 | FT | Lactating dairy cows | 120 μg AFB1/kg diet | ↓ reproductive efficiency, ↓ milk production | [158] | |
| AFB1 | FT | Lactating dairy cows | 100 μg AFB1/kg diet | ↓ milk production | [159] | |
| AFB1 | ET | Lactating dairy cows | 100 and 300 μg of AFB1/kg of BW | ↓ feed intake → ↓ milk production | [160] | |
| AFB1 | ET | Lactating dairy cows | 13 mg of AFB1 (pure and impure from Aspergillus parasiticus in culture) | ↓ milk production | [161] | |
| AFB1 | ET | Sheep | 1.8 and 2.4 mg of AFB1/kg diet | none | [162] | Exposition period of 5 years |
| AFB1 | FT | Sheep | 0.75 mg of AFB1/kg diet | Inappetence, apathy, hepatic lesion, neurological signs and death. | [163] | |
| AFB1 | ET | Lambs | 2.6 mg of AFB1/kg diet | ↓ BW ↑ AST, GGT, prothrombin time, cholesterol, uric acid and triglyceride values ↓ albumin, glucose and urea nitrogen and urea-to-creatine ratio | [164] | |
| AFB1 | ET | Lambs | 2 mg of AFB1/kg diet | = BW ↓ ADG, immune response | [165] | |
| AFB1 | ET | Lambs | 350 μg AFB1/kg diet | = ADI and blood parameters ↓ ADG gain, serum Ca and P | [166] | Exposition period of 150 days |
| AFB1 | ET | Lambs | 0, 5.9, 11.8, 17.7, 23.5 μg AFB1/kg diet | = DMI, cellular immunity ↓ ADW | [167] | |
| AFB1 | ET | Lambs | 2.5 mg of AFB1/kg diet | ↓ feed intakes, daily gain, and gain/feed ↑ AST, GGT, total protein, cholesterol | [168] | |
| AFB1 | ET | Lactating dairy cows | 96 μg/cow/day | slightly ↑ GGT and serum protein | [169] | |
| AFB1&FB1+FB2 | ET | Heifers | C (1.9 μg of AFB1 and 3.8 mg of FBs/kg diet), A (12.0 μg of AFB1 and 6.6 mg of FBs/kg diet), A-F (19.9 μg of AFB1 and 23.2 mg of FBs/kg diet) diets | = BW, DMI ↑GGT delay in reproductive career | [170] | |
| AFB1, DON, ZEA, FB1, OTA, T-2 toxin | ET | Lactating dairy cows | 38 AFB1 and 270 T-2 μg/kg; 720 DON, 701 FB1, 541 ZEA, 501 OTA mg/kg | ↓ DMI, milk yield, CP and NDF digestibilities, impact on haematological parameters and immunosuppression | [171] | |
| Maltoryzine | Lactating dairy cows | unknown | general poison | [172] | ||
| β-nitropropionic acid | Sheep and Cattle | unknown | emphysema and difficulty in locomotion | [173] | ||
| DON | ET | Lactating and no lactating dairy cows | 0.3 or 3.4/4.4 mg of DON/kg diet | ↓ NDF digestibility and slightly ↓ in microbial crude protein | [146] | Two level of F:C ratio, being 40:60 or 70:30 |
| DON | ET | Lactating dairy cows | 4.4 or 5.3 mg DON/kg DM | ↑ DMI ↓ Milk Fat | [174] | |
| DON | ET | Lactating dairy cows | 4.4 or 5.3 mg DON/kg DM | ↑ valerate ↓ pH, acetate and isobutyrate | [175] | |
| DON | ET | Lactating dairy cows | 0.59, 42, and 104 mg of DON/cow/day | none | [176] | |
| DON | ET | Lactating dairy cows | 8 mg of DON/kg diet | none | [177] | |
| DON | ET | Non lactating cows | about 8 or 35 mg of DON/cow/day | none, except slightly ↓ ingestion of contaminated feed | [178] | |
| DON | ET | Lactating dairy cows | 66 mg of DON/kg diet | none | [176] | |
| DON | ET | Non lactating cows | 4 or 3.6 mg of DON/kg diet and 0.13 or 0.05 mg of ZEA/kg in experiments 1 and 2, respectively | = rumen pH and VFA production ↓ microbial protein and ↑rumen NH3-N concentration and | [179] | |
| DON | ET | Lactating dairy cows | 3.5 mg of DON/kg diet and 0.24 mg of ZEA/kg diet | = DMI and milk production; Influence on metabolic parameters and immune response | [180,181] | |
| DON | ET | Lactating dairy cows | Group CON (0.02 mg ZEA and 0.07 mg DON/ kg DM), group FUS-50 (0.33 mg ZEA and 2.62 mg DON/kg DM), group FUS-100 (0.66 mg ZEA and 5.24 mg DON/ kg DM) | none | [182] | |
| DON | ET | Lactating dairy cows | The average daily intake of DON in group K was 12.4 mg, in group T 14.1 mg and in group M 14.3 mg and ZEA in group K was 12.4 mg, in group T 0.67 mg and in group M 0.68 mg | slightly ↑ in AST and LDH | [183] | |
| AFB1 & DAS | ET | Lambs | Group control (uncontaminated), group AFB1-contaminated (2.5 mg /kg), group DAS-contaminated (5 mg/kg from chemical standard) and group AFB1/DAS co-contaminated (2.5 mg of AFB1 and 5 mg of diet/kg) diets | ↓ Feed ingestion, BW | [184] | |
| FBs | ET | Lactating dairy cows | 75 mg of FBs/kg and 3 mg FB1/kg BW | none | [185] | |
| FB1 | ET | Steers | 94 mg FB1/kg diet | ↑ AST, GGT, hepatocellular injury and biliary epithelial hyperplasia | [23] | Exposition period of 253 days |
| FBs | ET | Claves | 15, 31 or 148 mg FBs/kg diet | = Feed ingestion, BW ↑AST, GGT, LDH, bilirubin and cholesterol | [186] | |
| FB1 | ET | Milk-fed calves | 1 mg of FB1/kg BW intravenous administered | Liver and kidney lesions ↑ serum AST, ALP, GGT and sorbitol dehydrogenase | [187] | |
| FBs | ET | Lambs | 0, 11.1, 22.2 or 45.5 mg of FBs/kg BW | Death, ↑ alkaline phosphatase, GGT, AST, cholesterol, triglyceride, urea nitrogen and creatinine | [188] | |
| ZEA | ET | Heifers | 250 mg ZEA/heifer | ↓ Conception rate, no other effects | [189] | |
| ZEA | ET | Dairy cow | from 0 to 500 mg ZEA/cow | None | [190] | |
| DON & ZEA | FT | Heifers | About 500 μg of DON/kg diet and 750 μg of ZEA/kg diet | unsynchronized ovarian cycles, vaginitis and early development of mammary gland in the prepubertal heifers | [191] | |
| ZEA | ET | Ewes | 1.5, 3, 6, 12, or 24 mg ZEA/ewe | reproductive disorders, lower lambing percentages and infertility. | [192] | |
| OTA | ET | Sheep | 0, 1.4, or 3.5 mg of OTA/kg diet | =feed intake and nutrient utilization | [193] | |
| OTA | ET | Sheep | 14 mg of OTA/kg diet | ↓ feed intake | [193] | Preliminary ET |
| Mychopenolic acid | ET | Sheep (male) | 0, 10, 70, 300 mg of MPA/sheep/day | none | [194] | |
| Mychopenolic acid | ET | Sheep | 300 mg of MPA/sheep/day | Slightly signs of immunosuppression in jejunum, white blood cells, ileum | [195] | |
| Mychopenolic acid | ET | Sheep | 300 mg of MPA/sheep/day | none | [196] | |
| Roquefortine C | FT | Cow | about 4–8 mg of RC/kg diet | Reversible paralytic effects | [197] | |
| Roquefortine C | ET | Sheep | 0, 10 and 50 mg of RC/sheep/day | None ↓ rumen pH | [95] | |
| Citrinin | FT | Sheep | Presence of visible moldy feeds in diets contaminated by both citrinin (2–10 mg/kg) and OTA (0–20 mg/kg) | fever, diarrhea and uraemia | [198] | |
| Citrinin, monacolin K, pravastatin and mevastatin | ET | Sheep | Monascus fermented rice | None ↓ rumen methane production | [154] | |
| Patulin | FT | Beef | Suspected Patulin | neurotoxicosis, comprising tremors, ataxia, paresis, recumbency and death | [199] |
a: aflatoxin B1, AFB1; ammonia nitrogen, NH3-N; average daily gain, ADG; average daily intake, ADI; average daily weight, ADW; aspartate aminotransferase, AST; body weight, BW; deoxynivalenol, DON; diacetoxyscirpenol, DAS; dry matter intake, DMI; dry matter, DM; forage to concentrate ratio, F:C; fumonisin B1, FB1; fumonisin B2, FB2; γ-glutamyltransferase, GGT; lactate dehydrogenase, LDH; Mychopenolic acid, MPA; ochratoxin A, OTA; Roquefortine C, RC; volatile fatty acids, VFA; zearalenone, ZEA.
In our opinion, the lack of unequivocal information regarding mycotoxin effects on ruminants should be related to the complexity to plan specific animal trials since a multitude of confounding effects exist. Among these, there are: (1) effect of mycotoxin on cattle and other ruminants depends by several factors, such as toxin-related (type and level of mycotoxin ingested as well as duration of intoxication period), diet-related (inclusion level of mycotoxin contaminated feeds, diet composition, forage to concentrate ratio, diet physical form, digestibility of dry matter or other nutrients, rate of passage, etc.), animal-related (species, sex, age, breed, dry matter intake level, general health, immune status, nutritional strategies) and environmental-related (farm management, hygiene, temperature, etc.) factors [134]; (2) for feeding experiments, it is strongly recommended to feed animals a known quantity of mycotoxins and to monitor individual daily mycotoxin intake because the main objective of these types of trials is to clarify the effect of one or at least few mycotoxins. However, feeds may be contaminated by more than one known and several unknown or unchecked mycotoxins. The toxic responses and clinical signs observed in animals ingesting multiple-contaminated feeds are more complex and diversified with respect to animals assuming feeds contaminated by one/two mycotoxins (rare) or their chemical standards (unrealistic). In particular, when mycotoxins are present simultaneously, some interactive effects, classified as additive, antagonistic or synergistic, could occur [18,200,201]. For instance, in the CAST report [4], authors reviewed 33 studies on mycotoxin interaction effects in farm animals, indicating that additive or antagonist effects were the predominant effects (78%). However, only two studies were carried out on ruminants, lambs in particular [164,184]; (3) mycotoxins can be modified mainly by plant and conjugated with polar compounds such as glucose, malonic acid and glutathione [124,126]. Modified mycotoxins are produced via enzymatic transformations related to plant detoxification processes and have been related to a resistance mechanism to counteract pathogen invasion [118,123,124,125]. Up to now, little is known about bioavailability of modified forms of mycotoxins, beyond DON and to some extent ZEA [127]. Evidences suggest they can be hydrolyzed and absorbed in the gastrointestinal tract of animals thus contributing to the overall exposure. Based on the few data currently available, the modified forms of a mycotoxin probably exert the same toxicity as the parent compound and when assessing the toxicity of modified mycotoxins it is important to determine the percentage of modified mycotoxin hydrolyzed in the intestinal tract [127].
3.1. Alternaria Derived Toxins
On 2011, the European Food Safety Authority (EFSA) reviewed information regarding safety of Alternaria derived toxins in food and feed, such as alternariol, alternariol monomethyl ether, tenuazonic acid, iso-tenuazonic acid, altertoxins, tentoxin, altenuene and AAL-toxins [74]. Generally, alternariol and alternariol monomethyl ether are genotoxic for bacteria and mammalian cells in vitro, whereas altertoxins are mutagenic for bacteria and induce cell transformation. Tenuazonic acid and tentoxin are not mutagenic for bacteria [202,203]. As clearly stated in the EFSA scientific opinion [74], the estimation of intake levels was limited to chicken, the only species for which some toxigenic data suitable for risk assessment exist. No information about exposure and toxicity due to Alternaria derived toxins were currently available for livestock, ruminants in particular. Consequently, information on susceptibility of farm animals to Alternaria derived compounds is needed, as these are largely detected in food and feeds [48,204,205,206,207]. The EFSA report [74] has been seriously questioned especially with respect to AAL toxins, numerous undocumented claims being found [72].
3.2. Aspergillus Derived Toxins
AFs as group are considered the most potent carcinogenic natural substances and have been classified as group 1 carcinogens by International Agency for Research on Cancer [208]. When ingested, AFs are rapidly adsorbed in the gastro-intestinal tract and quickly appear as metabolites in blood just after 5 min [209] and in milk at first milking [139,169,210,211]. The principal oxidized metabolite of AFB1 (i.e., AFM1) can be found in milk of lactating animals, thus representing a risk for human health. Consequently, ingestion safety levels for AFB1 in lactating dairy cows should be assessed on carry over rate of parent molecules into milk as a function of specific legislation [169,210,212]. Mechanism of action, toxic properties, human and animal exposures to AFs ingestion and risk due to milk contamination were extensively reviewed [4,139,213,214,215,216,217]. In the rumen, AFB1 is converted to aflatoxicol, AFM1 and many other hydroxylated metabolites [3,31,218,219] or sequestered by different rumen fluid components such as chlorophyllin structures, bacteria and yeast cell walls [29,30,219,220]. Despite the protection activity of rumen fluid, in vitro studies [141,144] suggested presence of increasing AFB1 levels in rumen fluid reduced gas production, ammonia N and VFA concentrations, showing therefore an antibacterial activity. Anyway, a hypothetical AFB1 diet concentration ranging from about 650 to 2000 μg/kg DM, estimated by considering a fixed rumen fluid volume of 50 L and a DMI of 23.5 kg/cow/day, could be calculated from AFB1 doses tested in these works. Similarly, Westlake et al. [142] showed presence of AFB1 in rumen fluid drastically reduced rumen digestibility of alfalfa by about 50% and 67% at 1 and 10 μg AFB1/mL buffered rumen fluid, respectively. Conversely, Auerbach et al. [143] reported a rumen AFB1 content of 9.5 ng/mL did not modify in vitro digestion of alfalfa and VFA productions. Consequently, the amount of AFs affecting animal performance and impairing their health is much greater than the dietary amounts associated with milk residues [134,169]. However, sheep [164,165,166,167] and dairy cows [169] exposed to AFs-contaminated diets reduced ingestion and presented alteration of hepatic activity and immune-suppression also at relatively low levels of mycotoxin ingestion. Furthermore, replacement heifers exposed to diets co-contaminated by AFs and FBs at increasing levels showed an important delay in reproductive career along with a slow growth [170].
Among about 500 cases submitted for necropsy at the Department of Pharmacology and Pathobiology of the Royal Veterinary and Agricultural University of Denmark from 1987 to 1992, 30 were diagnosed as Aspergillosis caused by A. fumigatus and zygomycosis by fungi of the class Zygomycetes [129]. The main target organs for invasive fungal infection were omasum followed by the rumen-reticulum and abomasum. Furthermore, Frisvad et al. [92] reported A. fumigatus produces several metabolites with antimicrobial, antifungal or antiprotozoal effects. Among these, gliotoxin is immunosuppressive, causing apoptosis of lymphocytes and macrophages or ROS could be generated and could cause damage to healthy cells in the organs of the host [8,221,222]. In CAST report [4] were summarized several observations relating presence of this mycotoxin to pathogenesis of Aspergillosis. Anyway, evidences supporting gliotoxin is the cause of mycotoxicosis in livestock are unverified and, as discussed previously, it is not likely to be formed in cereals. Concerning the antimicrobial effects of gliotoxin, Morgavi et al. [149] stated that only very high levels of gliotoxin, up to 80 μg/mL of rumen inoculum, influenced DM degradation, gas and VFA productions. Following same static assumptions reported above (50 L rumen fluid volume and 23.5 DMI), the level of 80 μg/mL of rumen inoculum results in a diet contaminated by a gliotoxin level of 140 mg/kg DM, three times higher than what expected by using in dairy cow diet the highest gliotoxin contaminated MS found by Pereyra et al. [81]. Anyway, an extract containing 8.8 μg of gliotoxin/mL decreased DM degradation, gas and VFA productions by 28%, 46% and 35% [149]. On farm conditions, A. fumigatus has been proposed as the pathogenic agent associated with mycotic haemorrhagic bowel syndrome in dairy cattle cases occurring in US and often attributed to Clostridium infections [137,223]. However, this piece of information was not supported by scientific evidences.
Other toxic compounds produced by Aspergillus strains are kojic acid, β-nitropropionic acid and cyclopiazonic acid [84]. Report of US Environment Protection Agency [172] stated these toxins possess antibacterial and antifungal activities. In particular, maltoryzine was associated with poisoning in dairy cows, but this information was not supported by references. Cyclopiazonic acid is toxic for several animal species and causes disruption of calcium homeostasis, degeneration and necrosis of the liver, lesions of myocardium, degeneration or death of cells and neurotoxins effects [224]. Anyway, all experiments reporting these effects were carried out on monogastric species [4]. Last, β-nitropropionic acid is a neurotoxin and its mode of action is an apparently irreversible succinate dehydrogenase inhibition [172]. Chronic or acute intoxications by β-nitropropionic acid on sheep and cattle [173] caused emphysema and difficulty in locomotion. Furthermore, microscopic lesions in the lungs, cells of central nervous system and Wallerian degeneration of the spinal cord were reported. No other information on livestock are currently available for this compound.
There is an extensive literature on the toxicokinetics, metabolism and tissue distribution of OTA [225]. In ruminants, OTA is largely degraded by ruminal microflora into the less toxic ochratoxin α [3,8,31,131,226,227] due mainly to the activity of protozoa [33,151,228,229]. In young calves, more than 90% of orally administered OTA is excreted in urine as metabolite ochratoxin α [230]. Blank et al. [231] investigated the metabolism of OTA feeding sheep 0, 9.5, 19.0 and 28.5 μg OTA/kg BW. Serum concentrations of OTA increased with exposition levels of animals and small amounts of ochratoxin α were detected in plasma, suggesting OTA could bypass rumen undegraded [27,232]. Similar results were reported by Höhler et al. [193] fed sheep 0, 1.4, or 3.5 mg of OTA/kg diet, even if no effects on feed intake and nutrient digestibility were reported. However, in a preliminary trial, the same authors reported sheep fed 14 mg of OTA/kg diet reduced feed ingestion. Even though OTA can escape ruminal degradation and traces were found in milk of experimentally exposed ewes, Boudra et al. [233] concluded the low carryover of OTA in milk minimizes the risk for consumers. Ribelin et al. [234] indicated that the lethal single oral dose of OTA in cattle is probably higher than 13 mg/kg of BW, but not recent upgrades have been reported.
Niederberger et al. [235] reported 5 heifers from one farm in Germany were affected by muscular tremor, hyperexcitability and hypersensitivity. Histological examination of animals revealed degeneration of neurons in the brainstem. Analyzing silage, presence of Aspergillus clavatus, a mold capable of producing neurotoxic tremorgenic mycotoxins, patulin and maltoryzin [236], was detected.
3.3. Fusarium Derived Toxins
The toxicological effects of Fusarium derived toxins in farm animals are deeply described [2,25,26,216,237,238]. DON and other trichothecenes, such as T-2 and HT-2 toxins, DAS and nivalenol, have been suspected to be implicated in farm animal disease outbreaks in many areas of the World [239]. Generally, trichothecenes type B are considered to be more toxic than type A for ruminants [83]. The number of ascertained cases of intoxication by Fusarium derived toxins remains low on field conditions, being this toxicosis often characterized by non-specific clinical symptoms [3,25,240].
Although DON is not suspected to cause acute toxicity in ruminants, it is considered to be the major cause of economic losses due to reduction of animal performance [241]. Clinical signs due to contaminated DON feed ingestion include gastrointestinal problems, soft stools, diarrhea, immunosuppression and a general decrease of performances probably due to feed refusal [3,242]. Generally, dairy cattle are retained more sensitive to the effects of DON compared to beef cattle and sheep [134]. Charmley et al. [176] carried out an experiment to determine the effect of DON on cow performance. The increasing daily intakes of DON were 0.59, 42, and 104 mg/cow/day. However, no effects were measured on intake and milk production of lactating animals. Only milk fat was drastically reduced (lowest value for intermediate treatment). Trenholm et al. [178] reported no lactating dairy cows consuming a wheat-oat DON-contaminated concentrate (1 kg/100 kg BW with a DON contamination of 6.4 mg/kg) slightly reduced ingestion of feed, even if no signs of illness as well as BW gain decrease were recorded. Similar absence of signs was reported by other authors [177,182,243]. Dänicke et al. [179] reported an increase in rumen ammonia concentration and a reduction in duodenal flow of microbial protein feeding rumen-duodenal fistulated no lactating dairy cows with a Fusarium toxin contaminated (DON and ZEA) wheat. The influence of DON on fermentation parameters, in particular on interruption of microbial protein synthesis or alteration of pH, was successively confirmed by Jeong et al. [147] carrying out an in vitro trial and by Keese et al. [174,175] directly on lactating dairy cows.
Other in vitro data [145,146] indicated the incubation of DON (5 mg of DON/kg diet) and other Fusarium toxins (ZEA, nivalenol, scirpentriol, 15-acetyldeoxynivalenol, and 3-acetyldeoxynivalenol) in diluted rumen fluid did not alter normal fermentation activity of rumen inocula, except for the activity of cellulosolitic bacteria. Korosteleva et al. [180,181] reported Fusarium contaminated diets (3.5 mg of DON/kg diet and 0.24 mg of ZEA/kg diet) affected metabolic parameters and immunity of lactating dairy cows, even if no effect on DM intake or milk performance was reported. Kiyothong et al. [171] reported lactating dairy cows fed a diet naturally contaminated with AFB1 and several Fusarium derived toxins showed lower DMI and nutrient digestibility than cows fed the same diet supplemented with a mycotoxin deactivating product. Furthermore, both hematological and immune parameters were adversely affected in cows receiving contaminated diet without product supplementation. Consequently, the impact of DON ingestion in lactating dairy cows is still controversial and needs future clarifications. These controversial results could be attributed to a different rumen activity in converting DON parent molecula into less toxic de-epoxidized metabolites [31,135,244]. Last, fusaric acid and DON were tested for antimicrobial activity against Ruminococcus albus and Methanobrevibacter ruminantium. The growth of both organisms was inhibited by fusaric acid but not by DON and consequently no synergistic inhibitory effect was observed [148].
Concerning health hazard due to ingestion of nivalenol, Hedman and Pettersson [245] reported ruminal microbiota was able to produce a de-epoxidised metabolite of nivalenol, thus suggesting a possible detoxification mechanism. Despite in EFSA scientific report [246] nivalenol exposure levels for lactating dairy cows and beefs are described, no information about the effects of its ingestion in livestock are currently available. A similar lack of information is present for fusarenon X.
Concerning trichothecenes type A, the adverse effects due to ingestion of diet contaminated by T-2 and HT-2 toxins have been extensively reviewed [247], but the majority of researchers carried out studies between 70s to 80s and they tested effects of trichothecene mycotoxins on young ruminants (in particular calves and lambs) [248,249,250,251,252,253]. The main effects referred to hemorrhages and lesions in the gastrointestinal tract, enteritis or bloody feces as well as changed in metabolic and immune status of animals. Effects of T-2 and HT-2 on semen quality have been suspected in bulls [254]. To the best of our knowledge, no information are currently available for lactating dairy cows or beef. Accordingly, the ESFA scientific report [247] concluded saying exposure level equal or higher than 0.3 mg T-2 toxin/kg BW per day may result in gastrointestinal lesions, altered serum proteins and hematological alterations in calves or lambs, whereas the limited data on lactating dairy cows do not allow to set a safety level of ingestion.
Concerning DAS, an experience was reported by Harvey et al. [184]. In this trail, lambs were fed for 14 days with control (uncontaminated), AFB1-contaminated (2.5 mg/kg), DAS-contaminated (5 mg/kg from chemical standard) and AFB1/DAS co-contaminated (2.5 mg of AFB1 and 5 mg of DAS/kg) diets. Animals receiving contaminated diets reduced feed ingestion by 7% to 12% thus probably causing a decrease in BW during intoxication period (difference between initial and final BW of 0.1, −0.6 and −2.7 kg for AFB1-, DAS- and AFB1/DAS-contaminated diets, respectively).
Among Fusarium derived toxins, FBs are cytotoxic, hepatotoxic and nephrotoxic to animals, even if mechanism of action is not completely elucidated [4,135,216,255]. Furthermore, they are inhibitors of cellular sphingosine (sphinganine) N-acetyltransferase that resulted in accumulation of sphinganine and sphingosine and a depletion of complex sphingolipids in eukaryotic cells, which in turn results in impairment of cell cycle regulation, cellular differentiation and in oxidative stress as well as apoptosis and necrosis [256]. In contrast to many other mycotoxins, FBs are poorly degraded in rumen compartment [31,150,257]. Major clinical signs of FBs poisoning in livestock are decreased appetite accompanied by serum biochemical and histologic evidences of hepatic damage. However, lactating dairy Jersey cows fed a diet contaminated at a level of 75 mg/kg as well as two cows consuming 3 mg FB1/kg BW did not show any clinical or hematological changes. Only transient diarrhea at the beginning of intoxication period and an increase in serum cholesterol were reported [185]. In a successive experiment, Holstein steers were fed a diet with a contamination level of 94 mg FB1/kg for 253 days and increases in serum aspartate aminotransferase (AST) and γ-glutamyltransferase (GGT) as well as hepatocellular injury and hyperplastic biliary epithelial cells were reported [23]. Likewise, peripubertal heifers fed diets contaminated by high levels of both AFs and FBs changed some parameters of plasma metabolic profile [170]. Similar metabolic changes, such as serum increase of AST, GGT, lactate dehydrogenase (LDH), bilirubin or cholesterol and histological changes were reported when calves were fed diets containing 15, 31 or 148 mg FBs/kg diet for 31 days [186]. However, no effects were measured on feed intake or weight gain, even if feed containing the highest FBs level seemed to be less palatable. At the highest dose, lymphocyte blastogenesis was significantly impaired at the end of intoxication period. To examine the effects of acute exposure to FBs, lambs were intraruminally dosed with increasing levels of FBs from Fusarium verticillioides culture material. The treatments were 0, 11.1, 22.2 or 45.5 mg of FBs/kg BW for 4 days and death occurred in the two highest dose groups [188]. For survival animals, increases in alkaline phosphatase, GGT, AST and LDH activities as well as in cholesterol, triglyceride, urea nitrogen and creatinine levels were observed. Furthermore, histological examination at the end of the trial revealed renal tubular necrosis and a mild hepatopathy.
The ZEA is converted in rumen compartment into two hydroxyl-metabolites, being α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL) [131,258], with about 90% of parent molecules converted into α-ZOL [31]. The α-ZOL is more oestrogenic than parent molecula but it is slowly absorbed in the liver and could be converted by this organ to the less potent β-ZOL [138]. About effects of ingestion of ZEA-contaminated diets in livestock, experimental studies are lacking, but some case reports indicated that after exposure to high doses of ZEA, animal could present reproductive problems, such as decrease in embryo survival, edema and hypertrophy of the genitalia in pre-pubertal females, decrease in production of luteinizing hormone and progesterone, changes in morphology of uterine tissues, feminization of young males due to decrease of testosterone production, and more generally infertility [2,134,138,259]. Either mode of action and toxicological studies of ZEA were reviewed [260]. The effects of ZEA were studied on heifers [189] and dairy cows [190]. In both studies, pure ZEA (250 mg/heifers and from 0 to 500.0 mg/no pregnant dry cows) was orally administered to animals. The only effect measured was a lower conception rate in treated heifers with respect to control. No effects on the reproductive organs and no changes in the progesterone blood concentrations were detected. In a dairy herd, animals receiving a diet contaminated with both DON and ZEA at levels of about 500 and 750 μg/kg, respectively, showed unsynchronized ovarian cycles, vaginitis and early development of mammary gland in heifers [191]. However, heifers fed a diet with a ZEA concentration of 1.25 mg/kg diet did not show reproductive problems. In addition, several field or case reports in which a direct relationship between ZEA exposition levels and symptoms of estrogenic effects was not found were reported [260], suggesting this might reflect the variability in rumen degradation of ZEA. Recent experiences tried to relate exposure of dairy cows to ZEA contaminated diet on herd level by measuring urinary metabolites [261,262,263]. Authors suggested that monitoring urinary ZEA concentrations could represent an useful tool to predict animal exposure to ZEA and other Fusarium toxins. Smith et al. [192], feeding ewes with increasing ZEA level (1.5, 3, 6, 12, or 24 mg ZEA/ewe), measured reproductive disorders, lower lambing percentages and infertility. At the highest doses, increases in oestrus duration or uterus and ovarian weights were observed too. Fink-Gremmels and Malekinejad [25] reported α-ZOL is used in many Countries as growth promoting agent in fattening cattle and lambs.
No information are currently available concerning effects of other Fusarium derived toxins, such as beauvericins, enniatins and moniliformin [8,206].
3.4. Penicillium Derived Toxins
P. roqueforti and P. paneum produce several secondary metabolites with immunosuppressive, antibacterial and other not well-defined toxicological effects for animals [3,8,11,24,130]. Insufficient and controversial information have been reported concerning effects of these mycotoxins on animals. Furthermore, different authors referred feeding forages contaminated by Penicillium strains can cause loss of appetite and impact nutrient efficiency, increase in somatic cell counts, ketosis, abomasal ulcer, laminitis, gastroenteritis, paralysis and abortion, probably due to the production of their toxic metabolites [3,11,128,195,264,265]. However, no adverse effects on animal health and blood parameters were detected in sheep fed 300 mg/day of mycophenolic acid [194]. Recent experiences carried out by Dzidic et al. [195,196] indicated that sheep fed 300 mg of mycophenolic acid/sheep/day from contaminated silage did not show any immunodepression effects. Furthermore, Penicillium derived toxins such as citrinin, OTA, patulin, mycophenolic acid, penicillic acid or a combination of one of these mycotoxins with OTA could inhibit activity of macrophage up to 25%, thus confirming immunomodulatory properties of these toxins and possible increase of the risk of disease susceptibility in cattle consuming contaminated diets [266].
Santos and Fink-Gremmels [16] verified the effect of ingestion of moldy silages on cows by the individuation of several biomarkers helpful for characterizing mycotoxin syndrome in cattle. In particular, three farms were selected on the basis of the clinical diagnosis of the local veterinarians who observed that animals showed loss in body condition score, poor feces consistence, signs of lameness without a clear disease condition and an irregular increase in somatic cell counts along with an unexpected low milk yield. The GS fed to animals in these farms were sampled and analyzed for mycotoxin contaminations. All these silages resulted highly contaminated by mycophenolic acid and by others Aspergillus (i.e., cyclopiazonic acid, gliotoxin and β-nitropropionic acid) or Fusarium (i.e., DON, ZEA, FB1) derived mycotoxins. The use of biomarkers to verify mycotoxicosis exposure for humans has been proposed mainly for regulated mycotoxins [267,268,269,270,271]. On the other hand, Santos and Fink-Gremmels [16] selected specific markers of oxidative stress, lipid metabolism and liver function for monitoring mycotoxin effects in lactating dairy cows. In these specific conditions, oxidative stress as well as a dysfunction of lipid metabolism were observed in animals ingesting these moldy silages. Effects measured into blood of animals were decreases of glutathione peroxidase activity level, glucose-6-phosphate-dehydrogenase concentrations, trolox equivalent antioxidant capacity, activity of phospholipid transfer protein and lecithin-cholesterol acyltransferase along with an increase in free cholesterol concentration. We retained this approach could be useful to clarify effects of mycotoxins on livestock. Consequently, these aspects should be further investigated to improve understanding of the pathophysiological changes associated with the multiple mycotoxin exposure in dairy cows, thus allowing for refined assessment of intervention strategies.
An in vitro trial was carried out by Gallo et al. [130] to verify effects of some Penicillum derived mycotoxins (i.e., mychopenolic acid, roquefortine C and PR toxin) on rumen fermentation parameters as well as to assess their stability in the rumen environment. Mycotoxin doses ranging from 0.1 to 2 μg/mL rumen fluid or from 0 to 2 μg/mL rumen fluid were tested in two successive trials. The concomitant presence of mycophenolic acid, roquefortine C and PR toxin in trial 1 or only mycophenolic acid and roquefortine C in trial 2 was tested to verify combined/synergic effects of these mycotoxins. Both mycophenolic acid and roquefortine C influenced curve parameters and decreases final gas production of about 13%–15% at the highest concentrations. These mycotoxins, extracted from highly-contaminated MS (contaminations higher than 10 mg/kg for at least one of two mycotoxins), had 30%–40% higher depressing effects on gas and VFA production than those predicted by the model developed by using pure mycotoxins (Gallo, data not reported). These findings suggested other secondary metabolites or the release of bound (modified) mycotoxins during incubation could worsen the effect of these toxins on rumen microorganisms. Furthermore, the stabilities of these two toxins after 48 h of rumen fluid incubation were similar and on average equal to about 50%. PR toxin did not interfere with rumen fermentation pattern and it was not detected after 48 h of incubation. Consequently, it was verified that mycophenolic acid and roquefortine C from standards additively interfered with rumen microorganisms at relatively low levels and were stable in rumen environment after 48 h of incubation, suggesting these mycotoxins could interfere with digestive processes and might represent a potential risk for ruminants. In vivo, reversible paralytic effects were reported in cows that ingested P. roqueforti-contaminated feed grains containing an average roquefortine C concentration of 25.3 mg/kg [197]. Tiwary et al. [272] reported roquefortine C did not appear to be responsible for tremorgenic effects and could be quantified as biomarker for penitrem A exposure. Tüller et al. [95] feeding sheep 0, 10 and 50 mg/sheep/day of roquefortine C did not report any effects on chemical and hematological parameters. However, the rumen pH decreased of 0.5 after intoxication. Lastly, even if PR has been suspected to be associated with cattle disorders [137,273], effects due its ingestion by farm animals have not yet been thoroughly investigated.
Patulin is produced by P. paneum as well as B. nivea. Patulin drastically interfered with rumen activity [152,153], even if effects of its ingestion on ruminants are actually unknown. Only Sabater-Vilar et al. [199] reported severe cases of neurotoxicosis, comprising tremors, ataxia, paresis, recumbence and death concomitantly occurred in several herds of beef cattle in Belgium. As described by these authors, Aspergillus clavatus was found to be the dominant fungal species in a feed containing malting residues and consumed by all these herds. The isolated fungus produced patulin in culture medium and mycotoxixosis caused by this toxin was suspected. For either andrastins nor marcfortines, no information have been reported regarding their effects on livestock and their fate in rumen compartment [8].
3.5. Monascus Ruber Derived Toxins
Citrinin is a nephrotoxic mycotoxin produced by several species of the genera Aspergillus and Penicillium [274]. Citrinin can occur also as an undesirable contaminant in Monascus ruber fermentation products and concomitant occurrence of OTA in food or feed materials has been often reported [274,275]. Field experiments [198,276] suggested cows fed citrinin-contaminated diets showed signs of pruritus, pyrexia and hemorrhagic syndrome as well as fever, diarrhea and uremia. In these experiences, animals ingested visible moldy feeds contaminated by both citrinin (30–40 μg/kg or 2–10 mg/kg) and OTA (0–20 mg/kg). Based on in vitro results, Stec et al. [277] reported immunotoxic effects of citrinin only at very high doses. Experimental data regarding systemic toxic effects in ruminants were not available and it is assumed that citrinin is highly degraded and metabolized through the microbial activity in the forestomachs of ruminants [274]. However, an impairment of the rumen microflora due to the antibacterial effect of citrinin cannot be excluded. Recently, Morgavi et al. [154] verified that the antimethanogenic activity of metabolites produced from different Monascus spp., such as monacolin K, pravastatin, mevastatin and citrinin. These substances showed an inhibitory effect on methanogens thus decreasing methanogenesis in vitro and in short term in vivo studies, without affecting rumen fermentation pattern.
No toxin effect has been associated with the consumption of monacolins contaminated diets in farm animals [8].
3.6. Endophytic Fungal Toxins
Grasses have relatively few intrinsic toxins, relying more on growth habit to survive defoliation and endophytic fungal toxins as chemical defenses [5,57]. Endophytic toxins in grasses include ergot alkaloids in tall fescue and tremorgens in perennial ryegrass [137,223]. Although a number of tremorgens have been identified, the most important is lolitrem B, produced by endophytic fungus. Lolitrems cause neurological effects, producing the ryegrass staggers syndrome [3,4]. Cattle consuming tall fescue contaminated with endophytic fungi have also shown symptoms of stumbles, excitability, increased of rectal temperature and respiration rate as well as decrease in BW [1].
3.7. Intestinal Modulation of Mycotoxins
Fink-Gremmels [31] reported some mycotoxins could pass rumen unchanged (cyclopiazonic acid, FBs, patulin), almost completely metabolized in the rumen in less toxic compounds (OTA in ochratoxin-α, DON in de-epoxy-DON, ZEA in β-ZOL, AFB1 in AFM1) or in rumen compartment in converted in metabolites with similar or higher toxic activity than parent molecules (ZEA in α-ZOL, AFB1 in aflatoxicol). Consequently, rumen could have a great capability to inactivate mycotoxins and reduce health risk in cattle for some mycotoxins, whereas for others it results completely inefficient in protecting animals by negative effects due to mycotoxin ingestion. The protective effect of rumen could be compromised when health status of animals is altered, for any changes in diet composition or as function of mycotoxin exposition levels [31,217]. At the same time, different in vitro trials reported mycotoxins such as AFB1 [29,218], DON [147], gliotoxin [149], FBs [31], OTA [151] or mycophenolic acid and roquefortine C [130] resulted partially stable in rumen environment and thus could reach intestine unchanged with possible antimicrobial activity on intestinal microflora or toxic effects on host animals. Furthermore, Fink-Gremmels [31] and Flores-Flores et al. [217] recently summarized information concerning mycotoxin milk contamination with the aim to evaluate possible risk for humans. Other than AFM1, several mycotoxins such as other AFs (AFG1, AFG2, AFB1, AFB2, AFM2), cyclopiazonic acid, FB1, nivalenol, OTA and ochratoxin α, T-2 toxin, ZEA and its metabolites or DON and its de-epoxy metabolite have been found in milk and we refer to these reviews for extensively discussions of these aspects.
Generally, maintenance of a healthy intestine is crucial to assure adequate nutrient absorption, maintenance of the indigenous microflora and protection of host animals against pathogens thus guaranteeing a correct function of immune system. However, studies on the effect of these mycotoxins on the gastrointestinal tract are limited, in particular for ruminants. Several authors [18,239,278,279] reviewed how different mycotoxins such as AFs, OTA, DON, T-2 toxin, ZEA and FBs, impact digestive and absorptive functions, intestinal defences and intestinal microbioma composition. In particular, Fusarium derived toxins, mainly DON and FB1, could drastically alter the defences mechanisms of intestine, reducing epithelial integrity, cell proliferation and mucus production or increasing intestinal permeability, immunoglobulins and cytokine productions [239]. Data from many research studies carried out on monogastric animals showed that mycotoxins can compromise several intestinal functions, such as digestion, absorption, permeability, defences and can result in lower productivity and poor health of animals [239,278,280]. However, experiments elucidating the effects of mycotoxins on intestinal functionality as well as interference with intestinal microbiota are absent for ruminants and need to be verified in the future, even because the rumen could produce known and unknown mycotoxin metabolites absent in monogastric diets.
4. On Farm Strategies to Minimize Risk of Mycotoxin Contaminations in Forages
4.1. Prevention of Mycotoxin Contaminations of Crops in Field and during Storage
Generally, mycotoxin contamination of agricultural products should be prevented or counteracted by using pre-harvest or post-harvest strategies. Several strategies have been investigated to avoid mycotoxin occurrence in each ring of the food chain. The simplest strategy is based on the prevention of mycotoxin formation in feeds. At field level, different steps could be effective to prevent fungal infestation and consequently mycotoxin production. Among field actions, the most important to counteract fungal infestations are: opportune crop rotation, tillage, soil fertilizers, planting date, crop hybrid/variety selection, chemical/biological control of infestation, crop removal, insect and weed controls. These aspects were widely discussed [22,64,281,282,283,284,285,286].
Under farm conditions, the storage of crops represents another critical step [285]. In particular, grains should be preserved for physical integrity and properly stored, with a moisture content lower than 13% and at low temperature [6,287,288,289]. Despite all precautions, it may happen that stored grains could be damaged and infected by molds and probably by mycotoxins. As recommended by Jard et al. [285], the farmers must discard moldy grains and any material that is suspected of being contaminated with mycotoxins, including apparently clean grains in the vicinity of moldy parts.
Concerning silages, a low oxygen concentration and augmentation of carbon dioxide are efficient in preventing mold development [290]. Consequently, all ensiling stages, such as aerobic, fermentation, stable, feed-out or aerobic spoilage phases should be controlled and optimized as much as possible to assure adequate conservation of ensiled crops [40,290]. However, ensiling procedures are not standardized and farmers use different procedures to ensile and store silages [69,120]. As extensively reviewed by Muck [291] and Dunière et al. [140], uncorrected silo management conditions, such as inappropriate DM content of crop at harvest for its effect in influencing final silage packing density, inadequate particle length, slow silo filling, imperfect mass sealing, poor mass compression, delay in mass pH drop, air penetration in ensiled mass or inappropriate unloading equipment and techniques, could compromise any of aforementioned ensiling phases, thus exposing silages to risk of air penetration and consequent activity of aerobic spoilage microorganisms [68,292,293]. In particular, aerobic deterioration could cause nutrient and DM losses, heat damage of nutrients, excessive proteolysis, proliferation of undesirable microorganisms, such as mycotoxigenic fungi, and production of their toxins [82]. The negative effects due to aerobic activity could be more serious in specific areas of silage, especially in the peripheral (both lateral and apical) parts of ensiled crop, which are generally packed and sealed with difficulty [67,68,69]. Furthermore, when silo is opened for feeding, oxygen becomes available to the front of the mass and the activity of the yeasts and molds, as a result of survival of fungal spores or a re-colonization of these microorganisms, could reduce aerobic stability of ensiled mass, thus favoring potentially toxigenic fungi development [28,36,68,264]. On farms, the adoption of correct ensiling procedures enables to reduce the area exposed to risk of air penetration, such as proper humidity of crops at harvest, use of additives, proper particle sizes, adequate silo size, optimal mass compression, use of polythene wall sheet or different polythene sheets covering ensiled mass, uniform and adequate distribution of weight on the top of ensiled mass limiting oxygen contact in peripheral zones of silo, rapid progress through the silage face, represent the best strategies to guarantee the safety and fermentative quality of ensiled crops [69,140]. Alternative ways for improving or guaranteeing the aerobic stability of silages consist in applying acid-based additives [294]. However, the use of such type of additives may result expensive [295] and the efficiency in the improvement of aerobic stability has not been sufficiently demonstrated [296]. Microbial inoculants such as lactic acid bacteria (LAB) are currently used as economical and practical alternatives to acid-based additives [295,297]. The use of beneficial microbial inoculants to silages before ensiling could improve fermentation occurring during all ensiling phases. However, homofermentative LAB, such as Lactobacillus plantarum, can produce a silage that is poorly stable when exposed to air, because the low production of antifungal compounds such as acetic acid [295,298,299]. Even if heterolactic fermentation is less efficient in the conservation of nutrients than homolactic fermentation [295], the use of heterolactic LAB inoculants, such as L. buchneri, has showed the potential to improve the production of silage from easy, moderately difficult and difficult to ensile materials by reducing the pH, ammonia nitrogen and DM losses [295,297,300]. This because a high production of acetic acid, with a higher antimycotic activity than lactic acid, occurs [69,295].
To obtain information about the rate and extent of either favorable or adverse fermentations that occur in silages, fermentation end-products are commonly used. To this end, different fermentative quality indexes, such as Flieg-Zimmer’s or Vanbelle-Bertin’s scores [301], have been proposed to rank between well- and poorly preserved forages according to the relative amounts of lactic acid, acetic acid, butyric acid or ammonia nitrogen. Recently, Gallo et al. [69,120] developed an index by using a multivariate approach (factorial analysis) to evaluate fermentative quality of MS. This fermentative quality index resulted highly correlated to presence of yeasts and molds in silage, as well as to concentrations of mycotoxins produced by A. fumigatus, P. roqueforti, P. paneum and Fusarium spp. Anyway, if a mycotoxin contaminated forage is used, it should be recommended to discard moldy parts or any material that is contaminated by mycotoxins, to reduce its use in diets by substituting it with other available forages or fibrous by-products and to use adequate sorbent materials, as successively discussed. After economical and management evaluations, feeds proved to be too dangerous for animal health should not be used.
About ensiling methods, bunkers represent the most common system to ensiled crops, but also other methods are currently available, such as pile, silo bags, wrapped bales and tower silos [28,69]. For MS, González Pereyra et al. [302] reported occurrences of Aspergillus spp. and Fusarium spp. were higher in bunker silos, whereas Penicillium spp. incidence was higher in silo bags. Contrarily, Gallo et al. [69] did not report an effect of adopted ensiling procedures on contaminations of Aspergillus, Penicillum or Fusarium derived mycotoxins. Consequently, these aspects require further investigations.
4.2. Detoxification and Biodegradation of Mycotoxins on Farm Conditions
Because it is very difficult to prevent mycotoxin contamination either pre-harvest or during storage of feeds [4], several tools for neutralization of mycotoxins have been developed to protect animals from ingestion of contaminated feeds. Overall, the decontamination and detoxification procedures have to respect some guidelines [4,56,303]: be effective in the inactivation, destroy or removal of the mycotoxins; not result in the deposition of toxic or carcinogenic/mutagenic substances, metabolites or by-products in feeds and food; retain nutrient value and feed acceptability of the products or commodities; not result in significant alterations of the product’s technology properties; be economical and technologically convenient; not alter the cost of final product and destroy fungal spores to avoid a late contamination.
As recently reviewed, the inclusion of sorbent materials in animal diets or the addition of enzymes or microorganisms capable of detoxifying mycotoxins have been reported to be reliable methods for prevention of mycotoxicosis in farms [2,6,18,22,220,285,303,304,305,306,307]. In particular, mycotoxin sequestering agents are compounds able to bind mycotoxins in contaminated feeds without dissociating toxin-sequestering agent complex, thus it could pass through the gastrointestinal tract of animals and toxin could be eliminated via feces [29,303,308]. Many studies have been carried out on inorganic and organic binders and we refer to previous mentioned reviews for details. Among inorganic sequestering agents, clays are largely used as binding agents for reducing AFB1 intoxication of livestock and AFM1 carry over into milk for lactating animals [29,164,218,306,308,309,310,311,312]. In addition, organic sequestering agents, such as activated carbon and yeast cell wall products, have been reported to efficiently reduce AFM1 in milk of cows fed AFB1-contaminated feed [313,314], even if the efficacy to bind AFs of some yeast cell wall products is still controversially discussed [218,220]. Anyway, all in vivo studies, carried out on ruminants, tested the sequestering efficiency of different adsorbents against AFs [6] and some Fusarium derived toxins, such as DON, ZEA and 15-acetyl-deoxynivalenol [180,181]. Furthermore, a mycotoxin deactivating product was tested on lactating dairy cows fed diets naturally contaminated by AFs [315] or AFs and several Fusarium derived toxins, such as DON, ZEA, FB1, OTA and T-2 toxin [171] and now it is approved for its use in pig diet by European Union [18]. Its mode of action is based on three strategies [18,315,316]: (1) polar mycotoxins (e.g., AFs) are adsorbed by the inorganic components; (2) other mycotoxins not or poorly absorbed by the inorganic components (e.g., trichothecenes, ZEA) are biotransformed by biological constituents, namely, Eubacterium strain (BBSH 797) and a yeast strain (Trichosporon mycotoxinivorans MTV) able to alter mycotoxin structures into non-toxic metabolites which are excreted and (3) protective action against mycotoxins acts by mycotoxins phycophytic substances derived from a species of sea alga (Ascophyllum nodosum) and plant (Silybum marianum) extracts, as reported by Pietri et al. [315]. When supplemented to contaminated diets, the product reduced AFM1 extraction into milk without interfering with feed intake and milk production [315] or increase both milk yield and milk protein in cows fed multi-contaminated (both Aspergillus and Fusarium derived mycotoxins) diets [171]. Alternative to the use of sorbent materials in animal diets, vaccination strategies were recently explored to prevent negative effects of mycotoxin (i.e., AFs) ingestion in lactating dairy cows [317] and heifers [17] or to reduce carryover of AFM1 into milk and cheese.
Some in vitro trials were carried out to verify the activity of different adsorbents for mycotoxins different from AFs, such as DON, ZEA and FBs [313,318,319,320,321,322]. To the best of our knowledge, no information are currently available about sequestering efficiency of different products against many Alternaria, Aspergillus, Penicillum and Monascus derived toxins that could be detected in forages. Only recently, Santos and Fink-Gremmels [16] observed on commercial farms that the dietary supplementation of a glucomannan mycotoxin absorbent agent resulted efficient to prevent mycotoxincosis in dairy cattle exposed to ingestion of moldy silages. Consequently, in vitro and in vivo experiments are necessary to verify the efficacy of different commercially available sequestering agents on these kind of mycotoxins. In addition, for many contaminated diets the challenge is from the possible co-occurrence of a high number of mycotoxins, so what is required is to standardize sampling procedures, to use specific methods of analysis able to detect hundreds of mycotoxins simultaneously, as well as their modified forms, and to adopt opportune strategies for successfully mitigating the different negative effects of the wide range of mycotoxins contaminating animal diets.
5. Conclusions
Grazed forage, hay or silages are often contaminated by a wide range of mycotoxins and other fungal exometabolites produced by molds able to infect crops at the pre-harvest stage, during prolonged wilting in bad weather conditions or in silos, piles and bags post-harvest. Despite an increased awareness of mycotoxin occurrences in silages and other forage crops, data are still limited, and thus unsuitable for properly assessing the risk of mycotoxin exposure in cattle and other ruminant species. Consequently, it should be strongly recommended to analyze forages not only for nutritive and fermentative characteristics, but also for mycotoxin contaminations, being this aspect strongly related to the safety use of a given forage in animal diet.
Cases of performance reduction, illness and other diseases have been often associated with ingestion of mycotoxin contaminated forages, but a direct link between ingestion of these mycotoxins, such as those produced by Aspergillus fuminatus, Penicillium roqueforti, P. paneum and other mycotoxigenic molds able to grow on silages and other forage crops, and animal intoxication events are rarely reported and are often unconfirmed. Indeed, these filamentous fungi can produce several exometabolites with antimicrobial and immunosuppressive properties that cause indirect and difficult to observe sub-acute symptoms, such as a reduction in the rumen functionality or an increase in susceptibility of animals to infections.
Consequently, certain scientific evidences regarding negative effects of mycotoxin ingestion on health status and performance of cattle is scarce and still need to be proven. The lack of unequivocal information regarding mycotoxin effects on ruminants should be also related to the complexity to plan specific animal trials since a multitude of confounding effects, such as toxin-, animal-, diet- or environmental-related factors, mycotoxin co-occurrence in feeds and presence of modified mycotoxins, exist. Alternatively, modeling the mycotoxin effects in animals would be a worthwhile approach able to provide useful information and to identify critical research areas that should be investigated.
To prevent or, at least, counteract the negative effects of mycotoxin ingestion in cattle, farmers should adopt the best practices to grow and harvest crops in field, to store hay and grains before feeding or to ensile forages with the aim to reduce the zone exposed to risk of air penetration and aerobic instability. In particular, for many secondary metabolites produced by mycotoxigenic fungi usually detected in silage and other forage crops, few information are currently available about effectiveness of dietary supplementation of adsorbent materials. There is a need to carry out specific trials for investigating sequestering efficiency of different adsorbent products against many Alternaria, Aspergillus, Penicillium and Monascus derived toxins that are normally detected in forages.
Last, in order to provide information concerning risk of mycotoxin contamination in cattle diets and to verify the mycotoxin ingestion in animals, an international network including participants of each ring of the food chain should be created to monitor mycotoxin occurrence in silages and other forage crops, thus permitting to verify exposition levels to mycotoxin ingestion of ruminants. Furthermore, shared in vitro or in vivo trial protocols are strongly preferable to standardize methodology and data interpretation.
Acknowledgments
Financial support was provided by FILIGRANA project “Valorizzazione della produzione del Grana Padano DOP tramite il controllo di filiera e l’ottimizzazione dei processi produttivi” of MiPAAF (Ministero delle Politiche Agricole Alimentari e Forestali, Italy).
Author Contributions
A.G. wrote the review, analyzed and synthetized information from bibliographies and prepared tables and the figure. J.C.F. and K.F.N. made the critical review on producers, fungal identification and ecology. J.C.F., K.F.N. and T.B. made the critical review of analytical methods. A.G., G.G. and T.B. made the critical review on mycotoxin effects on animals, sampling procedures and on farm strategies to counteract negative effects of mycotoxins.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zain M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15:129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 2.Zinedine A., Soriano J.M., Moltó J.C., Mañes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Fink-Gremmels J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008;176:84–92. doi: 10.1016/j.tvjl.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Council for Agricultural Science and Technology (CAST) Mycotoxins: Risks in Plant, Animal, and Human Systems. CAST; Ames, IA, USA: 2003. [Google Scholar]
- 5.Scudamore K.A., Livesey C.T. Occurrence and Significance of Mycotoxins in Forage Crops and Silage: A Review. J. Sci. Food Agric. 1998;77:1–17. doi: 10.1002/(SICI)1097-0010(199805)77:1<1::AID-JSFA9>3.0.CO;2-4. [DOI] [Google Scholar]
- 6.Kabak B., Dobson A.D.W., Var I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006;46:593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- 7.Bryden W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. [DOI] [Google Scholar]
- 8.Storm I.M.L.D., Sørensen J.L., Rasmussen R.R., Nielsen K.F., Thrane U. Mycotoxins in silage. Stewart Postharvest Rev. 2008;4:1–12. doi: 10.2212/spr.2008.6.4. [DOI] [Google Scholar]
- 9.Jennessen J., Nielsen K.F., Houbraken J., Lyhne E.K., Schnorer J., Frisvad J.C., Samson R.A. Secondary metabolite and mycotoxin production by the Rhizopus microsporus group. J. Agric. Food Chem. 2005;53:1833–1840. doi: 10.1021/jf048147n. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien M., Nielsen K.F., O’Kiely P., Forristal P.D., Fuller H.T., Frisvad J.C. Mycotoxins and other secondary metabolites produced in vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom isolated from baled grass silage in Ireland. J. Agric. Food Chem. 2006;54:9268–9276. doi: 10.1021/jf0621018. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen K.F., Sumarah M.W., Frisvad J.C., Miller J.D. Production of metabolites from the Penicillium roqueforti complex. J. Agric. Food Chem. 2006;54:3756–3763. doi: 10.1021/jf060114f. [DOI] [PubMed] [Google Scholar]
- 12.Streit E., Schatzmayr G., Tassis P., Tzika E., Marin D., Taranu I., Tabuc C., Nicolau A., Aprodu I., Puel O., et al. Current situation of mycotoxin contamination and co-occurrence in animal feed focus on Europe. Toxins. 2012;4:788–809. doi: 10.3390/toxins4100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magan N., Medina A., Aldred D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011;60:150–163. doi: 10.1111/j.1365-3059.2010.02412.x. [DOI] [Google Scholar]
- 14.Cole R.J., Cox R.H. Handbook of Toxic Fungal Metabolites. Academic Press, INC.; New York, NY, USA: 1981. [Google Scholar]
- 15.Richard J.L. Some major mycotoxins and their mycotoxicoses-An overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Santos R.R., Fink-Gremmels J. Mycotoxin syndrome in dairy cattle: Characterisation and intervention results. World Mycotoxin J. 2014;7:357–366. doi: 10.3920/WMJ2013.1577. [DOI] [Google Scholar]
- 17.Giovati L., Gallo A., Masoero F., Cerioli C., Ciociola T., Conti S., Magliani W., Polonelli L. Vaccination of heifers with anaflatoxin improves the reduction of aflatoxin B1 carry over in milk of lactating dairy cows. PLoS ONE. 2014;9:e94440. doi: 10.1371/journal.pone.0094440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murugesan G.R., Ledoux D.R., Naehrer K., Berthiller F., Applegate T.J., Grenier B., Phillips T.D., Schatzmayr G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015;94:1298–1315. doi: 10.3382/ps/pev075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis B.B. Advances in Experimental Medicine and Biology. In: deVries J.W., Trucksess M.W., Jackson L.S., editors. Mycotoxins and Food Safety. Volume 504 Springer US; Boston, MA, USA: 2002. [Google Scholar]
- 20.Vieira M.L.A., Johann S., Hughes F.M., Rosa C.A., Rosa L.H. The diversity and antimicrobial activity of endophytic fungi associated with medicinal plant Baccharis trimera (Asteraceae) from the Brazilian savannah. Can. J. Microbiol. 2014;60:847–856. doi: 10.1139/cjm-2014-0449. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo I., Varsavky E., Haidukowski M., Frade H. Macrocyclic trichothecenes in Baccharis coridifolia plants and endophytes and Baccharis artemisioides plants. Toxicon. 1997;35:753–757. doi: 10.1016/S0041-0101(96)00149-3. [DOI] [PubMed] [Google Scholar]
- 22.Jouany J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007;137:342–362. doi: 10.1016/j.anifeedsci.2007.06.009. [DOI] [Google Scholar]
- 23.Baker D.C., Rottinghaus G.E. Chronic experimental fumonisin intoxication of calves. J. Vet. Diagn. Investig. 1999;11:289–292. doi: 10.1177/104063879901100315. [DOI] [PubMed] [Google Scholar]
- 24.Storm I.M.L.D., Rasmussen R.R., Rasmussen P.H. Occurrence of Pre- and Post-Harvest Mycotoxins and Other Secondary Metabolites in Danish Maize Silage. Toxins. 2014;6:2256–2269. doi: 10.3390/toxins6082256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink-Gremmels J., Malekinejad H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007;137:326–341. doi: 10.1016/j.anifeedsci.2007.06.008. [DOI] [Google Scholar]
- 26.Pestka J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007;137:283–298. doi: 10.1016/j.anifeedsci.2007.06.006. [DOI] [Google Scholar]
- 27.Mobashar M., Hummel J., Blank R., Südekum K.-H. Ochratoxin A in Ruminants—A Review on Its Degradation by Gut Microbes and Effects on Animals. Toxins. 2010;2:809–839. doi: 10.3390/toxins204809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheli F., Campagnoli A., Dell’Orto V. Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed Sci. Technol. 2013;183:1–16. doi: 10.1016/j.anifeedsci.2013.01.013. [DOI] [Google Scholar]
- 29.Gallo A., Masoero F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2009;9:109–116. doi: 10.4081/ijas.2010.e21. [DOI] [Google Scholar]
- 30.Niderkorn V., Morgavi D.P., Pujos E., Tissandier A., Boudra H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in an in vitro simulated corn silage model. Food Addit. Contam. 2007;24:406–415. doi: 10.1080/02652030601101110. [DOI] [PubMed] [Google Scholar]
- 31.Fink-Gremmels J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2008;25:172–180. doi: 10.1080/02652030701823142. [DOI] [PubMed] [Google Scholar]
- 32.Gallo A., Moschini M., Cerioli C., Masoero F. Use of principal component analysis to classify forages and predict their calculated energy content. Animal. 2013;7:930–939. doi: 10.1017/S1751731112002467. [DOI] [PubMed] [Google Scholar]
- 33.Giuberti G., Gallo A., Masoero F., Ferraretto L.F., Hoffman P.C., Shaver R.D. Factors affecting starch utilization in large animal food production system: A review. Starch/Stärke. 2014;66:72–90. doi: 10.1002/star.201300177. [DOI] [Google Scholar]
- 34.Boysen M.E., Jacobsson K.-G., Schnurer J. Molecular Identification of Species from the Penicillium roqueforti Group Associated with Spoiled Animal Feed. Appl. Environ. Microbiol. 2000;66:1523–1526. doi: 10.1128/AEM.66.4.1523-1526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumarah M.W., Miller J.D., Blackwell B.A. Isolation and metabolite production by Penicillium roqueforti, P. paneum and P. crustosum isolated in Canada. Mycopathologia. 2005;159:571–577. doi: 10.1007/s11046-005-5257-7. [DOI] [PubMed] [Google Scholar]
- 36.Driehuis F., Spanjer M.C., Scholten J.M., te Giffel M.C. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J. Dairy Sci. 2008;91:4261–4671. doi: 10.3168/jds.2008-1093. [DOI] [PubMed] [Google Scholar]
- 37.Driehuis F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013;22:16–34. [Google Scholar]
- 38.Casteel S.W., Rottinghaus G.E., Johnson G.C., Wicklow D.T. Liver disease in cattle induced by consumption of moldy hay. Vet. Hum. Toxicol. 1995;37:248–251. [PubMed] [Google Scholar]
- 39.Fink-Gremmels J., Diaz D.E. Mycotoxins in forages. In: Diaz D.E., editor. The Mycotoxin Blue Book. Nottingham University Press; Thrumpton, Nottingham, UK: 2005. pp. 249–268. [Google Scholar]
- 40.Dell’Orto V., Baldi G., Cheli F. Mycotoxins in silage: Checkpoints for effective management and control. World Mycotoxin J. 2015 doi: 10.3920/WMJ2014.1866. [DOI] [Google Scholar]
- 41.Placinta C.M., D’Mello J.P.F., MacDonald A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999;78:21–37. doi: 10.1016/S0377-8401(98)00278-8. [DOI] [Google Scholar]
- 42.Scott P.M. Mycotoxins in feeds and ingredients and their origin. J. Food Prot. 1978;14:385–398. doi: 10.4315/0362-028X-41.5.385. [DOI] [PubMed] [Google Scholar]
- 43.Filtenborg O., Frisvad J.C., Thrane U. Moulds in food spoilage. Int. J. Food Microbiol. 1996;33:85–102. doi: 10.1016/0168-1605(96)01153-1. [DOI] [PubMed] [Google Scholar]
- 44.Meister U., Springer M. Mycotoxins in cereals and cereal products: Occurrence and changes during processing. J. Appl. Bot. Food Qual. 2004;78:168–173. [Google Scholar]
- 45.Leung M.C.K., Díaz-Llano G., Smith T.K. Mycotoxins in pet food: A review on worldwide prevalence and preventative strategies. J. Agric. Food Chem. 2006;54:9623–9635. doi: 10.1021/jf062363+. [DOI] [PubMed] [Google Scholar]
- 46.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. doi: 10.1016/j.anifeedsci.2007.06.005. [DOI] [Google Scholar]
- 47.Reddy K.R.N., Abbas H.K., Abel C.A., Shier W.T., Oliveira C.A.F., Raghavender C.R. Mycotoxin contamination of commercially important agricultural commodities. Toxin Rev. 2009;28:154–168. doi: 10.1080/15569540903092050. [DOI] [Google Scholar]
- 48.Streit E., Naehrer K., Rodrigues I., Schatzmayr G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013;93:2892–2899. doi: 10.1002/jsfa.6225. [DOI] [PubMed] [Google Scholar]
- 49.Miller J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2008;25:219–230. doi: 10.1080/02652030701744520. [DOI] [PubMed] [Google Scholar]
- 50.Lazzaro I., Moretti A., Giorni P., Brera C., Battilani P. Organic vs conventional farming: Differences in infection by mycotoxin-producing fungi on maize and wheat in Northern and Central Italy. Crop Prot. 2015;72:22–30. doi: 10.1016/j.cropro.2015.03.001. [DOI] [Google Scholar]
- 51.Prandini A., Sigolo S., Filippi L., Battilani P., Piva G. Review of predictive models for Fusarium head blight and related mycotoxin contamination in wheat. Food Chem. Toxicol. 2009;47:927–931. doi: 10.1016/j.fct.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 52.European Commission (EC) Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Union. 2002;L140:10–21. [Google Scholar]
- 53.European Commission (EC) Commission recommendation (2006/576/EU) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 2006;L229:7–9. [Google Scholar]
- 54.Food and Drug Administration (FDA) Mycotoxin Regulatory Guidance. A Guide for Grain Elevators, Feed Manufacturers, Grain Processors and Exporters. National Grain and Feed Association; Washington, DC, USA: 2011. [Google Scholar]
- 55.Ashiq S. Natural Occurrence of Mycotoxins in Food and Feed: Pakistan Perspective. Compr. Rev. Food Sci. Food Saf. 2015;14:159–175. doi: 10.1111/1541-4337.12122. [DOI] [PubMed] [Google Scholar]
- 56.European Commission (EC) Commission Regulation (EU) 2015/786 of 19 May 2015 defining acceptability criteria for detoxification processes applied to products intended for animal feed as provided for in Directive 2002/32/EC of the European Parliament and of the Council. Off. J. Eur. Union. 2015;L125:10–14. [Google Scholar]
- 57.Cheeke P.R. Endogenous toxins and mycotoxins in forage grasses and their effects on livestock. J. Anim. Sci. 1995;73:909–918. doi: 10.2527/1995.733909x. [DOI] [PubMed] [Google Scholar]
- 58.Cheeke P.R. Natural Toxicants in Feeds, Forages, and Poisonous Plants. 2nd ed. Interstate Publishers, Inc.; Danville, IL, USA: 1998. [Google Scholar]
- 59.Alonso V.A., Pereyra C.M., Keller L.A.M., Dalcero A.M., Rosa C.A.R., Chiacchiera S.M., Cavaglieri L.R. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013;115:637–643. doi: 10.1111/jam.12178. [DOI] [PubMed] [Google Scholar]
- 60.Mostrom M.S., Jacobsen B.J. Ruminant mycotoxicosis. Vet. Clin. N. Am. Food Anim. Pract. 2011;27:315–344. doi: 10.1016/j.cvfa.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Skládanka J., Nedělník J., Adam V., Doležal P., Moravcová H., Dohnal V. Forage as a primary source of mycotoxins in animal diets. Int. J. Environ. Res. Public Health. 2011;8:37–50. doi: 10.3390/ijerph8010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keller L.A.M., González Pereyra M.L., Keller K.M., Alonso V.A., Oliveira A.A., Almeida T.X., Barbosa T.S., Nunes L.M.T., Cavaglieri L.R., Rosa C.A.R. Fungal and mycotoxins contamination in corn silage: Monitoring risk before and after fermentation. J. Stored Prod. Res. 2013;52:42–47. doi: 10.1016/j.jspr.2012.09.001. [DOI] [Google Scholar]
- 63.Medina A., Rodriguez A., Magan N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014;5:348–366. doi: 10.3389/fmicb.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Battilani P., Camardo Leggieri M., Rossi V., Giorni P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013;94:38–46. doi: 10.1016/j.compag.2013.03.005. [DOI] [Google Scholar]
- 65.Mansfield M.A., Kuldau G.A. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia. 2007;99:269–278. doi: 10.3852/mycologia.99.2.269. [DOI] [PubMed] [Google Scholar]
- 66.Pelhate J. Maize silage: Incidence of moulds during conservation. Folia Vet. Lat. 1977;7:1–16. [PubMed] [Google Scholar]
- 67.Vissers M.M.M., Driehuis F., Te Giffel M.C., de Jong P., Lankveld J.M.G. Concentrations of butyric acid bacteria spores in silage and relationships with aerobic deterioration. J. Dairy Sci. 2007;90:928–936. doi: 10.3168/jds.S0022-0302(07)71576-X. [DOI] [PubMed] [Google Scholar]
- 68.Borreani G., Tabacco E. The relationship of silage temperature with the microbiological status of the face of corn silage bunkers. J. Dairy Sci. 2010;93:2620–2629. doi: 10.3168/jds.2009-2919. [DOI] [PubMed] [Google Scholar]
- 69.Gallo A., Bertuzzi T., Giuberti G., Moschini M., Bruschi S., Cerioli C., Masoero F. New assessment based on the use of principal factor analysis to investigate corn silage quality from nutritional traits, fermentation end products and mycotoxins. J. Sci. Food Agric. 2015 doi: 10.1002/jsfa.7109. [DOI] [PubMed] [Google Scholar]
- 70.Andersen B., Nielsen K.F., Fernández Pinto V., Patriarca A. Characterization of Alternaria strains from Argentinean blueberry, tomato, walnut and wheat. Int. J. Food Microbiol. 2015;196:1–10. doi: 10.1016/j.ijfoodmicro.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 71.Andersen B., Krøger E., Roberts R.G. Chemical and morphological segregation of Alternaria arborescens, A. infectoria and A. tenuissima species-groups. Mycol. Res. 2002;106:170–182. doi: 10.1017/S0953756201005263. [DOI] [Google Scholar]
- 72.Andersen B., Krøger E., Roberts R.G. Chemical and morphological segregation of Alternaria alternata, A. gaisen and A. longipes. Mycol. Res. 2001;105:291–299. doi: 10.1017/S0953756201003446. [DOI] [Google Scholar]
- 73.Christensen K.B., van Klink J.W., Weavers R.T., Larsen T.O., Andersen B., Phipps R.K. Novel chemotaxonomic markers of the Alternaria infectoria species-group. J. Agric. Food Chem. 2005;53:9431–9435. doi: 10.1021/jf0513213. [DOI] [PubMed] [Google Scholar]
- 74.European Food Safety Authority (EFSA) Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011;9:1–97. [Google Scholar]
- 75.Yu W., Yu F.-Y., Undersander D.J., Chu F.S. Immunoassays of Selected Mycotoxins in Hay, Silage and Mixed Feed. Food Agric. Immunol. 1999;11:307–319. doi: 10.1080/09540109999690. [DOI] [Google Scholar]
- 76.Mansfield M.A., Archibald D.D., Jones A.D., Kuldau G.A. Relationship of sphinganine analog mycotoxin contamination in maize silage to seasonal weather conditions and to agronomic and ensiling practices. Phytopathology. 2007;97:504–511. doi: 10.1094/PHYTO-97-4-0504. [DOI] [PubMed] [Google Scholar]
- 77.Richard E., Heutte N., Bouchart V., Garon D. Evaluation of fungal contamination and mycotoxin production in maize silage. Anim. Feed Sci. Technol. 2009;148:309–320. doi: 10.1016/j.anifeedsci.2008.02.004. [DOI] [Google Scholar]
- 78.Cavallarin L., Borreani G., Tabacco E., Lúscher A., Jeangros B., Kessler W., Huguenin O., Lobsiger M., Millar N., Suter D. Mycotoxin occurrence in farm maize silages in northern Italy. In: Lüscher A., Jeangros B., Huguenin O., Lobsiger M., Millar N., Suter D., editors. Land Use Systems in Grassland Dominated Regions; Proceedings of the 20th General Meeting of the European Grassland Federation; Luzern, Switzerland. 21–24 June 2004; Zürich, Switzerland: Swiss Grassland Society; 2004. pp. 1023–1025. [Google Scholar]
- 79.Garon D., Richard E., Sage L., Bouchart V., Pottier D., Lebailly P. Mycoflora and multimycotoxin detection in corn silage: Experimental study. J. Agric. Food Chem. 2006;54:3479–3484. doi: 10.1021/jf060179i. [DOI] [PubMed] [Google Scholar]
- 80.Mansfield M.A., Jones A.D., Kuldau G.A. Contamination of fresh and ensiled maize by multiple penicillium mycotoxins. Phytopathology. 2008;98:330–336. doi: 10.1094/PHYTO-98-3-0330. [DOI] [PubMed] [Google Scholar]
- 81.Pereyra C.M., Alonso V.A., Rosa C.A.R., Chiacchiera S.M., Dalcero A.M., Cavaglieri L.R. Gliotoxin natural incidence and toxigenicity of Aspergillus fumigatus isolated from corn silage and ready dairy cattle feed. World Mycotoxin J. 2008;1:457–462. doi: 10.3920/WMJ2007.1012. [DOI] [Google Scholar]
- 82.Driehuis F., Spanjer M.C., Scholten J.M., Te Giffel M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. B. 2008;1:41–50. doi: 10.1080/19393210802236927. [DOI] [PubMed] [Google Scholar]
- 83.Schollenberger M., Müller H.M., Rüfle M., Suchy S., Plank S., Drochner W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia. 2006;161:43–52. doi: 10.1007/s11046-005-0199-7. [DOI] [PubMed] [Google Scholar]
- 84.Storm I.M.L.D., Kristensen N.B., Raun B.M.L., Smedsgaard J., Thrane U. Dynamics in the microbiology of maize silage during whole-season storage. J. Appl. Microbiol. 2010;109:1017–1026. doi: 10.1111/j.1365-2672.2010.04729.x. [DOI] [PubMed] [Google Scholar]
- 85.Eckard S., Wettstein F.E., Forrer H.R., Vogelgsang S. Incidence of Fusarium species and mycotoxins in silage maize. Toxins. 2011;3:949–967. doi: 10.3390/toxins3080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oldenburg E., Eiiner F. Fusarium mycotoxins in forage maize—Detection and evaluation. Mycotoxin Res. 2005;21:105–107. doi: 10.1007/BF02954430. [DOI] [PubMed] [Google Scholar]
- 87.Mansfield M.A., de Wolf E.D., Kuldau G.A. Relationships Between Weather Conditions, Agronomic Practices, and Fermentation Characteristics with Deoxynivalenol Content in Fresh and Ensiled Maize. Plant Dis. 2005;89:1151–1157. doi: 10.1094/PD-89-1151. [DOI] [PubMed] [Google Scholar]
- 88.Kim E.-K., Maragos C.M., Kendra D.F. Liquid chromatographic determination of fumonisins B1, B2, and B3 in corn silage. J. Agric. Food Chem. 2004;52:196–200. doi: 10.1021/jf034934t. [DOI] [PubMed] [Google Scholar]
- 89.McElhinney C., Danaher M., Elliott C., O’Kiely P. The world Mycotoxin Forum. IBERS, Aberystwyth University; 2014. Identification and quantification of mycotoxins in silages: An Irish national survey; pp. 87–88. [Google Scholar]
- 90.Borreani G., Tabacco E., Antoniazzi S., Cavallarin L. Zearalenone contamination in farm maize silage. Ital. J. Anim. Sci. 2005;4:162–165. doi: 10.4081/ijas.2005.2s.162. [DOI] [Google Scholar]
- 91.Buckley T., Creighton A., Fogarty U. Analysis of Canadian and Irish forage, oats and commercially available equine concentrate feed for pathogenic fungi and mycotoxins. Ir. Vet. J. 2007;60:231–236. doi: 10.1186/2046-0481-60-4-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frisvad J.C., Rank C., Nielsen K.F., Larsen T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009;47(Suppl. 1):S53–S71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]
- 93.Schneweis I., Meyer K., Hormansdorfer S., Bauer J. Mycophenolic acid in silage. Appl. Environ. Microbiol. 2000;66:3639–3641. doi: 10.1128/AEM.66.8.3639-3641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Auerbach H., Oldenburg E., Weissbach F. Incidence of penicillium roqueforti and roquefortine C in silages. J. Sci. Food Agric. 1998;76:565–572. doi: 10.1002/(SICI)1097-0010(199804)76:4<565::AID-JSFA990>3.0.CO;2-6. [DOI] [Google Scholar]
- 95.Tüller G., Armbruster G., Wiedenmann S., Hänichen T., Schams D., Bauer J. Occurrence of roquefortine in silage—toxicological relevance to sheep. J. Anim. Physiol. Anim. Nutr. Berl. 1998;80:246–249. doi: 10.1111/j.1439-0396.1998.tb00536.x. [DOI] [Google Scholar]
- 96.Schneweis I., Meyer K., Hörmansdorfer S., Bauer J. Metabolites of Monascus ruber in silages. J. Anim. Physiol. Anim. Nutr. Berl. 2001;85:38–44. doi: 10.1046/j.1439-0396.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 97.Sassahara M., Pontes Netto D., Yanaka E.K. Aflatoxin occurrence in foodstuff supplied to dairy cattle and aflatoxin M1 in raw milk in the North of Paraná state. Food Chem. Toxicol. 2005;43:981–984. doi: 10.1016/j.fct.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Cheli F., Campagnoli A., Pinotti L., Fusi E., Dell’Orto V. Sampling feed for mycotoxins: Acquiring knowledge from food. Ital. J. Anim. Sci. 2010;8:5–22. doi: 10.4081/ijas.2009.5. [DOI] [Google Scholar]
- 99.Miraglia M., de Santis B., Minardi V., Debegnach F., Brera C. The role of sampling in mycotoxin contamination: An holistic view. Food Addit. Contam. 2005;22(Suppl. 1):31–36. doi: 10.1080/02652030500389055. [DOI] [PubMed] [Google Scholar]
- 100.Köppen R., Koch M., Siegel D., Merkel S., Maul R., Nehls I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010;86:1595–1612. doi: 10.1007/s00253-010-2535-1. [DOI] [PubMed] [Google Scholar]
- 101.Brera C., de Santis B., Prantera E., Debegnach F., Pannunzi E., Fasano F., Berdini C., Slate A.B., Miraglia M., Whitaker T.B. Effect of sample size in the evaluation of “in-field” sampling plans for aflatoxin B1 determination in corn. J. Agric. Food Chem. 2010;58:8481–8489. doi: 10.1021/jf1018356. [DOI] [PubMed] [Google Scholar]
- 102.Rivas Casado M., Parsons D.J., Weightman R.M., Magan N., Origgi S. Modelling a two-dimensional spatial distribution of mycotoxin concentration in bulk commodities to design effective and efficient sample selection strategies. Food Addit. Contam. A. 2009;26:1298–1305. doi: 10.1080/02652030903042517. [DOI] [PubMed] [Google Scholar]
- 103.European Commission (EC) Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006;L70:12–34. [Google Scholar]
- 104.European Commission (EC) Recomendations on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union. 2013;9:12–15. [Google Scholar]
- 105.Chang P.-K., Ehrlich K.C., Fujii I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins. 2009;1:74–99. doi: 10.3390/toxins1020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alonso V., Díaz Vergara L., Aminahuel C., Pereyra C., Pena G., Torres A., Dalcero A., Cavaglieri L. Physiological behaviour of gliotoxigenic Aspergillus fumigatus sensu stricto isolated from maize silage under simulated environmental conditions. Food Addit. Contam. A. 2015;32:236–244. doi: 10.1080/19440049.2014.996256. [DOI] [PubMed] [Google Scholar]
- 107.Bok J.W., Chung D., Balajee S.A., Marr K.A., Andes D., Nielsen K.F., Frisvad J.C., Kirby K.A., Keller N.P. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boudra H., Morgavi D.P. Mycotoxin risk evaluation in feeds contaminated by Aspergillus fumigatus. Anim. Feed Sci. Technol. 2005;120:113–123. doi: 10.1016/j.anifeedsci.2005.01.006. [DOI] [Google Scholar]
- 109.Frisvad J.C., Frank J.M., Kuijpers A.F.A., Houbraken J.A.M.P., Samson R.A. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004;50:45–61. [Google Scholar]
- 110.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium sbgenus Pemicillium: A guide to identification of food and air-bone teverticilliate penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–173. [Google Scholar]
- 111.Frisvad J.C., Thrane U., Samson R.A., Hoekstra E.S. Mycotoxin production by common filamentous fungi. In: Samson R.A., Hoekstra E.S., Frisvad J.C., editors. Introduction to Food- and Airborne Fungi. Centraalbureau voor Schimmelcultures (CBS); Utrecht, The Netherlands: 2004. pp. 321–331. [Google Scholar]
- 112.Meca G., Ritieni A. Production and analysis of ochratoxin A produced by Aspergillus ochraceus ITEM 5137 in submerged culture. Food Chem. 2009;117:470–472. doi: 10.1016/j.foodchem.2009.04.041. [DOI] [Google Scholar]
- 113.Samson R.A., Houbraken J., Thrane J., Frisvad J.C., Andersen B. Food and Indoor Fungi. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2010. [Google Scholar]
- 114.McCormick S.P., Stanley A.M., Stover N.A., Alexander N.J. Trichothecenes: From simple to complex mycotoxins. Toxins. 2011;3:802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chelkowski J. Fusarium: Mycotoxins, Taxonomy, Pathogenicity. Elsevier Science Publishers B.V.; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 116.European Food Safety Authority (EFSA) Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013;11 doi: 10.2903/j.efsa.2013.3379. [DOI] [Google Scholar]
- 117.Mansfield M.A. Ph.D. Thesis. Penn State University; University Park, PA, USA: 2005. Fungi and mycotoxins in fresh and ensiled maize and the effects of agronomic practices, wheatear conditions and silage characteristics. [Google Scholar]
- 118.Dall’Asta C., Falavigna C., Galaverna G., Battilani P. Role of maize hybrids and their chemical composition in Fusarium infection and fumonisin production. J. Agric. Food Chem. 2012;60:3800–3808. doi: 10.1021/jf300250z. [DOI] [PubMed] [Google Scholar]
- 119.Kim S.C., Adesogan A.T. Influence of ensiling temperature, simulated rainfall, and delayed sealing on fermentation characteristics and aerobic stability of corn silage. J. Dairy Sci. 2006;89:3122–3132. doi: 10.3168/jds.S0022-0302(06)72586-3. [DOI] [PubMed] [Google Scholar]
- 120.Gallo A., Giuberti G., Bruschi S., Fortunati P., Masoero F. Use of principal factor analysis to generate a corn silage fermentative quality index to rank well- or poorly-preserved forages. J. Sci. Food Agric. 2015 doi: 10.1002/jsfa.7272. [DOI] [PubMed] [Google Scholar]
- 121.Sørensen J.L., Nielsen K.F., Thrane U. Analysis of moniliformin in maize plants using hydrophilic interaction chromatography. J. Agric. Food Chem. 2007;55:9764–9768. doi: 10.1021/jf0715875. [DOI] [PubMed] [Google Scholar]
- 122.Boudra H., Morgavi D.P. Reduction in Fusarium toxin levels in corn silage with low dry matter and storage time. J. Agric. Food Chem. 2008;56:4523–4528. doi: 10.1021/jf800267k. [DOI] [PubMed] [Google Scholar]
- 123.Berthiller F., Dall’Asta C., Schuhmacher R., Lemmens M., Adam G., Krska R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005;53:3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- 124.Berthiller F., Crews C., Dall’Asta C., de Saeger S., Haesaert G., Karlovsky P., Oswald I.P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagl V., Woechtl B., Schwartz-Zimmermann H.E., Hennig-Pauka I., Moll W.-D., Adam G., Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014;229:190–197. doi: 10.1016/j.toxlet.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 126.Rychlik M., Humpf H.-U., Marko D., Dänicke S., Mally A., Berthiller F., Klaffke H., Lorenz N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014;30:197–205. doi: 10.1007/s12550-014-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.European Food Safety Authority (EFSA) Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011;9:1–124. [Google Scholar]
- 128.Nout M.J.R., Bouwmeester H.M., Haaksma J., van Dijk H. Fungal growth in silages of sugarbeet press pulp and maize. J. Agric. Sci. 1993;121:323–326. doi: 10.1017/S0021859600085506. [DOI] [Google Scholar]
- 129.Jensen H.E., Olsen S.N., Aalbaek B. Gastrointestinal aspergillosis and zygomycosis of cattle. Vet. Pathol. 1994;31:28–36. doi: 10.1177/030098589403100104. [DOI] [PubMed] [Google Scholar]
- 130.Gallo A., Giuberti G., Bertuzzi T., Moschini M., Masoero F. Study of the effects of PR toxin, mycophenolic acid and roquefortine C on in vitro gas production parameters and their stability in the rumen environment. J. Agric. Sci. 2015;153:163–176. doi: 10.1017/S0021859614000343. [DOI] [Google Scholar]
- 131.Kiessling K.H., Pettersson H., Sandholm K., Olsen M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984;47:1070–1073. doi: 10.1128/aem.47.5.1070-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yiannikouris A., Jouany J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002;51:81–99. doi: 10.1051/animres:2002012. [DOI] [Google Scholar]
- 133.Jouany J.P., Ushida K. The Role of Protozoa in Feed Digestion—Review - Asian Australas. J. Anim. Sci. 1999;12:113–128. doi: 10.5713/ajas.1999.113. [DOI] [Google Scholar]
- 134.Jouany J.P., Diaz D.E. Mycotoxins Blue Book. Nottingham University Press; Thrumpton, Nottingham, UK: 2005. Effects of mycotoxins in ruminants; pp. 295–321. [Google Scholar]
- 135.Hussein H. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 136.Rasmussen R.R., Rasmussen P.H., Larsen T.O., Bladt T.T., Binderup M.L. In vitro cytotoxicity of fungi spoiling maize silage. Food Chem. Toxicol. 2011;49:31–44. doi: 10.1016/j.fct.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 137.Whitlow L., Hagler W. Mold and mycotoxin issues in dairy cattle: Effects, prevention and treatment. Extention. 2010:1–22. [Google Scholar]
- 138.Santos R.R., Schoevers E.J., Roelen B.A.J., Fink-Gremmels J. Mycotoxins and female reproduction: In vitro approaches. World Mycotoxin J. 2013;6:245–253. doi: 10.3920/WMJ2013.1596. [DOI] [Google Scholar]
- 139.Pulina G., Battacone G., Brambilla G., Cheli F., Danieli P.P., Masoero F., Pietri A., Ronchi B. An update on the safety of foods of animal origin and feeds. Ital. J. Anim. Sci. 2014;13:845–856. doi: 10.4081/ijas.2014.3571. [DOI] [Google Scholar]
- 140.Dunière L., Sindou J., Chaucheyras-Durand F., Chevallier I., Thévenot-Sergentet D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013;182:1–15. doi: 10.1016/j.anifeedsci.2013.04.006. [DOI] [Google Scholar]
- 141.Mojtahedi M. Effect of aflatoxin B1 on in vitro rumen microbial fermentation responses using batch culture. Annu. Rev. Res. Biol. 2013;3:686–693. [Google Scholar]
- 142.Westlake K., Mackie R.I., Dutton M.F. In vitro metabolism of mycotoxins by bacterial, protozoal and ovine ruminal fluid preparations. Anim. Feed Sci. Technol. 1989;25:169–178. doi: 10.1016/0377-8401(89)90117-X. [DOI] [Google Scholar]
- 143.Auerbach H., Maas R.F.M., Op Den Camp H.J.M., Pol A., Fink Gremmels J. Biodegradation of aflatoxin B1 by bovine rumen microorganisms in vitro and its effects on rumen fermentation; Proceedings of the Mycotox 98. Mycotoxins in Food Chain: Processing and toxicological aspects (Mycotox 98. Les Mycotoxines dans la Chaine Alimentaire: Aspects toxicologiques et technologiques); Toulouse, France. 2–4 July 1998. [Google Scholar]
- 144.Jiang Y.H., Yang H.J., Lund P. Effect of aflatoxin B1 on in vitro ruminal fermentation of rations high in alfalfa hay or ryegrass hay. Anim. Feed Sci. Technol. 2012;175:85–89. doi: 10.1016/j.anifeedsci.2012.03.021. [DOI] [Google Scholar]
- 145.Boguhn J., Neumann D., Helm A., Strobel E., Tebbe C.C., Dänicke S., Rodehutscorda M. Effects of concentrate proportion in the diet with or without Fusarium toxin-contaminated triticale on ruminal fermentation and the structural diversity of rumen microbial communities in vitro. Arch. Anim. Nutr. 2010;64:467–483. doi: 10.1080/1745039X.2010.511515. [DOI] [PubMed] [Google Scholar]
- 146.Hildebrand B., Boguhn J., Dänicke S., Rodehutscord M. Effect of Fusarium toxin-contaminated triticale and forage-to-concentrate ratio on fermentation and microbial protein synthesis in the rumen. J. Anim. Physiol. Anim. Nutr. Berl. 2012;96:307–318. doi: 10.1111/j.1439-0396.2011.01143.x. [DOI] [PubMed] [Google Scholar]
- 147.Jeong J.S., Lee J.H., Simizu Y., Tazaki H., Itabashi H., Kimura N. Effects of the Fusarium mycotoxin deoxynivalenol on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2010;162:144–148. doi: 10.1016/j.anifeedsci.2010.09.009. [DOI] [Google Scholar]
- 148.May H.D., Wu Q., Blake C.K. Effects of the Fusarium spp. mycotoxins fusaric acid and deoxynivalenol on the growth of Ruminococcus albus and Methanobrevibacter ruminantium. Can. J. Microbiol. 2000;46:692–699. doi: 10.1139/cjm-46-8-692. [DOI] [PubMed] [Google Scholar]
- 149.Morgavi D.P., Boudra H., Jouany J.P., Michalet-Doreau B. Effect and stability of gliotoxin, an Aspergillus fumigatus toxin, on in vitro rumen fermentation. Food Addit. Contam. 2004;21:871–878. doi: 10.1080/02652030400002188. [DOI] [PubMed] [Google Scholar]
- 150.Gurung N.K., Rankins D.L., Shelby R.A. In vitro ruminal disappearance of fumonisin B1 and its effects on in vitro dry matter disappearance. Vet. Hum. Toxicol. 1999;41:196–199. [PubMed] [Google Scholar]
- 151.Ozpinar H., Augonyte G., Drochner W. Inactivation of ochratoxin in ruminal fluid with variation of pH-value and fermentation parameters in an in vitro system. Environ. Toxicol. Pharmacol. 1999;7:1–9. doi: 10.1016/S1382-6689(98)00041-6. [DOI] [PubMed] [Google Scholar]
- 152.Escoula L. Patulin production by Penicillium granulatum and inhibition of ruminal flora. J. Environ. Pathol. Toxicol. Oncol. 1992;11:45–48. [PubMed] [Google Scholar]
- 153.Tapia M.O., Stern M.D., Soraci A.L., Meronuck R., Olson W., Gold S., Koski-Hulbert R.L., Murphy M.J. Patulin-producing molds in corn silage and high moisture corn and effects of patulin on fermentation by ruminal microbes in continuous culture. Anim. Feed Sci. Technol. 2005;119:247–258. doi: 10.1016/j.anifeedsci.2004.12.002. [DOI] [Google Scholar]
- 154.Morgavi D.P., Martin C., Boudra H. Fungal secondary metabolites from Monascus spp. reduce rumen methane production in vitro and in vivo. J. Anim. Sci. 2013;91:848–860. doi: 10.2527/jas.2012-5665. [DOI] [PubMed] [Google Scholar]
- 155.Cook W.O., Richard J.L., Osweiler G.D., Trampel D.W. Clinical and pathologic changes in acute bovine aflatoxicosis: Rumen motility and tissue and fluid concentrations of aflatoxins B1 and M1. Am. J. Vet. Res. 1986;47:1817–1825. [PubMed] [Google Scholar]
- 156.Garrett W.N., Heitman H., Booth A.N. Aflatoxin Toxicity in Beef Cattle. Exp. Biol. Med. 1968;127:188–190. doi: 10.3181/00379727-127-32652. [DOI] [PubMed] [Google Scholar]
- 157.Jones M.G., Ewart J.M. Effects on milk production associated with consumption of decorticated extracted groundnut meal contaminated with aflatoxin. Vet. Rec. 1979;105:492–493. doi: 10.1136/vr.105.21.492. [DOI] [PubMed] [Google Scholar]
- 158.Guthrie L.D., Bedell D.M. Effects of aflatoxin in corn on production and reproduction in dairy cattle. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1979;83:202–204. [PubMed] [Google Scholar]
- 159.Patterson D.S., Anderson P.H. Recent aflatoxin feeding experiments in cattle. Vet. Rec. 1982;110:60. doi: 10.1136/vr.110.3.60. [DOI] [PubMed] [Google Scholar]
- 160.Mertens D.R., Wyatt R.D. Acute aflatoxicosis in lactating dairy cows. J. Dairy Sci. 1977;60:153–154. [Google Scholar]
- 161.Applebaum R.S., Brackett R.E., Wiseman D.W., Marth E.H. Responses of dairy cows to dietary aflatoxin: Feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 1982;65:1503–1508. doi: 10.3168/jds.S0022-0302(82)82374-6. [DOI] [PubMed] [Google Scholar]
- 162.Lewis G., Markson L.M., Allcroft R. The effect of feeding toxic groundnut meal to sheep over a period of five years. Vet. Proc. 1967;80:312–314. doi: 10.1136/vr.80.9.312. [DOI] [PubMed] [Google Scholar]
- 163.Suliman H.B., Mohamed A.F., Awadelsied N.A., Shommein A.M. Acute mycotoxicosis in sheep: Field cases. Vet. Hum. Toxicol. 1987;29:241–243. [PubMed] [Google Scholar]
- 164.Harvey R.B., Kubena L.F., Phillips T.D., Corrier D.E., Elissalde M.H., Huff W.E. Diminution of aflatoxin toxicity to growing lambs by dietary supplementation with hydrated sodium calcium aluminosilicate. Am. J. Vet. Res. 1991;52:152–156. [PubMed] [Google Scholar]
- 165.Fernàndez A., Hernaindez M., Verde M., Sanz M. Effect of aflatoxin on performance, hematology, and clinical immunology in lambs. Can. J. Vet. Res. 2000;64:53–58. [PMC free article] [PubMed] [Google Scholar]
- 166.Gowda N.K.S., Suganthi R.U., Malathi V., Raghavendra A. Efficacy of heat treatment and sun drying of aflatoxin-contaminated feed for reducing the harmful biological effects in sheep. Anim. Feed Sci. Technol. 2007;133:167–175. doi: 10.1016/j.anifeedsci.2006.08.009. [DOI] [Google Scholar]
- 167.Tripathi M.K., Mondal D., Karim S.A. Growth, haematology, blood constituents and immunological status of lambs fed graded levels of animal feed grade damaged wheat as substitute of maize. J. Anim. Physiol. Anim. Nutr. Berl. 2008;92:75–85. doi: 10.1111/j.1439-0396.2007.00712.x. [DOI] [PubMed] [Google Scholar]
- 168.Edrington T.S., Harvey R.B., Kubena L.F. Effect of aflatoxin in growing lambs fed ruminally degradable or escape protein sources. J. Anim. Sci. 1994;72:1274–1281. doi: 10.2527/1994.7251274x. [DOI] [PubMed] [Google Scholar]
- 169.Masoero F., Gallo A., Moschini M., Piva G., Diaz D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal. 2007;1:1344–1350. doi: 10.1017/S1751731107000663. [DOI] [PubMed] [Google Scholar]
- 170.Abeni F., Migliorati L., Terzano G.M., Capelletti M., Gallo A., Masoero F., Pirlo G. Effects of two different blends of naturally mycotoxin-contaminated maize meal on growth and metabolic profile in replacement heifers. Animal. 2014;8:1667–1676. doi: 10.1017/S1751731114001475. [DOI] [PubMed] [Google Scholar]
- 171.Kiyothong K., Rowlinson P., Wanapat M., Khampa S. Effect of mycotoxin deactivator product supplementation on dairy cows. Anim. Prod. Sci. 2012;52:832–841. doi: 10.1071/AN11205. [DOI] [Google Scholar]
- 172.U.S. Enviromental Protection Agency (EPA) Aspergillus oryzae Final Risk Assessment, Biotechnology Program Under Toxic Substances Control Act (TSCA) [(accessed on 5 May 2015)]; Available online: http://www.epa.gov/biotech_rule/pubs/fra/fra007.htm.
- 173.James L.F., Hartley W.J., Williams M.C., van Kampen K.R. Field and experimental studies in cattle and sheep poisoned by nitro-bearing Astragalus or their toxins. Am. J. Vet. Res. 1980;41:377–382. [PubMed] [Google Scholar]
- 174.Keese C., Meyer U., Rehage J., Spilke J., Boguhn J., Breves G., Dänicke S. On the effects of the concentrate proportion of dairy cow rations in the presence and absence of a fusarium toxin-contaminated triticale on cow performance. Arch. Anim. Nutr. 2008;62:241–262. doi: 10.1080/17450390802066435. [DOI] [PubMed] [Google Scholar]
- 175.Keese C., Meyer U., Rehage J., Spilke J., Boguhn J., Breves G., Dänicke S. Ruminal fermentation patterns and parameters of the acid base metabolism in the urine as influenced by the proportion of concentrate in the ration of dairy cows with and without Fusarium toxin-contaminated triticale. Arch. Anim. Nutr. 2008;62:287–302. doi: 10.1080/17450390802066443. [DOI] [PubMed] [Google Scholar]
- 176.Charmley E., Trenholm H.L., Thompson B.K., Vudathala D., Nicholson J.W., Prelusky D.B., Charmley L.L. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. J. Dairy Sci. 1993;76:3580–3587. doi: 10.3168/jds.S0022-0302(93)77697-3. [DOI] [PubMed] [Google Scholar]
- 177.Ingalls J.R. Influence of deoxynivalenol on feed consumption by dairy cows. Anim. Feed Sci. Technol. 1996;60:297–300. doi: 10.1016/0377-8401(96)00984-4. [DOI] [Google Scholar]
- 178.Trenholm H.L., Thompson B.K., Martin K.E., Greenhalgh R., McAllister A.J. Ingestion of vomitoxin (Deoxynivalenol)-contaminated wheat by nonlactating dairy cows. J. Dairy Sci. 1985;68:1000–1005. doi: 10.3168/jds.S0022-0302(85)80921-8. [DOI] [PubMed] [Google Scholar]
- 179.Dänicke S., Matthäus K., Lebzien P., Valenta H., Stemme K., Ueberschär K.-H., Böhm J., Razzazi-Fazeli E., Flachowsky G. Effects of Fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. J. Anim. Physiol. Anim. Nutr. Berl. 2005;89:303–315. doi: 10.1111/j.1439-0396.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 180.Korosteleva S.N., Smith T.K., Boermans H.J. Effects of feedborne Fusarium mycotoxins on the performance, metabolism, and immunity of dairy cows. J. Dairy Sci. 2007;90:3867–3873. doi: 10.3168/jds.2007-0162. [DOI] [PubMed] [Google Scholar]
- 181.Korosteleva S.N., Smith T.K., Boermans H.J. Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows. J. Dairy Sci. 2009;92:1585–1593. doi: 10.3168/jds.2008-1267. [DOI] [PubMed] [Google Scholar]
- 182.Winkler J., Kersten S., Meyer U., Engelhardt U., Dänicke S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food Chem. Toxicol. 2014;65:196–204. doi: 10.1016/j.fct.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 183.Hochsteiner W., Schuh M., Luger K., Baumgartner W. Effect of mycotoxin contaminated feed on production parameters of dairy cows. Berl. Munchener Tierarztliche Wochenschr. 2000;113:14–21. [PubMed] [Google Scholar]
- 184.Harvey R.B., Edrington T.S., Kubena L.F., Elissalde M.H., Corrier D.E., Rottinghaus G.E. Effect of aflatoxin and diacetoxyscirpenol in ewe lambs. Bull. Environ. Contam. Toxicol. 1995;54:325–330. doi: 10.1007/BF00195100. [DOI] [PubMed] [Google Scholar]
- 185.Richard J.L., Meerdink G., Maragos C.M., Tumbleson M., Bordson G., Rice L.G., Ross P.F. Absence of detectable fumonisins in the milk of cows fed Fusarium proliferatum (Matsushima) Nirenberg culture material. Mycopathologia. 1996;133:123–126. doi: 10.1007/BF00439124. [DOI] [PubMed] [Google Scholar]
- 186.Osweiler G.D., Kehrli M.E., Stabel J.R., Thurston J.R., Ross P.F., Wilson T.M. Effects of fumonisin-contaminated corn screenings on growth and health of feeder calves. J. Anim. Sci. 1993;71:459–466. doi: 10.2527/1993.712459x. [DOI] [PubMed] [Google Scholar]
- 187.Mathur S. Fumonisin B1 is hepatotoxic and nephrotoxic in milk-fed calves. Toxicol. Sci. 2001;60:385–396. doi: 10.1093/toxsci/60.2.385. [DOI] [PubMed] [Google Scholar]
- 188.Edrington T.S., Harvey R.B., Kubena L.F., Elissalde M.H., Rottinghaus G.E., Rottinghaust G.E. Acute hepatic and renal toxicity in lambs dosed with culture material. J. Anim. Sci. 1995;73:508–515. doi: 10.2527/1995.732508x. [DOI] [PubMed] [Google Scholar]
- 189.Weaver G.A., Kurtz H.J., Behrens J.C., Robison T.S., Seguin B.E., Bates F.Y., Mirocha C.J. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986;47:1395–1397. [PubMed] [Google Scholar]
- 190.Weaver G.A., Kurtz H.J., Behrens J.C., Robison T.S., Seguin B.E., Bates F.Y., Mirocha C.J. Effect of zearalenone on dairy cows. Am. J. Vet. Res. 1986;47:1826–1828. [PubMed] [Google Scholar]
- 191.Coppock R.W., Mostrom M.S., Sparling C.G., Jacobsen B., Ross S.C. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet. Hum. Toxicol. 1990;32:246–248. [PubMed] [Google Scholar]
- 192.Smith J.F., di Menna M.E., McGowan L.T. Reproductive performance of Coopworth ewes following oral doses of zearalenone before and after mating. J. Reprod. Fertil. 1990;89:99–106. doi: 10.1530/jrf.0.0890099. [DOI] [PubMed] [Google Scholar]
- 193.Höhler D., Südekum K.H., Wolffram S., Frohlich A., Marquardt R.R., Ho D. Metabolism and excretion of ochratoxin A fed to sheep. J. Anim. Sci. 1999;77:1217–1223. doi: 10.2527/1999.7751217x. [DOI] [PubMed] [Google Scholar]
- 194.Mohr A.I., Lorenz I., Baum B., Hewicker-Trautwein M., Pfaffl M., Džidić A., Meyer H.H.D., Bauer J., Meyer K. Influence of oral application of mycophenolic acid on the clinical health status of sheep. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2007;54:76–81. doi: 10.1111/j.1439-0442.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 195.Dzidic A., Prgomet C., Mohr A., Meyer K., Bauer J., Meyer H.H.D., Pfaffl M.W. Effects of mycophenolic acid on inosine monophosphate dehydrogenase I and II mRNA expression in white blood cells and various tissues in sheep. J. Vet. Med. Ser. A. 2006;53:163–169. doi: 10.1111/j.1439-0442.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 196.Dzidic A., Meyer H.H.D., Bauer J., Pfaffl M.W. Long-term effects of mycophenolic acid on the immunoglobulin and inflammatory marker-gene expression in sheep white blood cells. Mycotoxin Res. 2010;26:235–240. doi: 10.1007/s12550-010-0061-8. [DOI] [PubMed] [Google Scholar]
- 197.Haggblom P. Isolation of roquefortine C from feed grain. Appl. Environ. Microbiol. 1990;56:2924–2926. doi: 10.1128/aem.56.9.2924-2926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lloyd W.E. Citrinin and ochratoxin toxicoses in cattle in the United States; Proceedings of the 2nd International Symposium of Veterinary Laboratory Diagnosticians; Lucerne, Switzerland. 24–26 June 1980; pp. 435–439. [Google Scholar]
- 199.Sabater-Vilar M., Maas R.F.M., de Bosschere H., Ducatelle R., Fink-Gremmels J. Patulin produced by an Aspergillus clavatus isolated from feed containing malting residues associated with a lethal neurotoxicosis in cattle. Mycopathologia. 2004;158:419–426. doi: 10.1007/s11046-005-2877-x. [DOI] [PubMed] [Google Scholar]
- 200.Pedrosa K., Borutova R. Synergistic effects of mycotoxins discussed. Feedstuffs Repr. 2011;83:1–3. [Google Scholar]
- 201.Speijers G.J.A., Speijers M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004;153:91–98. doi: 10.1016/j.toxlet.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 202.Ostry V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008;1:175–188. doi: 10.3920/WMJ2008.x013. [DOI] [Google Scholar]
- 203.Wang W., Jones C., Ciacci-Zanella J., Holt T., Gilchrist D.G., Dickman M.B. Fumonisins and Alternaria alternata lycopersici toxins: Sphinganine analog mycotoxins induce apoptosis in monkey kidney cells. Proc. Natl. Acad. Sci. USA. 1996;93:3461–3465. doi: 10.1073/pnas.93.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Uhlig S., Eriksen G.S., Hofgaard I.S., Krska R., Beltrán E., Sulyok M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins. 2013;5:1682–1697. doi: 10.3390/toxins5101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Streit E., Schwab C., Sulyok M., Naehrer K., Krska R., Schatzmayr G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins. 2013;5:504–523. doi: 10.3390/toxins5030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Santos R.R., Schoevers E.J., Wu X., Roelen B.A.J., Fink-Gremmels J. The protective effect of follicular fluid against the emerging mycotoxins alternariol and beauvericin. World Mycotoxin J. 2015;1:1–6. doi: 10.3920/WMJ2014.1829. [DOI] [Google Scholar]
- 207.Siciliano I., Ortu G., Gilardi G., Gullino M., Garibaldi A. Mycotoxin Production in Liquid Culture and on Plants Infected with Alternaria spp. Isolated from Rocket and Cabbage. Toxins. 2015;7:743–754. doi: 10.3390/toxins7030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.International Agency for Research on Cancer (IARC) Aflatoxins. IARC Summ. Evaluation. 2002;82:1–171. [Google Scholar]
- 209.Gallo A., Moschini M., Masoero F. Aflatoxins absorption in the gastro-intestinal tract and in the vaginal mucosa in lactating dairy cows. Ital. J. Anim. Sci. 2008;7:53–63. doi: 10.4081/ijas.2008.53. [DOI] [Google Scholar]
- 210.Battacone G., Nudda A., Rassu S.P.G., Decandia M., Pulina G. Excretion pattern of aflatoxin M1 in milk of goats fed a single dose of aflatoxin B1. J. Dairy Sci. 2012;95:2656–2661. doi: 10.3168/jds.2011-5003. [DOI] [PubMed] [Google Scholar]
- 211.Battacone G., Nudda A., Palomba M., Mazzette A., Pulina G. The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J. Dairy Sci. 2009;92:4997–5004. doi: 10.3168/jds.2008-1684. [DOI] [PubMed] [Google Scholar]
- 212.Veldman A., Meijs J.A.C., Borggreve G.J., Heeres-van der Tol J.J. Carry-over of aflatoxin from cows’ food to milk. Anim. Prod. 1992;55:163–168. doi: 10.1017/S0003356100037417. [DOI] [Google Scholar]
- 213.European Food Safety Authority (EFSA) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004;39:1–27. [Google Scholar]
- 214.Miller D.M., Wilson D.M., Eaton D.L., Groopman J.D. Veterinary diseases related to aflatoxins. In: Eaton D.L., Groopman J.D., editors. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. Academic Press; San Diego, CA, USA: 1993. pp. 347–364. [Google Scholar]
- 215.Pier A.C. Major biological consequences of aflatoxicosis in animal production. J. Anim. Sci. 1992;70:3964–3967. doi: 10.2527/1992.70123964x. [DOI] [PubMed] [Google Scholar]
- 216.Bernabucci U., Colavecchia L., Danieli P.P., Basiricò L., Lacetera N., Nardone A., Ronchi B. Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicol. Vitr. 2011;25:684–691. doi: 10.1016/j.tiv.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 217.Flores-Flores M.E., Lizarraga E., López de Cerain A., González-Peñas E. Presence of mycotoxins in animal milk: A review. Food Control. 2015;53:163–176. doi: 10.1016/j.foodcont.2015.01.020. [DOI] [Google Scholar]
- 218.Moschini M., Gallo A., Piva G., Masoero F. The effects of rumen fluid on the in vitro aflatoxin binding capacity of different sequestering agents and in vivo release of the sequestered toxin. Anim. Feed Sci. Technol. 2008;147:292–309. doi: 10.1016/j.anifeedsci.2008.01.010. [DOI] [Google Scholar]
- 219.Wu Q., Jezkova A., Yuan Z., Pavlikova L., Dohnal V., Kuca K. Biological degradation of aflatoxins. Drug Metab. Rev. 2009;41:1–7. doi: 10.1080/03602530802563850. [DOI] [PubMed] [Google Scholar]
- 220.Diaz D.E., Smith T.K. The Mycotoxin Blue Book. Nottingham University Press; Thrumpton, Nottingham, UK: 2005. Mycotoxin sequestering agents: Practical tools for the neutralisation of mycotoxins; pp. 323–339. [Google Scholar]
- 221.Kroll M., Arenzana-Seisdedos F., Bachelerie F., Thomas D., Friguet B., Conconi M. The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome. Chem. Biol. 1999;6:689–698. doi: 10.1016/S1074-5521(00)80016-2. [DOI] [PubMed] [Google Scholar]
- 222.Tsunawaki S., Yoshida L.S., Nishida S., Kobayashi T., Shimoyama T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 2004;72:3373–3382. doi: 10.1128/IAI.72.6.3373-3382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Whitlow L., Hagler W.M. Mycotoxins in dairy cattle: Occurrence, toxicity, prevention and treatment. Proc. Southwest Nutr. Conf. 2005:124–138. [Google Scholar]
- 224.Huang X., Chu F.S. Production and characterization of monoclonal and polyclonal antibodies against the mycotoxin cyclopiazonic acid. J. Agric. Food Chem. 1993;41:329–333. doi: 10.1021/jf00026a038. [DOI] [Google Scholar]
- 225.European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004:1–36. [Google Scholar]
- 226.Hult K., Teiling A., Gatenbeck S. Degradation of ochratoxin A by a ruminant. Appl. Environ. Microbiol. 1976;32:443–444. doi: 10.1128/aem.32.3.443-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Battacone G., Nudda A., Pulina G. Effects of ochratoxin a on livestock production. Toxins. 2010;2:1796–1824. doi: 10.3390/toxins2071796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mobashar M., Blank R., Hummel J., Westphal A., Tholen E., Südekum K.-H. Ruminal ochratoxin A degradation—Contribution of the different microbial populations and influence of diet. Anim. Feed Sci. Technol. 2012;171:85–97. doi: 10.1016/j.anifeedsci.2011.10.002. [DOI] [Google Scholar]
- 229.Müller H.M., Lerch C., Müller K., Eggert W. Kinetic profiles of ochratoxin A and ochratoxin alpha during in vitro incubation in buffered forestomach and abomasal contents from cows. Nat. Toxins. 1998;6:251–258. doi: 10.1002/(SICI)1522-7189(199811/12)6:6<251::AID-NT35>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 230.Sreemannarayana O., Frohlich A.A., Vitti T.G., Marquardt R.R., Abramson D. Studies of the Tolerance and Disposition of Ochratoxin a in Young Calves. J. Anim. Sci. 1988;66:1703–1711. doi: 10.2527/jas1988.6671703x. [DOI] [PubMed] [Google Scholar]
- 231.Blank R., Rolfs J.-P., Südekum K.-H., Frohlich A.A., Marquardt R.R., Wolffram S. Effects of chronic ingestion of ochratoxin a on blood levels and excretion of the mycotoxin in sheep. J. Agric. Food Chem. 2003;51:6899–6905. doi: 10.1021/jf034547j. [DOI] [PubMed] [Google Scholar]
- 232.Denli M., Perez J.F. Ochratoxins in feed, a risk for animal and human health: Control strategies. Toxins. 2010;2:1065–1077. doi: 10.3390/toxins2051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Boudra H., Saivin S., Buffiere C., Morgavi D.P. Short communication: Toxicokinetics of ochratoxin A in dairy ewes and carryover to milk following a single or long-term ingestion of contaminated feed. J. Dairy Sci. 2013;96:6690–6696. doi: 10.3168/jds.2013-6707. [DOI] [PubMed] [Google Scholar]
- 234.Ribelin W.E., Fukushima K., Still P.E. The toxicity of ochratoxin to ruminants. Can. J. Comp. Med. 1978;42:172–176. [PMC free article] [PubMed] [Google Scholar]
- 235.Niederberger M., Oevermann A., Kirscher F., Meylan M. Tremorgenic syndrome in a cattle herd after feeding silage contaminated with A. clavatus. Schweiz. Arch. Tierheilkd. 2011;153:105–110. doi: 10.1024/0036-7281/a000163. [DOI] [PubMed] [Google Scholar]
- 236.Suzuki T., Takeda M., Tanabe H. A New Mycotoxin produced by Aspergillus clavatus. Chem. Pharm. Bull. Tokyo. 1971;19:1786–1788. doi: 10.1248/cpb.19.1786. [DOI] [PubMed] [Google Scholar]
- 237.Voss K.A., Smith G.W., Haschek W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007;137:299–325. doi: 10.1016/j.anifeedsci.2007.06.007. [DOI] [Google Scholar]
- 238.Guerre P. Fusariotoxins in avian species: Toxicokinetics, metabolism and persistence in tissues. Toxins. 2015;7:2289–2305. doi: 10.3390/toxins7062289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S., Haesebrouck F., van Immerseel F., Croubels S. The impact of Fusarium Mycotoxins on human and animal host susceptibility to infectious diseases. Toxins. 2014;6:430–452. doi: 10.3390/toxins6020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Marczuk J., Obremski K., Lutnicki K., Gajecka M., Gajecki M. Zearalenone and deoxynivalenol mycotoxicosis in dairy cattle herds. Pol. J. Vet. Sci. 2012;15:365–372. doi: 10.2478/v10181-012-0055-x. [DOI] [PubMed] [Google Scholar]
- 241.Morgavi D.P., Riley R.T. An historical overview of field disease outbreaks known or suspected to be caused by consumption of feeds contaminated with Fusarium toxins. Anim. Feed Sci. Technol. 2007;137:201–212. doi: 10.1016/j.anifeedsci.2007.06.002. [DOI] [Google Scholar]
- 242.European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004;73:1–42. [Google Scholar]
- 243.Côté L.M., Dahlem A.M., Yoshizawa T., Swanson S.P., Buck W.B. Excretion of deoxynivalenol and its metabolite in milk, urine, and feces of lactating dairy cows. J. Dairy Sci. 1986;69:2416–2423. doi: 10.3168/jds.S0022-0302(86)80681-6. [DOI] [PubMed] [Google Scholar]
- 244.Seeling K., Lebzien P., Dänicke S., Spilke J., Südekum K.-H., Flachowsky G. Effects of level of feed intake and Fusarium toxin-contaminated wheat on rumen fermentation as well as on blood and milk parameters in cows. J. Anim. Physiol. Anim. Nutr. Berl. 2006;90:103–115. doi: 10.1111/j.1439-0396.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 245.Hedman R., Pettersson H. Transformation of nivalenol by gastrointestinal microbes. Arch. Tierernahr. 1997;50:321–329. doi: 10.1080/17450399709386142. [DOI] [PubMed] [Google Scholar]
- 246.European Food Safety Authority (EFSA) Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed1. EFSA J. 2013;11:1–119. [Google Scholar]
- 247.European Food Safety Authority (EFSA) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011;9:1–187. [Google Scholar]
- 248.Hsu I.C., Smalley E.B., Strong F.M., Ribelin W.E. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl. Microbiol. 1972;24:684–690. doi: 10.1128/am.24.5.684-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Weaver G.A., Kurtz H.J., Mirocha C.J., Bates F.Y., Behrens J.C., Robison T.S., Swanson S.P. The failure of purified T-2 mycotoxin to produce hemorrhaging in dairy cattle. Can. Vet. J. 1980;21:210–213. [PMC free article] [PubMed] [Google Scholar]
- 250.Mann D.D., Buening G.M., Hook B., Osweiler G.D. Effects of T-2 mycotoxin on bovine serum proteins. Am. J. Vet. Res. 1983;44:1757–1759. [PubMed] [Google Scholar]
- 251.Mann D.D., Buening G.M., Osweiler G.D., Hook B.S. Effect of subclinical levels of T-2 toxin on the bovine cellular immune system. Can. J. Comp. Med. 1984;48:308–312. [PMC free article] [PubMed] [Google Scholar]
- 252.Buening G.M., Mann D.D., Hook B., Osweiler G.D. The effect of T-2 toxin on the bovine immune system: Cellular factors. Vet. Immunol. Immunopathol. 1982;3:411–417. doi: 10.1016/0165-2427(82)90023-X. [DOI] [PubMed] [Google Scholar]
- 253.Yoshizawa T., Mirocha C.J., Behrens J.C., Swanson S.P. Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet. Toxicol. 1981;19:31–39. doi: 10.1016/0015-6264(81)90300-X. [DOI] [PubMed] [Google Scholar]
- 254.Alm K., Dahlbom M., Säynäjärvi M., Andersson M.A., Salkinoja-Salonen M.S., Andersson M.C. Impaired semen quality of AI bulls fed with moldy hay: A case report. Theriogenology. 2002;58:1497–1502. doi: 10.1016/S0093-691X(02)01079-8. [DOI] [PubMed] [Google Scholar]
- 255.Spotti M., Caloni F., Fracchiolla L., Pompa G., Vigo D., Maffeo G. Fumonisin B1 carry-over into milk in the isolated perfused bovine udder. Vet. Hum. Toxicol. 2001;43:109–111. [PubMed] [Google Scholar]
- 256.European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to fumonisins as undesirable substances in animal feed. EFSA J. 2005;235:1–32. [Google Scholar]
- 257.Caloni F., Spotti M., Auerbach H., Op den Camp H., Gremmels J.F., Pompa G. In vitro metabolism of fumonisin B1 by ruminal microflora. Vet. Res. Commun. 2000;24:379–387. doi: 10.1023/A:1006422200226. [DOI] [PubMed] [Google Scholar]
- 258.Kennedy D.G., Hewitt S.A., McEvoy J.D., Currie J.W., Cannavan A., Blanchflower W.J., Elliot C.T. Zeranol is formed from Fusarium spp. toxins in cattle in vivo. Food Addit. Contam. 1998;15:393–400. doi: 10.1080/02652039809374658. [DOI] [PubMed] [Google Scholar]
- 259.Minervini F., Dell’Aquila M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008;9:2570–2584. doi: 10.3390/ijms9122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to Zearalenone as undesirable substance in animal feed. EFSA J. 2004;89:1–35. [Google Scholar]
- 261.Fushimi Y., Takagi M., Hasunuma H., Uno S., Kokushi E., Watanabe U., Liu J., Marey M.A., Miyamoto A., Otoi T., et al. Application of mycotoxin adsorbent to cattle feed contaminated with zearalenone: Urinary zearalenone excretion and association with anti-Müllerian hormone. World Mycotoxin J. 2014;7:367–378. doi: 10.3920/WMJ2013.1672. [DOI] [Google Scholar]
- 262.Takagi M., Uno S., Kokushi E., Shiga S., Mukai S., Kuriyagawa T., Takagaki K., Hasunuma H., Matsumoto D., Okamoto K., et al. Measurement of urinary zearalenone concentrations for monitoring natural feed contamination in cattle herds: On-farm trials. J. Anim. Sci. 2011;89:287–296. doi: 10.2527/jas.2010-3306. [DOI] [PubMed] [Google Scholar]
- 263.Takagi M., Hirai T., Shiga S., Uno S., Kokushi E. Relationship between urinary zearalenone concentration and embryo production in superovulated cattle. Arch. Tierernahr. 2013;56:360–366. [Google Scholar]
- 264.Pereyra M.L.G., Alonso V.A., Sager R., Morlaco M.B., Magnoli C.E., Astoreca A.L., Rosa C.A.R., Chiacchiera S.M., Dalcero A.M., Cavaglieri L.R. Fungi and selected mycotoxins from pre- and postfermented corn silage. J. Appl. Microbiol. 2008;104:1034–1041. doi: 10.1111/j.1365-2672.2007.03634.x. [DOI] [PubMed] [Google Scholar]
- 265.Veselý D., Veselá D., Adámková M. Occurrence of PR-toxin-producing Penicillium roqueforti in corn silage. Vet. Med. Praha. 1981;26:109–115. [PubMed] [Google Scholar]
- 266.Oh S.Y., Balch C.G., Cliff R.L., Sharma B.S., Boermans H.J., Swamy H.V.L.N., Quinton V.M., Karrow N.A. Exposure to Penicillium mycotoxins alters gene expression of enzymes involved in the epigenetic regulation of bovine macrophages (BoMacs) Mycotoxin Res. 2013;29:235–243. doi: 10.1007/s12550-013-0174-y. [DOI] [PubMed] [Google Scholar]
- 267.Solfrizzo M., Gambacorta L., Lattanzio V.M.T., Powers S., Visconti A. Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal. Bioanal. Chem. 2011;401:2831–2841. doi: 10.1007/s00216-011-5354-z. [DOI] [PubMed] [Google Scholar]
- 268.Meky F., Turner P.C., Ashcroft A.E., Miller J.D., Qiao Y.-L., Roth M.J., Wild C.P. Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem. Toxicol. 2003;41:265–273. doi: 10.1016/S0278-6915(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 269.Ediage E.N., di Mavungu J.D., Song S., Wu A., van Peteghem C., de Saeger S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta. 2012;741:58–69. doi: 10.1016/j.aca.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 270.Choi J., Mørck T.A., Polcher A., Knudsen L.E., Joas A. Review of the state of the art of human biomonitoring for chemical substances and its application to human exposure assessment for food. EFSA J. 2015:1–321. [Google Scholar]
- 271.Solfrizzo M., Gambacorta L., Visconti A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins. 2014;6:523–538. doi: 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tiwary A.K., Puschner B., Poppenga R.H. Using roquefortine C as a biomarker for penitrem A intoxication. J. Vet. Diagn. Investig. 2009;21:237–239. doi: 10.1177/104063870902100210. [DOI] [PubMed] [Google Scholar]
- 273.Pedrosa K., Griessler K. Toxicity, occurrence and negative effects of PR toxin—The hidden enemy. [(accessed on 13 May 2015)]. Available online: https://www.yumpu.com/en/document/view/34544639/download.
- 274.European Food Safety Authority (EFSA) Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012;10:1–82. [Google Scholar]
- 275.Bouslimi A., Bouaziz C., Ayed-Boussema I., Hassen W., Bacha H. Individual and combined effects of ochratoxin A and citrinin on viability and DNA fragmentation in cultured Vero cells and on chromosome aberrations in mice bone marrow cells. Toxicology. 2008;251:1–7. doi: 10.1016/j.tox.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 276.Griffiths I., Done S. Citrinin as a possible cause of the pruritis, pyrexia, haemorrhagic syndrome in cattle. Vet. Rec. 1991;129:113–117. doi: 10.1136/vr.129.6.113. [DOI] [PubMed] [Google Scholar]
- 277.Stec J.A.N., Rachubik J.Ł.A.W., Szczotka M., Mak J.K.U.Ź. Effects of penicillium mycotoxins: Citrin, ochratoxin A, and patulin on in vitro proliferation of bovine lymphocytes. Bull. Vet. Inst. Pulawy. 2008;52:163–167. [Google Scholar]
- 278.Grenier B., Applegate T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bouhet S., Oswald I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005;108:199–209. doi: 10.1016/j.vetimm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 280.Maresca M., Fantini J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon. 2010;56:282–294. doi: 10.1016/j.toxicon.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 281.Cleveland T.E., Dowd P.F., Desjardins A.E., Bhatnagar D., Cotty P.J. United States Department of Agriculture—Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 2003;59:629–642. doi: 10.1002/ps.724. [DOI] [PubMed] [Google Scholar]
- 282.Shinha K.K., Bhatnagar D. Mycotoxins in Agriculture and Food Safety. Marcel Dekker, Inc.; New York, NY, USA: 1998. [Google Scholar]
- 283.Eeckhout M., Landschoot S., Deschuyffeleer N., de Laethauwer S., Haesaert G. Guidelines for prevention and control of mould growth and mycotoxin production in cereals. [(accessed on 14 June 2015)]. Available online: http://synagra.be/Download.ashx?ID=6421.
- 284.Food and Agriculture Organization of the United Nations (FAO) [(accessed on 14 July 2004)]. Available online: http://www.codexalimentarius.org/input/download/standards/10099/CXP_056e.pdf.
- 285.Jard G., Liboz T., Mathieu F., Guyonvarc’h A., Lebrihi A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. A. 2011;28:1590–1609. doi: 10.1080/19440049.2011.595377. [DOI] [PubMed] [Google Scholar]
- 286.Edwards S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004;153:29–35. doi: 10.1016/j.toxlet.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 287.Schrödter R. Influence of harvest and storage conditions on trichothecenes levels in various cereals. Toxicol. Lett. 2004;153:47–49. doi: 10.1016/j.toxlet.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 288.Chulze S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. A. 2010;27:651–657. doi: 10.1080/19440040903573032. [DOI] [PubMed] [Google Scholar]
- 289.Sabatini A., Danieli P.P., Bernabucci U., Ronchi B. Evaluation of mycotoxins contamination in intensive beef cattle production system. Italy J. Anim. Sci. 2010;6:466–468. [Google Scholar]
- 290.Oude Elferink S.J.W.H., Driehuis F., Gottschal J., Spoelstra S. Paper 2.0: Silage fermentation processes and their manipulation. FAO Plant P. 2000;161:17–30. [Google Scholar]
- 291.Muck R. Recent advances in silage microbiology. Agric. Food Sci. 2013;22:3–15. [Google Scholar]
- 292.Ali M., Cone J.W., Khan N.A., Hendriks W.H., Struik P.C. Effect of temperature and duration of ensiling on in vitro degradation of maize silages in rumen fluid. J. Anim. Physiol. Anim. Nutr. Berl. 2015;99:251–257. doi: 10.1111/jpn.12244. [DOI] [PubMed] [Google Scholar]
- 293.Orosz S., Wilkinson J.M., Wigley S., Bíró Z., Galló J. Microbial status, aerobic stability and fermentation of maize silage sealed with an oxygen barrier film or standard polyethylene film. Agric. Food Sci. 2013;22:182–188. [Google Scholar]
- 294.Kung L., Sheperd A.C., Smagala A.M., Endres K.M., Bessett C.A., Ranjit N.K., Glancey J.L. The effect of preservatives based on propionic acid on the fermentation and aerobic stability of corn silage and a total mixed ration. J. Dairy Sci. 1998;81:1322–1330. doi: 10.3168/jds.S0022-0302(98)75695-4. [DOI] [PubMed] [Google Scholar]
- 295.Ranjit N.K., Kung L. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000;83:526–535. doi: 10.3168/jds.S0022-0302(00)74912-5. [DOI] [PubMed] [Google Scholar]
- 296.European Food Safety Authority (EFSA) Scientific Opinion on the safety and efficacy of propionic acid, sodium propionate, calcium propionate and ammonium propionate for all animal species. EFSA J. 2011;9:1–22. [Google Scholar]
- 297.Tabacco E., Piano S., Revello-Chion A., Borreani G. Effect of Lactobacillus buchneri LN4637 and Lactobacillus buchneri LN40177 on the aerobic stability, fermentation products, and microbial populations of corn silage under farm conditions. J. Dairy Sci. 2011;94:5589–5598. doi: 10.3168/jds.2011-4286. [DOI] [PubMed] [Google Scholar]
- 298.Rust S.R., Kim H.S., Enders G.L. Effects of a microbial inoculant on fermentation characteristics and nutritional value of corn silage. J. Prod. Agric. 1989;2:235–241. doi: 10.2134/jpa1989.0235. [DOI] [Google Scholar]
- 299.Weinberg Z.G., Ashbell G., Hen Y., Azrieli A. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J. Appl. Bacteriol. 1993;75:512–518. doi: 10.1111/j.1365-2672.1993.tb01588.x. [DOI] [Google Scholar]
- 300.EFSA Scientific Opinion on the safety and efficacy of Lactobacillus brevis (DSM 23231), Lactobacillus buchneri (DSM 22501), Lactobacillus buchneri (NCIMB 40788—CNCM I—4323), Lactobacillus buchneri (ATCC PTA—6138) and Lactobacillus buchneri (ATCC PPTA—2) EFSA J. 2013;11:1–16. [Google Scholar]
- 301.Woolford M.K. The Silage Fermentation. Marcel Dekker, Inc.; NewYork, NY, USA: 1984. [Google Scholar]
- 302.González Pereyra M.L., Chiacchiera S.M., Rosa C.A., Sager R., Dalcero A.M., Cavaglieri L. Comparative analysis of the mycobiota and mycotoxins contaminating corn trench silos and silo bags. J. Sci. Food Agric. 2011;91:1474–1481. doi: 10.1002/jsfa.4336. [DOI] [PubMed] [Google Scholar]
- 303.Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. [(accessed on 14 June 2009)]. Available online: http://www.efsa.europa.eu/it/supporting/doc/22e.pdf.
- 304.Awad W.A., Ghareeb K., Bohm J., Zentek J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2010;27:510–520. doi: 10.1080/19440040903571747. [DOI] [PubMed] [Google Scholar]
- 305.Galvano F., Piva A., Ritieni A., Galvano G. Dietary Strategies to Counteract the Effects of Mycotoxins: A Review. J. Food Prot. 2001;12:120–131. doi: 10.4315/0362-028x-64.1.120. [DOI] [PubMed] [Google Scholar]
- 306.Gallo A., Masoero F., Bertuzzi T., Piva G., Pietri A. Effect of the inclusion of adsorbents on aflatoxin B1 quantification in animal feedstuffs. Food Addit. Contam. A. 2010;27:54–63. doi: 10.1080/02652030903207219. [DOI] [PubMed] [Google Scholar]
- 307.Ramos A.J., Hernández E. Prevention of aflatoxicosis in farm animals by Means of hydrated sodium calcium aluminosilicate addition to feedstuffs: A review. Anim. Feed Sci. Technol. 1997;65:197–206. doi: 10.1016/S0377-8401(96)01084-X. [DOI] [Google Scholar]
- 308.Masoero F., Gallo A., Diaz D., Piva G., Moschini M. Effects of the procedure of inclusion of a sequestering agent in the total mixed ration on proportional aflatoxin M1 excretion into milk of lactating dairy cows. Anim. Feed Sci. Technol. 2009;150:34–45. doi: 10.1016/j.anifeedsci.2008.07.009. [DOI] [Google Scholar]
- 309.Smith E.E., Phillips T.D., Ellis J.A., Harvey R.B., Kubena L.F., Thompson J., Newton G. Dietary hydrated sodium calcium aluminosilicate reduction of aflatoxin M1 residue in dairy goat milk and effects on milk production and components. J. Anim. Sci. 1994;72:677–682. doi: 10.2527/1994.723677x. [DOI] [PubMed] [Google Scholar]
- 310.Phillips T.D., Clement B.A., Kubena L.F., Harvey R.B. Detection and detoxification of aflatoxins: Prevention of aflatoxicosis and aflatoxin residues with hydrated sodium calcium aluminosilicate. Vet. Hum. Toxicol. 1990;32(Suppl.):15–19. [PubMed] [Google Scholar]
- 311.Kutz R.E., Sampson J.D., Pompeu L.B., Ledoux D.R., Spain J.N., Vázquez-Añón M., Rottinghaus G.E. Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J. Dairy Sci. 2009;92:3959–3963. doi: 10.3168/jds.2009-2031. [DOI] [PubMed] [Google Scholar]
- 312.Queiroz O.C.M., Han J.H., Staples C.R., Adesogan A.T. Effect of adding a mycotoxin-sequestering agent on milk aflatoxin M1 concentration and the performance and immune response of dairy cattle fed an aflatoxin B1 contaminated diet. J. Dairy Sci. 2012;95:5901–5908. doi: 10.3168/jds.2011-5287. [DOI] [PubMed] [Google Scholar]
- 313.Galvano F., Pietri A., Bertuzzi T., Bognanno M., Chies L., de Angelis A., Galvano M. Activated Carbons: In Vitro Affinity for Fumonisin B1 and Relation of Adsorption Ability to Physicochemical Parameters. J. Food Prot. 1997;7:985–991. doi: 10.4315/0362-028X-60.8.985. [DOI] [PubMed] [Google Scholar]
- 314.Diaz D.E., Hagler W.M., Blackwelder J.T., Eve J.A., Hopkins B.A., Anderson K.L., Jones F.T., Whitlow L.W. Aflatoxin Binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia. 2004;157:233–241. doi: 10.1023/B:MYCO.0000020587.93872.59. [DOI] [PubMed] [Google Scholar]
- 315.Pietri A., Bertuzzi T., Piva G., Binder E.M., Schatzmayr D., Rodrigues I. Aflatoxin Transfer from Naturally Contaminated Feed to Milk of Dairy Cows and the Efficacy of a Mycotoxin Deactivating Product. Int. J. Dairy Sci. 2009;4:34–42. [Google Scholar]
- 316.Grenier B., Bracarense A.-P.F.L., Schwartz H.E., Lucioli J., Cossalter A.-M., Moll W.-D., Schatzmayr G., Oswald I.P. Biotransformation approaches to alleviate the effects induced by fusarium mycotoxins in swine. J. Agric. Food Chem. 2013;61:6711–6719. doi: 10.1021/jf400213q. [DOI] [PubMed] [Google Scholar]
- 317.Polonelli L., Giovati L., Magliani W., Conti S., Sforza S., Calabretta A., Casoli C., Ronzi P., Grilli E., Gallo A., et al. Vaccination of lactating dairy cows for the prevention of aflatoxin B 1 carry over in milk. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0026777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Döll S., Dänicke S., Valenta H., Flachowsky G. ro studies on the evaluation of mycotoxin detoxifying agents for their efficacy on deoxynivalenol and zearalenone. Arch. Anim. Nutr. 2007;58:311–324. doi: 10.1080/00039420412331273268. [DOI] [PubMed] [Google Scholar]
- 319.Kong C., Shin S., Kim B. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: An in vitro approach. Springerplus. 2014;3:346–350. doi: 10.1186/2193-1801-3-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tomašević-Čanović M., Daković A., Rottinghaus G., Matijašević S., Duričić M. Surfactant modified zeolites-new efficient adsorbents for mycotoxins. Microporous Mesoporous Mater. 2003;61:173–180. doi: 10.1016/S1387-1811(03)00365-2. [DOI] [Google Scholar]
- 321.Hahn I., Kunz-Vekiru E., Twarużek M., Grajewski J., Krska R., Berthiller F. Aerobic and anaerobic in vitro testing of feed additives claiming to detoxify deoxynivalenol and zearalenone. Food Addit. Contam. A. 2015:1–12. doi: 10.1080/19440049.2015.1023741. [DOI] [PubMed] [Google Scholar]
- 322.Avantaggiato G., Solfrizzo M., Visconti A. Recent advances on the use of adsorbent materials for detoxification of Fusarium mycotoxins. Food Addit. Contam. 2005;22:379–388. doi: 10.1080/02652030500058312. [DOI] [PubMed] [Google Scholar]