Abstract
AIM: To investigate the protective effect of paricalcitol and enalapril on renal inflammation and oxidative stress in ApoE-knock out mice.
METHODS: Animals treated for 4 mo as group (1) ApoE-knock out plus vehicle, group (2) ApoE-knock out plus paricalcitol (200 ng thrice a week), (3) ApoE-knock out plus enalapril (30 mg/L), (4) ApoE-knock out plus paricalcitol plus enalapril and (5) normal. Blood pressure (BP) was recorded using tail cuff method. The kidneys were isolated for biochemical assays using spectrophotometer and Western blot analyses.
RESULTS: ApoE-deficient mice developed high BP (127 ± 3 mmHg) and it was ameliorated by enalapril and enalapril plus paricalcitol treatments but not with paricalcitol alone. Renal malondialdehyde concentrations, p22phox, manganese-superoxide dismutase, inducible nitric oxide synthase (NOS), monocyte chemoattractant protein-1, tumor necrosis factor-alpha and transforming growth factor-β1 levels significantly elevated but reduced glutathione, CuZn-SOD and eNOS levels significantly depleted in ApoE-knock out animals compared to normal. Administration of paricalcitol, enalapril and combined together ameliorated the renal inflammation and oxidative stress in ApoE-knock out animals.
CONCLUSION: Paricalcitol and enalapril combo treatment ameliorates renal inflammation as well as oxidative stress in atherosclerotic animals.
Keywords: Atherosclerosis, Enalapril, Paricalcitol, Renal inflammation, Oxidative stress
Core tip: Although the protective efficacy of vitamin D and angiotensin converting enzyme inhibitors (ACEIs) have been studied in the cardiovascular system of atherosclerotic mice. However this is the first report to investigate the renal protection by paricalcitol and enalapril, alone or in combination in ApoE-deficient atherosclerotic mice. This innovative study clearly shows that vitamin D, ACEI and their combo ameliorated the renal inflammation and oxidative stress in ApoE-knock out animals by depleting the inflammatory and oxidative stress markers as well as restoring the renal antioxidant defense system in atherosclerotic mice. The combination of paricalcitol and enalapril warrants the clinical usefulness in renal atherosclerotic patients.
INTRODUCTION
Atherosclerosis is implicated of morbidity and mortality in patients with cardiorenal diseases worldwide. Epidemiological as well as clinical investigations demonstrated a strong relationship between vitamin D deficiency and cardiorenal risk factors such as hypertension, chronic kidney disease (CKD), diabetes mellitus, atherosclerosis, myocardial infarction, stroke, and congestive heart failure[1,2]. It could be suggested that vitamin D deficiency contributes to the development of renal and cardiovascular diseases via its strong association with risk factors such as diabetes, hyperlipidemia and hypertension[3-5]. However, direct effects of vitamin D on the renal and cardiovascular system may also be involved. Activation of vitamin D receptors not only regulates the parathyroid hormone but also alters the renin-angiotensin system (RAS), cardiac hypertrophy, inflammation, and calcium levels[6-12]. The molecular mechanism for how vitamin D may improve cardiorenal disease outcomes, specifically cardiorenal atherosclerotic lesions, is not well understood.
A number of studies documented an essential role of RAS in both cardiovascular pathophysiology, including atherosclerosis, and renal pathophysiology[13-15]. RAS inhibitors and blockers have therapeutic potentials in chronic cardiorenal dysfunctions[13,16-18]. Angiotensin II causes oxidants/antioxidants imbalance in the kidney, heart and vascular system inducing reactive oxygen species (ROS) production via the activation of NADPH oxidase[19,20]. These ROS initiate vascular membrane lipid peroxidation leading to inflammation and the generation of inflammatory cytokines (TNF-α) through NF-κB activation[21,22] and other mediators such as vascular cell adhesion molecule-1 (VCAM-1), MCP-1, TGF- β1, Matrix metalloproteinase 9 (MMP9), iNOS and Mn-SOD[23-25]. ROS oxidize cellular biomolecules (lipids, proteins and nucleic acids) leading to renal, heart and vascular impairments[26]. But the cellular ROS are scavenged by endogenous antioxidants including antioxidant enzymes such as SOD, catalase, and glutathione peroxidase (GSHPx), reduced glutathione (GSH) and vitamins A, C, and E[27]. Most importantly, depletion of a major cellular antioxidant such as GSH has been reported to cause renal and cardiovascular dysfunction in rats [28,29]. Antioxidant therapy has been shown to ameliorate renal and cardiovascular oxidative stress by scavenging excess ROS and upregulating the antioxidant defense system[27,30]. However, since the protective mechanisms of angiotensin converting enzyme inhibitors (ACEIs) in the atherosclerotic lesions of mouse kidney are not completely understood, we set out to investigate this aspect in the present study.
Paricalcitol, a vitamin D analog, has been associated not only with the regulation of early stages of atherogenesis but also with vascular calcification. Treatments with vitamin D analogs at therapeutic dosages ameliorate the secondary elevation of parathyroid hormones and vascular calcium levels[31-33]. In clinical studies, paricalcitol reduced proteinuria, inflammation, and the mortality rate in CKD patients[34-36]. Our recent study demonstrated that when paricalcitol combined with enalapril ameliorated the oxidative cardiovascular injury by suppressing ROS-generating enzyme NADPH oxidase activity and by upregulating the antioxidant defense system in a uremic rat model and mouse model of atherosclerosis[29,37-39]. It is not known, however, whether vitamin D analogs can protect against inflammatory and oxidative stress in the kidney of atherosclerotic mice. RAS is implicated in atherosclerosis and vitamin D suppresses it, the combination of vitamin D and RAS inhibitor most likely elevated the therapeutic potentials in the kidney of ApoE-deficient mice. Therefore, the present study aimed to investigate the protective efficacy of a vitamin D analog, i.e., paricalcitol, and an ACE inhibitor, i.e., enalapril on inflammation and oxidants/antioxidants imbalance in the kidney of ApoE-deficient atherosclerotic mice.
MATERIALS AND METHODS
Animals
Sixty female mice (20-25 g) were procured from (Taconic Company, Hudson, NY, United States). Mice were given food and water ad libitum. They were maintained at a room temperature (25 °C with 50% humidity) and on 12/12-h light/dark cycle. After one week acclimatization they were separated into five groups and treated as follows:
Group 1 (ApoE deficient + vehicle): Mice were administered a vehicle (100 μL of propylene glycol, i.p.) 3 times a week for 16 wk (n = 12).
Group 2 (ApoE deficient + paricalcitol): Mice were given paricalcitol (200 ng in propylene glycol, i.p.) 3 times a week for 16 wk (n = 12).
Group 3 (ApoE deficient + enalapril): Mice were given enalapril (30 mg/kg) in their drinking water for 16 wk (n =12).
Group 4 (ApoE deficient + paricalcitol + enalapril): Mice were given paricalcitol + enalapril for 16 wk (n = 12).
Group 5 (wild-type normal control): Wild-type control mice were given vehicle only 3 times a week for 16 wk (n = 12).
The use of ApoE-deficient mice as a model of atherosclerotic lesions and drug treatment were reported earlier[40,41]. BP was monitored after drug treatment using a Non-Invasive Blood Pressure System NIBP800 (Columbus Instruments, Columbus, Ohio, United States) as described earlier[37-39]. After 16 wk, mice were euthanized by decapitation and kidneys were isolated. The kidneys (n = 6) from each group of mice were rinsed with PBS, immediately placed in liquid nitrogen then preserved at -80 °C. The remaining kidneys (n = 6) from each group of mice were fixed in buffered formalin for histological study. All Procedures involving animals were reviewed including care to minimize the pain and discomfort by the Ponce School of Medicine’s Institutional Animal Care and Use Committee (IACUC). The animal protocol #LF-07 was approved by the committee. The drugs were obtained from Sigma Chemical Company (St. Louis, Mo, United States) and Abbott Pharmaceuticals (Abbott Park, IL, United States).
Determination of glutathione
Glutathione (GSH) was determined as previously described[37-39] using commercial kit (Cayman Company, Ann Arbor, MI, United States).
Lipid peroxidation assay
The end product of lipid peroxidation malondialdehyde (MDA) levels were estimated according to Ohkawa and colleague’s method[42]. For the standard, 1, 1, 3, 3-tetraethoxypropane was used.
Protein assay
Total protein levels were determined using Coomassie reagent according to the method of Read and Northcole[43]. Bovine serum albumin (BSA) was used as the standard.
Protein extraction
Total protein extraction from frozen tissues was performed using M-PER buffer (Pierce, Rockford, IL, United States).
Western blot analysis
Total proteins (40 μg) was resolved by 12.5% SDS polyacrylamide gel electrophoresis (SDS PAGE) running gel and a 5% stacking gel as described previously[36-38].
Statistical analysis
The data are represented as mean ± SEM. The data were statistically analyzed by one-way analysis of variance (ANOVA) followed by a post-hoc, Schaffer test. P < 0.05 was considered significant. The statistical analysis of the data has been reviewed by Dr. Hernandez of Ponce School of Medicine.
RESULTS
As depicted in Figure 1, ApoE-deficient atherosclerotic mice in group 1 receiving vehicle alone developed significant hypertension (increased mean BP) compared to normal wild type controls in group 5 (P < 0.05) after 16 wk of treatment. Treatment with enalapril or enalapril in combination with paricalcitol for 16 wk prevented the increase in mean BP (P < 0.05). However, paricalcitol alone treatment for 16 wk did not lower the mean BP of mice in group 2.
Figure 1.
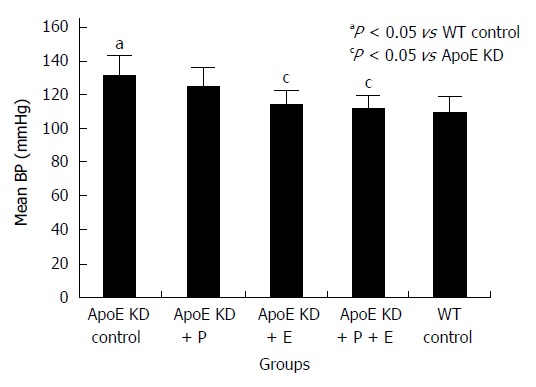
Effect of paricalcitol and enalapril alone and in combination for 16-wk on mean blood pressure (mmHg) in ApoE-deficient atherosclerotic mice. There was a significant (P < 0.05) increase in mean BP in atherosclerotic mice compared to wild type control (n = 12). Enalapril (n = 12) alone and in combination with paricalcitol (n = 12) significantly (P < 0.05) reduced mean blood pressure (BP) in atherosclerotic mice (n = 12). Paricalcitol (n = 12) slightly but not significantly decreased mean BP in atherosclerotic mice.
Figure 2 shows that the levels of the renal lipid peroxidation end product, malondialdehyde (MDA), increased by 200% (P < 0.001) in atherosclerotic mice in group 1 compared to wild type controls in group 5, indicating the renal oxidative injury in atherosclerosis. Paricalcitol and Enalapril alone significantly reduced renal MDA levels (33% and 58%, respectively) compared to group 1 (P < 0.05). Combining these two drugs, however, decreased greater MDA levels by 67% (P < 0.01) than either drug alone in atherosclerotic mice.
Figure 2.
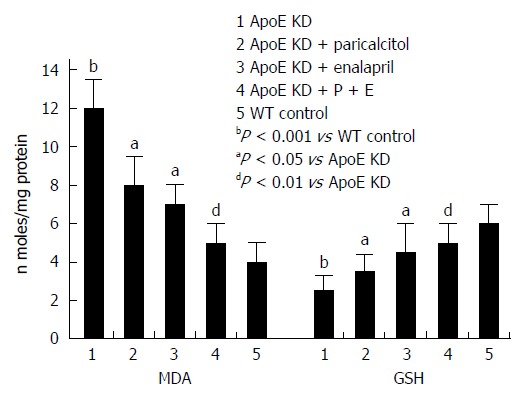
Effect of paricalcitol and enalapril alone and in combination for 16-wk on mono and combination for 16-wk on renal malondialdehyde and glutathione levels in ApoE-deficient atherosclerotic mice. There was a significant (P < 0.001) increase in the renal MDA levels in atherosclerotic mice compared to wild type control (group 5). Paricalcitol (n = 6) and Enalapril (n = 6) alone significantly (P < 0.05 and P < 0.05) reduced aortic malondialdehyde (MDA) levels. However combination of the two (n = 6) decreased greater and significant (P < 0.01) aortic MDA levels than either drug alone in atherosclerotic mice (n = 6). There was a significant (P < 0.001) decrease in aortic glutathione (GSH) levels in atherosclerotic mice compared to wild type control (group 5). Paricalcitol (n = 6) and enalapril (n = 6) alone significantly (P < 0.05) increased aortic GSH levels. However combination of the two (n = 6) increased greater and significant (P < 0.01) aortic GSH levels than either drug alone in atherosclerotic mice (n = 6).
There was a marked decrease in renal reduced glutathione (GSH) (58%, P < 001) in vehicle-treated atherosclerotic mice in group 1 compared to wild type controls in group 5 (Figure 2) indicating an oxidative injury. Paricalcitol and Enalapril alone significantly increased renal GSH levels (40% and 80%, respectively) compared to group 1 (P < 0.05). Combining of the two drugs, however, increased greater GSH levels by 100% (P < 0.01) than either drug alone in atherosclerotic mice. All three therapies protected against the reduction in renal GSH levels, indicating an up-regulation of the renal antioxidant system.
Renal p22phox level was significantly elevated (150%) in ApoE-deficient mice compared to normal controls (Figure 3). Paricalcitol treatment decreased the subunit expression (38%) in the kidney compared to group 1. Enalapril and drug combo significantly decreased p22phox (76% and 68% of group 1) thus lowers the oxidative damage of the kidney.
Figure 3.
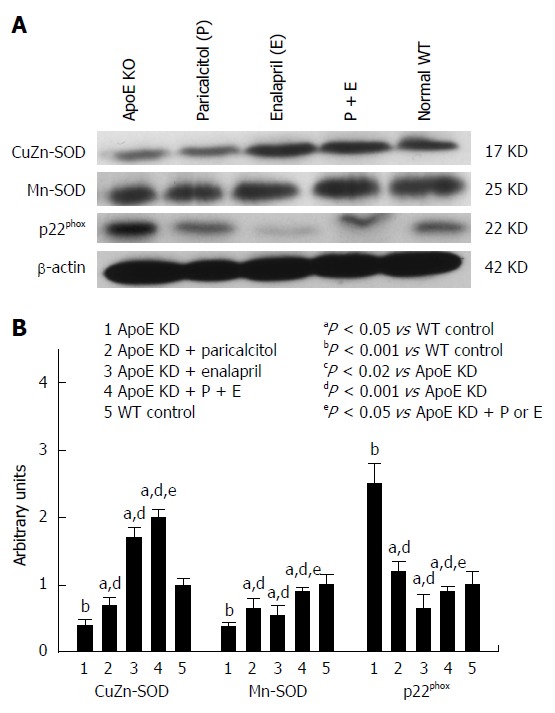
Western blot analysis and densitometry of protein band analysis to paricalcitol and enalapril alone and in combination for 16-wk on renal NADPH oxidase subunit p22phox, manganese-superoxide dismutase and copper/zinc-superoxide dismutase protein expression in ApoE-deficient atherosclerotic mice. A: Western blot analysis of the effect of paricalcitol and enalapril alone and in combination for 16-wk on renal NADPH oxidase subunit p22phox, manganese-superoxide dismutase (Mn-SOD) and copper/zinc-superoxide dismutase (CuZn-SOD) protein expression in ApoE-deficient atherosclerotic mice; B: Densitometry of protein band analysis show that renal NADPH oxidase subunit p22phox and Mn-SOD expression significantly increased (P < 0.001) whereas CuZn-SOD protein expression significantly decreased (P < 0.001) in atherosclerotic mice (n = 3) compared to controls (n = 3). Paricalcitol (n = 3), enalapril (n = 3) and combination of the two (n = 3) significantly (P < 0.02 and P < 0.001) ameliorated the oxidative stress by inhibiting the induction of NADPH oxidase and Mn-SOD expression (38%, 76% and 68%, respectively) and up-regulating the CuZn-SOD protein expression (52%, 342% and 398%, respectively) in atherosclerotic mice. The data represent mean ± SE of three independent Western blot experiments.
As depicted in Figure 3 renal Mn-SOD level significantly elevated (98%) and CuZn-SOD level significantly depleted (55%) in atherosclerotic animals compared to group 5. Both drug separately as well as combined together depleted Mn-SOD levels (52%, 76% and 68%, respectively) and induced CuZn-SOD levels (52%, 342% and 398%, respectively) compared to group 1.
The renal inflammatory markers (TNF-α, MCP-1, TGF-β1 and iNOS) were significantly elevated (190%, 198%, 158% and 992%, respectively) and eNOS levels significantly depleted (55%) in ApoE-deficient animals compared to group 5 (Figures 4 and 5). Both drugs alone as well as combined together significantly depleted iNOS (69%, 72% and 76%, respectively), MCP-1 (72%, 79%, 76%, respectively), TNF-α (79%, 88%, and 72%, respectively) and TGF- β1 (24%, 82% and 93%, respectively) compared to group 1. Both drug separately as well as combined together ameliorated the renal endothelial damage via induction of eNOS (98%, 147% and 342%, respectively) compared to group 1.
Figure 4.
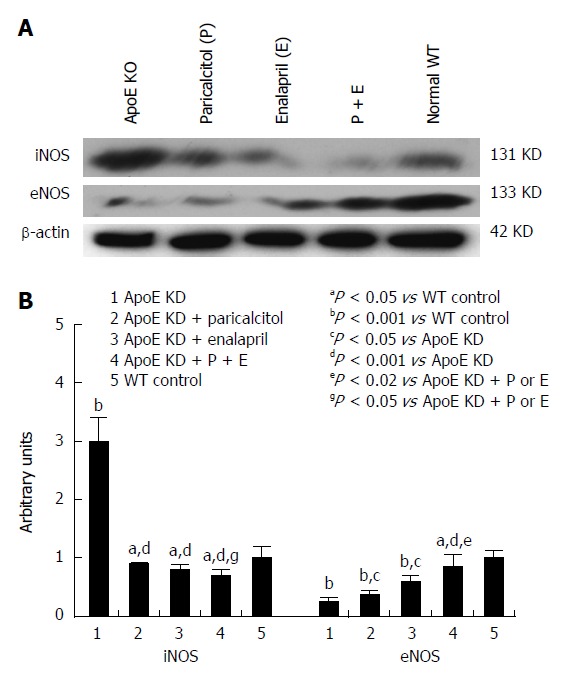
Western blot analysis and densitometry of protein band analysis to paricalcitol and enalapril alone and in combination for 16-wk on renal iNOS and eNOS protein expression in ApoE-deficient atherosclerotic mice. A: Western blot analysis of the effect of paricalcitol and enalapril alone and in combination for 16-wk on renal iNOS and eNOS protein expression in ApoE-deficient atherosclerotic mice; B: Densitometry of protein band analysis show that renal iNOS expression was significantly enhanced (P < 0.001) whereas eNOS protein expression was significantly decreased (P < 0.001) in ApoE-deficient atherosclerotic mice (n = 3) compared to controls (n = 3). Pricalcitol (n = 3), enalapril (n = 3) and the combination of the two (n = 3) significantly (P < 0.05 and P < 0.001) ameliorated the alterations in iNOS and eNOS expression in atherosclerotic mice. The data represent mean ± SE of three independent Western blot experiments.
Figure 5.
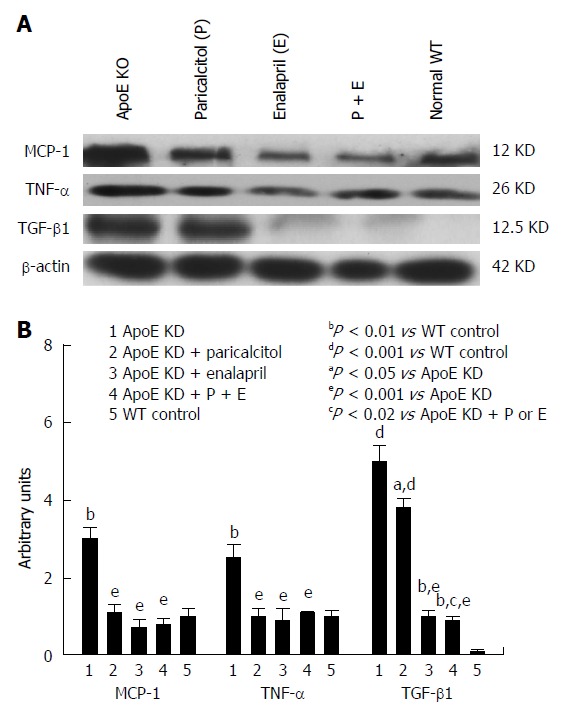
Western blot analysis and densitometry of protein band analysis to paricalcitol and enalapril alone and in combination for 16-wk on renal inflammatory proteins MCP-1, TNF-α and TGF-β1 expression in ApoE-deficient atherosclerotic mice. A: Western blot analysis of the effect of paricalcitol and enalapril alone and in combination for 16-wk on renal inflammatory proteins MCP-1, TNF-α and TGF-β1 expression in ApoE-deficient atherosclerotic mice; B: Densitometry of protein band analysis show that renal MCP-1, TNF-α and TGF-β1 expression was significantly enhanced (P < 0.01, P < 0.01 and P < 0.001) in ApoE-deficient atherosclerotic mice (n = 3) compared to controls (n = 3). Pricalcitol (n = 3) and enalapril (n = 3) alone and in combination of the two (n = 3) significantly (P < 0.05 and P < 0.001) exerted anti-inflammatory response by down-regulating the MCP-1, TNF-α and COX-2 expression in atherosclerotic mice. The data represent mean ± SE of three independent Western blot experiments.
DISCUSSION
This study reveals the protective efficacy of the vitamin D analog, paricalcitol, and ACE inhibitor, enalapril, either alone or in combination, on renal inflammatory and oxidative stress in ApoE-deficient atherosclerotic mouse model. A number of preclinical as well as clinical studies have demonstrated the beneficial effects of RAS blockade in renal and cardiovascular diseases including atherosclerosis[13,16-18,29,37-39,44]. However, the mechanisms by which ACEIs provide protection against cardiovascular diseases are not fully understood. The role of inflammatory and oxidative injuries in the disorders of the kidney, heart and blood vessels such as CKD, renal and cardiac failure, atherosclerosis and hypertension is well known[18,34,37-39,45]. Chronically increased angiotensin II activity has been implicated in promoting oxidative stress, inflammation and atherosclerosis[46-48]. The inflammatory and oxidative stress response in atherosclerotic mice can induce the generation of oxidants and inhibit the endogenous antioxidants[27,30,37,39]. Angiotensin II has been shown to induce vascular oxidative stress through the activation of NADPH oxidase, which leads to the generation of superoxide[19,20,22,40]. The present data in an ApoE-deficient mouse model of atherosclerosis show that a profound increase in renal NADPH oxidase subunit p22phox protein expression corresponds to enhanced MDA levels and elevated mean BP. The down-regulation of NADPH oxidase by enalapril, paricalcitol and the combination, clearly suggests a role for RAS in the progression of renal oxidative stress. Interestingly the vitamin D analog, paricalcitol, which has been shown to be efficacious in renal and cardiovascular diseases[12,31,34-36], has an antioxidant effect by decreasing the superoxide generating enzyme (NADPH oxidase) and MDA levels in the kidney. This action may be related to the negative regulation of RAS by vitamin D analogs[21,49]. Earlier studies have shown that excess superoxide generation due to NADPH oxidase activation causes inflammation and further generation of inflammatory cytokines (TNF-alpha) through NF-κB activation[21,22,50]. The role of NF-κB activation is also demonstrated in atherosclerosis[21,51] and in renal disease[52]. An earlier report demonstrated that 1, 25-dihydroxyvitamin D3 blocks hyperglycemia-induced renal injury by blunting NF-κB activation[53]. Our recent studies demonstrate that renal and cardiovascular expression of pro-inflammatory substances (MCP-1, TNF-α, and COX-2) and macrophage infiltration are profoundly depressed when paricalcitol was combined with the ACE inhibitor, enalapril, in uremic rats and in atherosclerotic mice[29,38]. In preclinical studies, the treatment of animals with vitamin D ameliorated fibrosis in the kidney by inhibiting TGFβ and inducing hepatocyte growth factor[5,54]. TGFβ has been shown to inhibit interstitial fibrosis via down regulation of myofibroblast induction[54,55]. Preclinical report demonstrated that paricalcitol administered intravenously inhibits interstitial fibrosis in the kidneys of animals with obstructive nephropathy[35], and a clinical study has shown oral administration protected the kidney function in the CKD patients[56]. Therefore, the current study demonstrating the depletion of renal inflammatory factors by drugs treatment in atherosclerotic mice, together with previous data, suggests that the renal protection provided by paricalcitol or enalapril and combination of the two is most likely due to their anti-inflammatory actions.
Inflammation and oxidative stress in the kidney of atherosclerotic mice may also be related to an alteration in the cellular antioxidant defense system. Glutathione (GSH), a major non-protein thiol antioxidant, maintains the cellular integrity[28,57]. It directly scavenges excess ROS and also regenerates cellular antioxidant vitamins[28,48]. Our data show a significant depletion of renal GSH in atherosclerotic mice, an indicator of oxidative stress[48,57]. The depletion of GSH is known to cause failed antioxidant defense leading to lipid peroxidation[28,48,57]. The suppression of cellular glutathione by an inhibitor of glutathione synthesis causes hypertension and cardiovascular oxidative stress in rats[28]. Therefore, preservation of physiological levels of GSH is a vital cellular mechanism for the prevention of oxidative injury to the kidney. A lower level of antioxidants in the tissues of patients with renal and cardiovascular diseases has also been reported[16,18,56,58]. The data further show that both paricalcitol and enalapril alone and in combination significantly elevates renal GSH levels suggesting the antioxidant actions of the treatments. The present data show that renal mitochondrial Mn-SOD protein expression is significantly increased in ApoE-deficient atherosclerotic mice suggesting a defense mechanism for scavenge of excess superoxides. SODs are the primary scavengers of the endogenous ROS in the cells. There are three isozymes of SOD exist in mammalian cells: cytosolic CuZn-SOD, mitochondrial Mn-SOD and extracellular ecSOD and CuZn-SOD activity is inactivated by free radicals[59]. The inflammatory and oxidative stress in the renal tissues of atherosclerotic mice induced accumulation of oxidant species leading to protein oxidation and degradation of enzyme protein. Importantly, activation or inhibition of SOD activities are known to be due to cellular accumulation of oxidant species[60]. Mitochondrial Mn-SOD has been reported to be regulated by pro-inflammatory cytokine TNF-alpha[61] and both drugs paricalcitol and enalapril have anti-inflammatory actions[16,29,33,34,37-39]. Hence the combination of the two drugs would be more effective in the amelioration of renal inflammatory injury in atherosclerosis. The data further show that renal eNOS protein expression down-regulated but iNOS protein expression up-regulated in ApoE-deficient atherosclerotic mice compared to controls suggesting oxidative and inflammatory injury to the renal vascular endothelium. Cellular nitric oxide (NO) is produced by eNOS in the endothelium and by iNOS in response to inflammation[62]. Treatment with paricalcitol and enalapril attenuated the changes in eNOS and iNOS levels in atherosclerotic mice. The present data as well as our earlier studies[29,33,37-39] clearly show that vitamin D and ACE inhibitor combo ameliorated the renal inflammation and oxidants/antioxidants imbalance in animal model of atherosclerosis than either drug alone.
In summary, we demonstrated that renal inflammatory and oxidative stress in ApoE-deficient mice is due to enhanced TNF-α, TGFβ1, MCP-1, p22phox, Mn-SOD and iNOS levels and lipid peroxidation and to a reduction in GSH levels and CuZn-SOD protein expression. Treatment with either vitamin D and ACE inhibitor alone or their combo attenuates renal inflammation and oxidative damage in ApoE-deficient animals by depleting TNF-α, TGFβ1, MCP-1, p22phox, Mn-SOD and iNOS levels and lipid peroxidation and by preserving GSH, CuZn-SOD and eNOS levels. In conclusion, the combination of paricalcitol and enalapril is more efficacious than either drug alone in ameliorating renal inflammatory and oxidative stress in atherosclerosis.
COMMENTS
Background
Atherosclerosis is the primary cause of renal, heart and vascular diseases worldwide. There is a strong association between vitamin D deficiency and renin-angiotensin system (RAS) in atherosclerosis. Although the cardiovascular protection by vitamin D and RAS inhibitors have been reported in the atherosclerosis. However the protection against renal inflammation and oxidative stress in atherosclerosis by these agents are not reported.
Research frontiers
The study aimed to investigate the protective efficacy of a vitamin D analog, i.e., paricalcitol, and an ACE inhibitor, i.e., enalapril, either separately or in combo, on inflammation and oxidant damage in the kidney of ApoE-deficient atherosclerotic mice.
Innovations and breakthroughs
This study demonstrates for the first time that renal inflammation and oxidative injury is protected by the combination of paricalcitol and enalapril in ApoE-deficient atherosclerotic mice.
Applications
The study hypothesized that renal inflammation and oxidative stress (oxidants/antioxidants imbalance) in atherosclerosis is ameliorated by the combination of vitamin D and RAS inhibitor in mice. This combination is applicable to be used in the prevention and treatment of atherosclerotic renal injury in patients.
Terminology
The renal inflammatory and oxidative stress in ApoE-deficient mice is due to enhanced inflammatory markers (MCP-1, TNF-α, TGFβ1) oxidants (p22phox, Mn-SOD and iNOS and MDA) and depletion of antioxidants (GSH, CuZn-SOD and eNOS). Administration of vitamin D and ACE inhibitor either separately or in combo attenuates renal inflammation and oxidant damage in ApoE-deficient animals by suppressing inflammatory and oxidant markers and restoring the antioxidant defense system.
Peer-review
The paper “Effect of paricalcitol and enalapril on renal inflammation and oxidative stress in mouse model of atherosclerosis” is very well written research paper. In their study they concluded that paricalcitol and enalapril combination therapy affords protection against renal inflammation and oxidative stress in atherosclerosis.
Footnotes
Supported by A research grant from Abbott Pharmaceutical, United States.
Institutional review board statement: The animal protocol was designed to minimize pain and discomfort to the animals. The animals were acclimatized to laboratory conditions (25 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for one week.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Ponce School of Medicine (Protocol #LF-07).
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Data sharing statement: This is a non-clinical study hence the technical appendix, statistical code, and data sets are not available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 28, 2015
First decision: March 6, 2015
Article in press: June 11, 2015
P- Reviewer: Lin GM, Petrovic D, Tatulian S S- Editor: Song XX L- Editor: A E- Editor: Wang CH
References
- 1.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QY, Jiang CM, Sun C, Tang TF, Jin B, Cao DW, He JS, Zhang M. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J Nephrol. 2015;28:471–476. doi: 10.1007/s40620-014-0167-8. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515–1522. doi: 10.2215/CJN.02260409. [DOI] [PubMed] [Google Scholar]
- 5.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9:337–347. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 6.Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8:e54625. doi: 10.1371/journal.pone.0054625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol. 2013;304:C1027–C1039. doi: 10.1152/ajpcell.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrés V. Vitamin D puts the brakes on angiotensin II-induced oxidative stress and vascular smooth muscle cell senescence. Atherosclerosis. 2014;236:444–447. doi: 10.1016/j.atherosclerosis.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Rammos G, Tseke P, Ziakka S. Vitamin D, the renin-angiotensin system, and insulin resistance. Int Urol Nephrol. 2008;40:419–426. doi: 10.1007/s11255-007-9244-4. [DOI] [PubMed] [Google Scholar]
- 10.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005;68:1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Cheng XW, Hu L, Hao CN, Hayashi M, Takeshita K, Hamrah MS, Shi GP, Kuzuya M, Murohara T. Renin inhibition reduces atherosclerotic plaque neovessel formation and regresses advanced atherosclerotic plaques. Atherosclerosis. 2014;237:739–747. doi: 10.1016/j.atherosclerosis.2014.10.098. [DOI] [PubMed] [Google Scholar]
- 13.Pacurari M, Kafoury R, Tchounwou PB, Ndebele K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam. 2014;2014:689360. doi: 10.1155/2014/689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci. 2003;326:15–24. doi: 10.1097/00000441-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 15.da Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, Rutledge JC, Vergona R, Sullivan ME, Wang YX. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis. 2005;178:9–17. doi: 10.1016/j.atherosclerosis.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Mason RP. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2011;7:405–416. doi: 10.2147/VHRM.S20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 18.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 19.Hattori Y, Akimoto K, Nishikimi T, Matsuoka H, Kasai K. Activation of AMP-activated protein kinase enhances angiotensin ii-induced proliferation in cardiac fibroblasts. Hypertension. 2006;47:265–270 [. doi: 10.1161/01.HYP.0000198425.21604.aa. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Ma Y, Zhang J, Cheng J, Du J. A new cellular signaling mechanism for angiotensin II activation of NF-kappaB: An IkappaB-independent, RSK-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol. 2005;25:1148–1153. doi: 10.1161/01.ATV.0000164624.00099.e7. [DOI] [PubMed] [Google Scholar]
- 21.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Zeng HS, Guo Y, Zhou ZB, Tang BS, Li FK. The expression of matrix metalloproteinases-9, transforming growth factor-beta1 and transforming growth factor-beta receptor I in human atherosclerotic plaque and their relationship with plaque stability. Chin Med J (Engl) 2004;117:1825–1829. [PubMed] [Google Scholar]
- 23.Gustafsson S, Lind L, Söderberg S, Zilmer M, Hulthe J, Ingelsson E. Oxidative stress and inflammatory markers in relation to circulating levels of adiponectin. Obesity (Silver Spring) 2013;21:1467–1473. doi: 10.1002/oby.20097. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Jiang JP, Hu J, Wang J, Zheng MZ. Curcumin protects against lipopolysaccharide-induced vasoconstriction dysfunction via inhibition of thrombospondin-1 and transforming growth factor-β1. Exp Ther Med. 2015;9:377–383. doi: 10.3892/etm.2014.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamza SM, Dyck JR. Systemic and renal oxidative stress in the pathogenesis of hypertension: modulation of long-term control of arterial blood pressure by resveratrol. Front Physiol. 2014;5:292. doi: 10.3389/fphys.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues SD, França KC, Dallin FT, Fujihara CK, Nascimento AJ, Pecoits-Filho R, Nakao LS. N-acetylcysteine as a potential strategy to attenuate the oxidative stress induced by uremic serum in the vascular system. Life Sci. 2015;121:110–116. doi: 10.1016/j.lfs.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 28.Finch JL, Suarez EB, Husain K, Ferder L, Cardema MC, Glenn DJ, Gardner DG, Liapis H, Slatopolsky E. Effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria, and renal oxidative stress in uremic rats. Am J Physiol Renal Physiol. 2012;302:F141–F149. doi: 10.1152/ajprenal.00293.2011. [DOI] [PubMed] [Google Scholar]
- 29.Ivanovski O, Szumilak D, Nguyen-Khoa T, Ruellan N, Phan O, Lacour B, Descamps-Latscha B, Drüeke TB, Massy ZA. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005;67:2288–2294. doi: 10.1111/j.1523-1755.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 30.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509–1519. doi: 10.1681/ASN.2007080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repo JM, Rantala IS, Honkanen TT, Mustonen JT, Kööbi P, Tahvanainen AM, Niemelä OJ, Tikkanen I, Rysä JM, Ruskoaho HJ, et al. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007;72:977–984. doi: 10.1038/sj.ki.5002458. [DOI] [PubMed] [Google Scholar]
- 32.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18:1796–1806. doi: 10.1681/ASN.2006091028. [DOI] [PubMed] [Google Scholar]
- 33.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 34.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 35.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 36.Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol. 2009;29:465–472. doi: 10.1159/000178251. [DOI] [PubMed] [Google Scholar]
- 37.Husain K, Suarez E, Isidro A, Ferder L. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol. 2010;32:296–304. doi: 10.1159/000319445. [DOI] [PubMed] [Google Scholar]
- 38.Suarez-Martinez E, Husain K, Ferder L. Adiponectin expression and the cardioprotective role of the vitamin D receptor activator paricalcitol and the angiotensin converting enzyme inhibitor enalapril in ApoE-deficient mice. Ther Adv Cardiovasc Dis. 2014;8:224–236. doi: 10.1177/1753944714542593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 40.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 41.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 42.Read SM, Northcote DH. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981;116:53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- 43.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 45.Sata M, Fukuda D. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. J Med Invest. 2010;57:12–25. doi: 10.2152/jmi.57.12. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 47.Husain K, Vazquez M, Ansari RA, Malafa MP, Lalla J. Chronic alcohol-induced oxidative endothelial injury relates to angiotensin II levels in the rat. Mol Cell Biochem. 2008;307:51–58. doi: 10.1007/s11010-007-9583-6. [DOI] [PubMed] [Google Scholar]
- 48.Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D(3) as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol. 2005;96:59–66. doi: 10.1016/j.jsbmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Wu M, Jiang H, Hao J, Zhang Q, Zhu Q, Saren G, Zhang Y, Meng X, Yue X. Angiotensin II upregulates endothelial lipase expression via the NF-kappa B and MAPK signaling pathways. PLoS One. 2014;9:e107634. doi: 10.1371/journal.pone.0107634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 53.Panizo S, Barrio-Vázquez S, Naves-Díaz M, Carrillo-López N, Rodríguez I, Fernández-Vázquez A, Valdivielso JM, Thadhani R, Cannata-Andía JB. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant. 2013;28:2735–2744. doi: 10.1093/ndt/gft268. [DOI] [PubMed] [Google Scholar]
- 54.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16:1371–1383. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 57.Younes M, Siegers CP. Mechanistic aspects of enhanced lipid peroxidation following glutathione depletion in vivo. Chem Biol Interact. 1981;34:257–266. doi: 10.1016/0009-2797(81)90098-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 59.Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- 60.Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 61.Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. J Biol Chem. 2003;278:23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- 62.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]


