Abstract
AIM: To investigate the impact of agitation speed on pectinase production and morphological changing of Aspergillus niger (A. niger) HFD5A-1 in submerged fermentation.
METHODS: A. niger HFM5A-1 was isolated from a rotted pomelo. The inoculum preparation was performed by adding 5.0 mL of sterile distilled water containing 0.1% Tween 80 to a sporulated culture. Cultivation was carried out with inoculated 1 × 107 spores/mL suspension and incubated at 30 °C with different agitation speed for 6 d. The samples were withdrawn after 6 d cultivation time and were assayed for pectinase activity and fungal growth determination. The culture broth was filtered through filter paper (Whatman No. 1, London) to separate the fungal mycelium. The cell-free culture filtrate containing the crude enzyme was then assayed for pectinase activity. The biomass was dried at 80 °C until constant weight. The fungal cell dry weight was then expressed as g/L. The 6 d old fungal mycelia were harvested from various agitation speed, 0, 50, 100, 150, 200 and 250 rpm. The morphological changing of samples was then viewed under the light microscope and scanning electron microscope.
RESULTS: In the present study, agitation speed was found to influence pectinase production in a batch cultivation system. However, higher agitation speeds than the optimal speed (150 rpm) reduced pectinase production which due to shear forces and also collision among the suspended fungal cells in the cultivation medium. Enzyme activity increased with the increasing of agitation speed up to 150 rpm, where it achieved its maximal pectinase activity of 1.559 U/mL. There were significant different (Duncan, P < 0.05) of the pectinase production with the agitation speed at static, 50, 100, 200 and 250 rpm. At the static condition, a well growth mycelial mat was observed on the surface of the cultivation medium and sporulation occurred all over the fungal mycelial mat. However with the increased in agitation speed, the mycelial mat turned slowly to become a single circular pellet. Thus, it was found that agitation speed affected the morphological characteristics of the fungal hyphae/mycelia of A. niger HFD5A-1 by altering their external as well as internal cell structures.
CONCLUSION: Exposure to higher shear stress with an increasing agitation speed could result in lower biomass yields as well as pectinase production by A. niger HFD5A-1.
Keywords: Aspergillus niger, Agitation, Submerged culture, Pectinase, Microbial growth, Dissolved oxygen
Core tip: We report the influence of agitation speed on pectinase production on our newly isolate Aspergillus niger (A. niger) HFM5A-1 in submerged fermentation. The agitation speed was found to influence pectinase production in a batch cultivation system. However, higher agitation speeds than the optimal speed (150 rpm) reduced pectinase production which due to shear forces and also collision among the suspended fungal cells in the cultivation medium. It was found that agitation speed affected the morphological characteristics of the fungal hyphae/mycelia of A. niger HFD5A-1 by altering their external as well as internal cell structures.
INTRODUCTION
Pectinases constitute is a unique group of enzymes that cleave the glycosidic bonds of galacturonic acid residues of pectic substances, which are the complex structural polysaccharides of plant cells. Major pectinases are refer to polygalacturonase, pectin lyase, pectate lyase and pectin esterase[1]. Pectinases are useful industrial enzyme and are largely used in various industrial processes such as for solubilization of the cell wall of plants, wood, fruit or paper[2,3].
There are several parameters influencing the maximal enzyme production in submerged fermentation, usually it consist of physical and chemical parameters. Physical parameters involving the optimization of initial medium pH, cultivation temperature, inoculum sizes and also agitation speed. Microorganisms differ in their oxygen requirement. Oxygen acts as a terminal electron acceptor for oxidative reactions to provide energy for cellular activities. The rate of the agitation speed could influenced the extent of mixing in the shake flasks system and affected the nutrient availability as well[4]. Agitation speed has been affected many enzymes activities in different strains of bacteria and fungi[5,6] as well as microalgae[7].
Agitation provides adequate mixing, mass, heat transfer and also improving dissolved oxygen in the culture medium. At lower agitation speed insufficient oxygen in the culture medium usually affects the microbial growth, whereas higher agitation speeds sometimes also lowering the enzymes production[8]. Higher agitation speed develop shear forces among the suspended microbial cells in the culture medium and the production drops due to cell damages which results from cell collision. Shear forces also can give several effects on the fungal cell. It can cause the morphological changes to the fungal by damaging the external and internal cell structures, variation in fungal growth and yield formation[9]. Therefore, the optimal agitation speed is necessary to be determined in order to obtain maximal enzyme production. The aim of this study was to determine the influence of agitation speed on pectinase production and morphology of Aspergillus niger (A. niger) HFD5A-1 in submerged fermentation using a flask system.
MATERIALS AND METHODS
Microorganisms, culture maintenance and inoculum preparation
A. niger HFM5A-1 which was isolated from a rotted pomelo was supplied by the Industrial Biotechnology Research Laboratory, School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia. The fungal culture was maintained on potato dextrose agar (Merck, Germany) slant supplemented with 1.0% citrus pectin (w/v) (Sigma, Denmark) at 30 °C for five days aerobically (until sporulation) before storing them at 4 °C until further use. The cultures were sub-cultured regularly at every month to maintain viability.
The inoculum preparation was performed by adding 5.0 mL of sterile distilled water containing 0.1% Tween 80 to a sporulated culture. The spores were dislodged by using a sterile inoculation loop and shaking vigorously. The spore suspension was adjusted to 1 × 107 spores/mL using haemocytometer chamber (Neubauer Germany) and 1.0 mL was taken as the inoculum.
Cultivation medium and fermentation
The cultivation medium used for fermentation was a modified medium of Maldonado and Strasser de Saad[10]. The medium consisted of (w/v): KH2PO4, 0.4%; Na2HPO4, 0.2%; FeSO4.7H2O, 0.02%; CaCl2, 0.001%; peptone, 0.4%; MnSO4.7H2O, 0.007%; H3BO3, 0.001% and citrus pectin, 1.8%. The initial pH of the medium was adjusted to 4.5. Cultivation was carried out after inoculated with 1 × 107 spores/mL suspension and incubated at 30 °C with different agitation speed for 6 d. The samples were withdrawn after 6 d cultivation time and were assayed for pectinase activity and fungal growth determination.
Enzyme recovery and fungal biomass determination
The culture broth was filtered through filter paper (Whatman No. 1, London) to separate the fungal mycelium. The cell-free culture filtrate containing the crude enzyme was then assayed for pectinase activity.
The filter paper containing biomass was dried at 80 °C until constant weight and the fungal cell dry weight was obtained by deducting the weight of filter paper and the fungal biomass from the weight of filter paper alone. The fungal cell dry weight was then expressed as g/L. All the experiments were performed in triplicate and the values were reported as standard deviations.
Assay for pectinase activity
Pectinase assay was carried out by measuring reducing sugars release from pectin hydrolyzation. One aliquot of sample was added to a solution containing one aliquot of 1% pectin in 0.1 mol/L citrate buffer with pH 4.5. After incubation at 45 °C for 30 min, reducing sugars were determined by the dinitrosalicylic acid (DNS) method[11] using galacturonic acid as a reference. The enzymatic activity was expressed in terms of Unit (U). One unit of enzyme activity was defined as the amount of enzyme that catalyzes the release of 1 μmol of galacturonic acid per mL of culture filtrate per minute under the assay conditions.
Scanning electron microscopy studies
The 6 d old fungal mycelia were harvested from various agitation speed, 0, 50, 100, 150, 200 and 250 rpm, washed three times with sterile distilled water and then blotted dry in Whatman filter papers. The samples for scanning electron microscopy (SEM) were prepared as described previously by Darah et al[5] and Darah and Ibrahim[12] and observed under FESEM LEO Supra 50VP, Carl Zeiss, Germany.
Statistical analysis
In order to evaluate and determine the significant of the findings and also to compare the differences among the findings, the statistical analysis was used. One way Analysis of Variance (ANOVA) and Duncan Multiple Range Test (DMRT) with PASW Statistics 18 version were used to analyze the significant different of the mean of experimental data. A 5% confidence level or α = 0.05 were used to test all experimental data. All enzyme activities and fungal growth were made in triplicates. The error bars showed the standard deviation of triplicates.
RESULTS
Enzyme activity rises up with the increasing of agitation speed up to 150 rpm (Figure 1), where it achieves its maximum pectinase activity of 1.559 U/mL. There were significant different (Duncan, P < 0.05) of the pectinase production with the agitation speed at static, 50, 100, 200 and 250 rpm. However, its highest fungal growth was showed at 200 rpm with 1.938 g/L. The agitation speed which not at 150 rpm resulted in low pectinase production. The lowest pectinase production was from the non-agitated culture (static condition) with about 0.111 U/mL and of 0.972 g/L of fungal growth.
Figure 1.
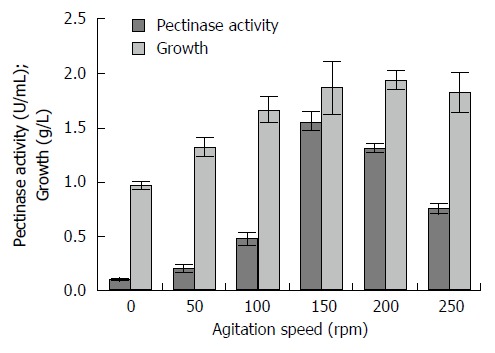
Effect of agitation speeds on pectinase, fungal growth and protein content by Aspergillus niger HFD5A-1 at 6 d cultivation time.
The influence of agitation speed was found had a great impact on the morphology of A. niger HFD5A-1 (Figure 2). At static condition, a mycelial mat was discovered on the top of the cultivation broth (Figure 2A) and sporulation occurred all over the fungal mycelial mat. Nevertheless increased in agitation speed, the mycelial mat turned to form a single spherical pellet. It was noted that at 50, 100 and 150 rpm of agitation produced slimy mycelial clumps that were small (2.8 cm; Figure 2B), medium (3.6 cm; Figure 2C) and big (4.7 cm; Figure 2D) sizes, respectively. There were no sporulation observed at 50, 100 and 150 rpm. However, mycelial clumps formed at 150 rpm were in intense yellow in coloration. At 200 rpm, a few spherical and elongated pellet of fungal growth with the sizes between 0.6-1.0 mm were formed (Figure 2E). Besides, at 250 rpm a mixture of small and bigger sizes of fungal pellets were formed, and the small fungal pellet were those that detached from big pellets (Figure 2F).
Figure 2.
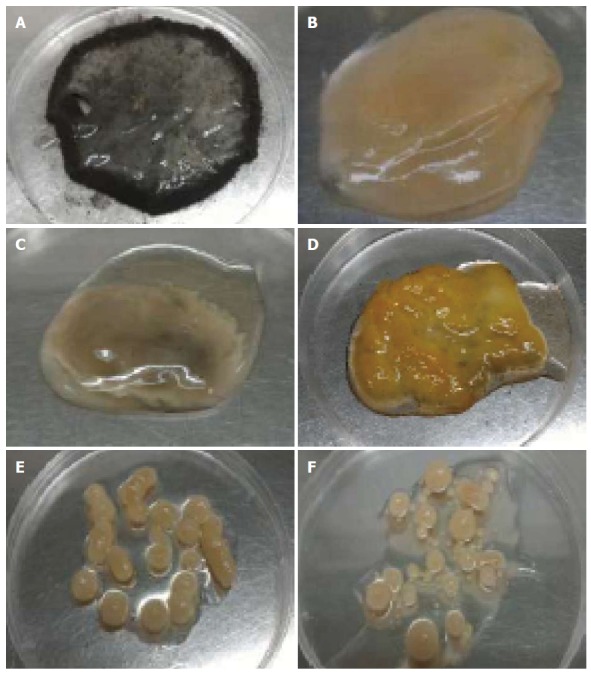
Mycelial morphology of Aspergillus niger HFD5A-1 observed with naked eyes at different agitation speeds at 6 d cultivation time. A: Static condition; B: 50 rpm; C: 100 rpm; D: 150 rpm; E: 200 rpm; F: 250 rpm.
In order to have a closer view of the fungal pellets, they were further analyzed under stereo light microscope at different agitation speeds (Figure 3). Figure 3A (static condition) shows loosely packed mycelium with sporulation, but the density of the mycelium were observed to increase with increasing agitation speeds from 50 rpm to 150 rpm (Figure 3B-D). Conversely, at 200 and 250 rpm (Figure 3E and F) there were decreasing in density of mycelium.
Figure 3.
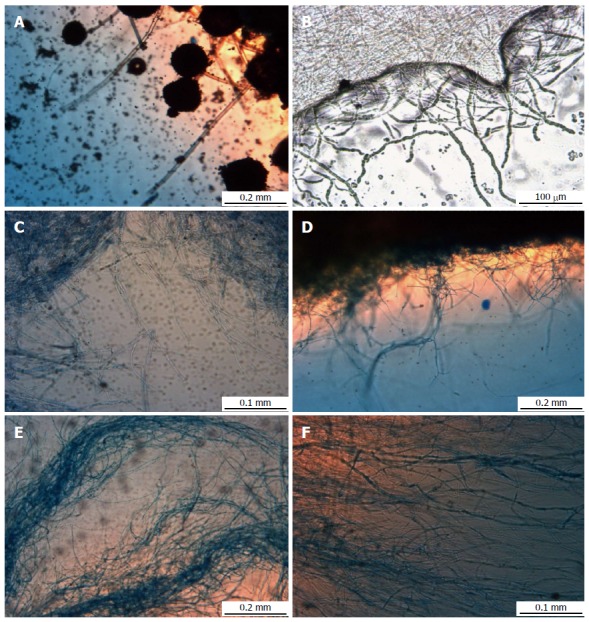
Mycelial morphology of Aspergillus niger HFD5A-1 observed under light microscope at different agitation speeds at 6 d cultivation time. A: Static condition; B: 50 rpm; C: 100 rpm; D: 150 rpm; E: 200 rpm; F: 250 rpm.
Observations under scanning electron microscope were also carried out to have a clearer view of Figure 3. SEM micrographs (Figure 4) show the mycelia of fungal are cylindrical in shape and branched with fruiting bodies. This condition occurred to the fungal culture agitated at low agitation speeds, i.e., at static (Figure 4A), 100 rpm (Figure 4B) and 150 rpm (Figure 4C), whereas at higher agitation speeds of 200 rpm (Figure 4D) the fungal culture exhibited lesser branching mycelia. There was no formation of fruiting bodies at 200 rpm. In addition, fungus grown in static condition showed branched mycelia and numerous formations of fruiting bodies (Figure 4A). At 100 rpm (Figure 4B) fungal growth more on the formation of hyphae with less fruiting body. This condition can be seen in the formation of tight packing structures. However, agitation speed of 150 rpm (Figure 4C) exhibited dense and branched mycelia with increasing formation of fruiting bodies. The fungal growth was best at the agitation of 200 rpm (Figure 4D) which exhibited smoother, not well rounded of hyphae and dense with high branched mycelia. The formations of chlamydospores also observed at 200 rpm.
Figure 4.
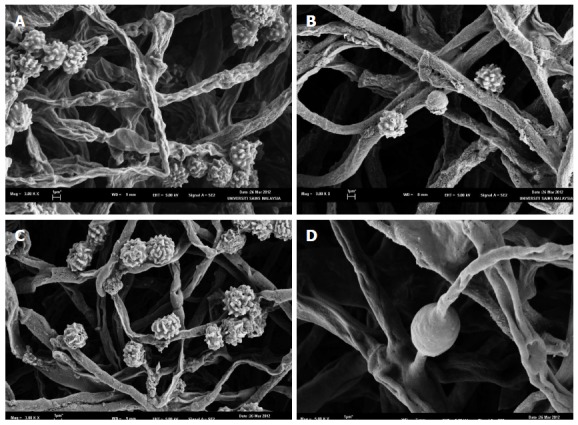
Scanning electron microscopy micrographs of the mycelial morphology Aspergillus niger HFD5A-1 at different agitation speed at 6 d cultivation time. A: 0 rpm; B: 50 rpm; C: 150 rpm; D: 200 rpm.
DISCUSSION
Generally, filamentous fungi have a typical polarized growth pattern in the form of cannular filaments name as hyphae[9]. The hyphae then grow in branches to form mycelium. In submerged cultures, the fungal culture exhibits two major morphologies observed as pellets and free mycelium. The pellets are clumps and dense agglomerates of hyphae which may be spherical or ellipsoidal agglomerates[13,14], whereas the free mycelia, are freely spread throughout the culture medium[9]. These morphologies are very much influenced by some environmental and genetic factors such as type of organism, pH and composition of the media, inoculation ratio, agitation speed and aeration rate, feeding rate and genetic factors of the culture[15,16]. The morphology of filamentous fungi in submerged fermentation has great effect on metabolite production as well as productivity. These two morphologies exhibit significant differences during the cultivation period[17].
Agitation speed has been shown to affect many enzyme productions by microorganisms. In the present investigation, A. niger HFD5A-1 showed highest pectinase activity at 150 rpm after 6 d of cultivation. This is probably due to aeration of the culture medium was increased and dissolved oxygen in the media was sufficient. Nutrient uptake by fungus increased resulting in increased pectinase production. At 200 rpm, the enzyme production was dropped even though the fungal growth increased considerably. The adequate aeration and nutrient might caused the fungus to grow well but shear forces and cell damage finally had negative effect on enzyme production[18]. However, the different strains of fungus demonstrated different optimal agitation speeds for maximize the enzyme production yield in submerged fermentation. Darah et al[19] reported that A. niger FETL FT3 has shown the maximum production of tannase activity at 200 rpm agitation speed instead of 150 rpm agitation speed.
Agitation speed and dissolved oxygen concentration on pectinase production are important factors leading to successful progress of fermentation. Sufficient dissolved oxygen in medium is an important for microbial cells in mass transfer characteristics example substrate and product[5]. Agitation provides adequate mixing, mass and heat transfer. However, agitation also creates shear forces, causing morphological changes, and eventually damage to the cell structure[5,20].
In industrial applications submerged cultures of filamentous fungi are widely used to produce commercially products and pellet form is usually preferred in downstream fermentation process due to the non viscosity of fermentation medium[14]. Hence, the mass transfer of oxygen and nutrients is much easier and also the separation of the pellets from the medium is noncomplex[21]. Furthermore agitation and aeration are simple technique in fermentation system, therefore the operating cost is not costly. Thus, agitation rate need to be controlled in order to obtain maximum production.
Agitation speed is an important factor in enhancing pectinase yield produce by A. niger HFD5A-1 in submerged fermentation. However, agitation speeds beyond than the optimum speed (150 rpm) resulted in low pectinase production.
COMMENTS
Background
Agitation speed is a crucial factor affected the enzyme production in submerged fermentation system. Therefore, the optimal agitation speed is necessary to be determined in order to obtain maximal enzyme production. The purpose of this study was therefore to evaluate the influence of agitation speed on pectinase production as well as morphology of Aspergillus niger (A. niger) HFD5A-1 in submerged fermentation using a flask system which has not been studied yet.
Research frontiers
The author aimed to to evaluate the influence of agitation speed on pectinase production as well as morphology of A. niger HFD5A-1 in submerged fermentation using a flask system.
Innovations and breakthroughs
This study demonstrates agitation speed plays an important role in enhancing pectinase production by A. niger HFD5A-1 in submerged fermentation. However, higher or lower agitation speeds than the optimum speed (150 rpm) resulted in low pectinase production. Even though agitation increases the dissolved oxygen level in the cultivation medium, higher agitation speed can cause damage to the fungal cells that was due to shear forces and collision among the fungal pellets. Therefore, the relationship among morphology and rheology as well as the factors influencing them has to be fully investigated.
Applications
The authors hypothesize that agitation speed plays an important role in enhancing pectinase production by A. niger HFD5A-1 in submerged fermentation. The information obtained should assist progress towards the large scale production of pectinase by submerged cultivation of A. niger HFD5A-1.
Peer-review
In this paper, Darah et al report the effect of agitation speed on the morphology of A. nige HFD5A-1 hyphae and its pectinase production in submerged fermentation. The findings are interesting.
Footnotes
Supported by Universiti Sains Malaysia Scientific Research Fund.
Institutional review board statement: Our experiment was not related to the stated fields. Thus, no document can be provided.
Conflict-of-interest statement: No conflict of interest to declare.
Data sharing statement: We do not have data sharing statement to be attached.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 7, 2015
First decision: April 27, 2015
Article in press: July 2, 2015
P- Reviewer: Freire-De-Lima CG, Kitagawa S S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
References
- 1.Ernesto FT, Tania VS, Gustavo VG. Production of hydrolytic depolymerising pectinases. Food Technol Biotechnol. 2006;44:221–227. [Google Scholar]
- 2.Scott J, Untereiner WA, Wong B, Straus NA, Malloch D. Genotypic variation in Penicillium chysogenum from indoor environments. Mycologia. 2004;96:1095–1105. [PubMed] [Google Scholar]
- 3.Sarvamangala RP, Agasar D. Exploration of regional Agro-wastes for the production of Pectinase by Aspergillus niger. Food Technol Biotechnol. 2006;44:289–292. [Google Scholar]
- 4.Venugopal T, Jayachandra KA, Appaiah A. Effect of aeration on the production of endopectinase from coffee pulp by a novel thermophilic fungi, Mycotypha sp. strain no. AKM1801. Biotechnol. 2007;6:245–250. [Google Scholar]
- 5.Darah I, Sumathi G, Jain K, Lim SH. Influence of agitation speed on tannase production and morphology of Aspergillus niger FETL FT3 in submerged fermentation. Appl Biochem Biotechnol. 2011;165:1682–1690. doi: 10.1007/s12010-011-9387-8. [DOI] [PubMed] [Google Scholar]
- 6.Mukesh-Kumar DJ, Saranya GM, Suresh K, Anda PD, Rajakumar R, Kalaichelvan PT, Production and optimization of pectinase from Bacillus sp. MFW 7 using cassava waste. Asian J Plant Sci Research. 2012;2:369–375. [Google Scholar]
- 7.Sobczuk TM, Camacho FG, Grima EM, Chisti Y. Effects of agitation on the microalgae Phaeodactylum tricornutum and Porphyridium cruentum. Bioprocess Biosyst Eng. 2006;28:243–250. doi: 10.1007/s00449-005-0030-3. [DOI] [PubMed] [Google Scholar]
- 8.Seth M, Chand S. Biosynthesis of tannase and hydrolysis of tannins to gallicacid by Aspergillus awamori-optimisation of process parameter. Process Biochem. 2000;36:39–44. [Google Scholar]
- 9.Znidarsic P, Pavko A. The morphology of filamentous fungi in submerged cultivations as a bioprocess parameter. Food Technol Biotechnol. 2001;39:237–252. [Google Scholar]
- 10.Maldonado MC, Strasser de Saad AM. Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Ind Microbiol Biotechnol. 1998;20:34–38. doi: 10.1038/sj.jim.2900470. [DOI] [PubMed] [Google Scholar]
- 11.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem. 1959;31:426–428. [Google Scholar]
- 12.Darah I, Ibrahim CO. Effect of agitation on lignin degrading enzyme production by Phanerochaete chrysosporium grown in shake flask cultures. Asia Pacific J Mol Biol Biotechnol. 1996;4:174–182. [Google Scholar]
- 13.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv. 2004;22:189–259. doi: 10.1016/j.biotechadv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Casas López JL, Sánchez Pérez JA, Fernández Sevilla JM, Rodríguez Porcel EM, Chisti Y. Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. J Biotechnol. 2005;116:61–77. doi: 10.1016/j.jbiotec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.García-Soto MJ, Botello-Álvarez E, Jiménez-Islas H, Navarrete-Bolaños J, Barajas-Conde E, Rico-Martínez R. In: Guevara-González G, Torres-Pacheco I, editors. Growth morphology and hydrodynamics of filamentous fungi in submerged cultures. Kerala: Agricultural and food biotechnology; 2006. pp. 17–34. [Google Scholar]
- 16.Oncus S, Unluturk S, Tari C, Gogus N. Various factors affecting the pellet morphology, brothe reology and pectinase enzyme production in submerged fermentation of Aspergillus sojae. 13th World Congress of Food Science & Technology. Turkey: IUFoST World Congress; 2006. p. 0271. [Google Scholar]
- 17.Cui YQ, Ouwehand JNW, Van DL, Giuseppin MLF, Luyben KCAM. Aspects of the use of complex media for submerged fermentation of Aspergillus awamori. Enzyme Microb Technol. 1998;23:168–177. [Google Scholar]
- 18.Whitaker A, Allan K, Peter H. Principles of Fermentation Technology. New York: Pergamon Press; 1984. pp. 108–191. [Google Scholar]
- 19.Darah I, Sumathi G, Jain K, Sheh-Hong L. Involvement of physical parameters in medium improvement for tannase production by Aspergillus niger FETL FT3 in submerged fermentation. Biotechnol Res Int. 2011;2011:897931. doi: 10.4061/2011/897931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Liu W, Tian B, Zhang C. Fluid flow induced shear stress affects cell growth and total flavone production by Phellinus igniarius in stirred-tank bioreactor. Chiang Mai J Sci. 2012;39:69–75. [Google Scholar]
- 21.Porcel EMR, Lopez JLC, Perez JAS, Sevilla JMF, Chisti Y. Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus. Biochem Eng J. 2005;26:139–144. [Google Scholar]


