Abstract
Early repolarization syndrome (ERS), demonstrated as J-point elevation on an electrocardiograph, was formerly thought to be a benign entity, but the recent studies have demonstrated that it can be linked to a considerable risk of life - threatening arrhythmias and sudden cardiac death (SCD). Early repolarization characteristics associated with SCD include high - amplitude J-point elevation, horizontal and/or downslopping ST segments, and inferior and/or lateral leads location. The prevalence of ERS varies between 3% and 24%, depending on age, sex and J-point elevation (0.05 mV vs 0.1 mV) being the main determinants. ERS patients are sporadic and they are at a higher risk of having recurrent cardiac events. Implantable cardioverter-defibrillator implantation and isoproterenol are the suggested therapies in this set of patients. On the other hand, asymptomatic patients with ERS are common and have a better prognosis. The risk stratification in asymptomatic patients with ERS still remains a grey area. This review provides an outline of the up-to-date evidence associated with ERS and the risk of life - threatening arrhythmias. Further prospective studies are required to elucidate the mechanisms of ventricular arrhythmogenesis in patients with ERS.
Keywords: Early repolarization syndrome, Early repolarization, Sudden cardiac death, J-wave
Core tip: Early repolarization syndrome (ERS), demonstrated as J-point elevation on an electrocardiograph, was formerly thought to be a benign entity, but the recent studies have demonstrated that it can be linked to a higher risk of ventricular arrhythmias and sudden cardiac death. The prevalence of ERS varies between 3% and 24%, depending on age, sex and J-point elevation (0.05 mV vs 0.1 mV) being the main determinants. ERS patients are sporadic and they are at a higher risk of having recurrent cardiac events. Implantable cardioverter-defibrillator implantation and isoproterenol are the suggested therapies in this set of patients. On the other hand, asymptomatic patients with ERS are common and have a better prognosis. The risk stratification in asymptomatic patients with ERS still remains a grey area. This review provides an outline of the up-to-date evidence associated with ERS and the risk of life - threatening arrhythmias. Further prospective studies are required to elucidate the mechanisms of ventricular arrhythmogenesis in patients with ERS.
INTRODUCTION
Sudden cardiac death (SCD) is defined as natural death due to cardiac causes in a person who may or may not have previously recognized heart disease but in whom the time and mode of death are unexpected[1]. In the context of time, “sudden” is defined for most clinical and epidemiologic purposes as 1 h or less between a change in clinical status heralding the onset of the terminal clinical event and the cardiac arrest itself[1]. The overwhelming majority of SCD cases are related to cardiac arrhythmias[2]. The commonest electrophysiologic mechanisms leading to SCD are ventricular arrhythmias. About 10% of the cases of SCD are related to primary electrophysiological disorders with known (e.g., Brugada syndrome) or unknown (e.g., idiopathic VF) ion-channel abnormalities[1,3-8].
Early repolarization (ER), also recognized as “J-waves” or “J-point elevation”’ is an electrocardiographic abnormality consistent with elevation of the junction between the end of the QRS complex and the beginning of the ST segment in 2 contiguous leads[9,10]. Grant et al[11] are considered to be the first who used the term ER to describe ST-segment deviations and related T wave inversion and premature repolarization was thought to be the underlying aetiology.
The so - called “early repolarization syndrome (ERS)” was unanimously and indisputably regarded as “normal,” a “normal variant”, or a “benign early repolarization” until 2000[12]. However, numerous more recent reports have suggested a relationship between ER and an increased risk of death from cardiac arrhythmias[8,13-19].
ERS is an electrocardiographic (ECG) entity characterized by J-point elevation manifested either as either QRS slurring (at the transition from the QRS segment to the ST-segment) or notching (a positive deflection inscribed on terminal S wave), ST segment elevation with upper concavity and prominent T-waves in at least two contiguous leads[20] (Figure 1).
Figure 1.
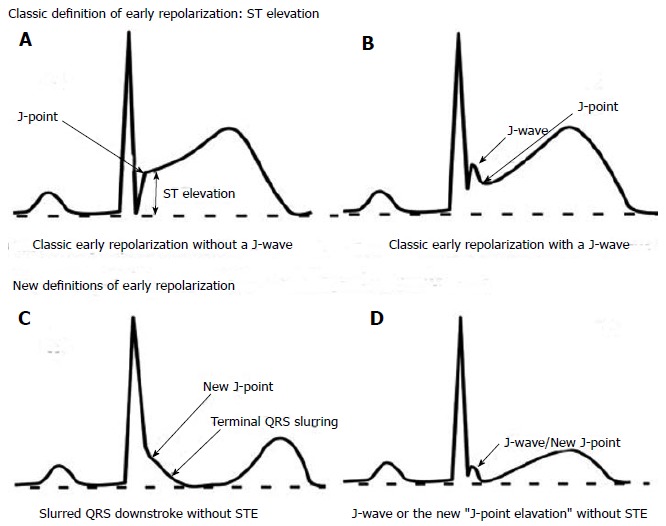
Examples of the classic and new definitions of early repolarization. Examples of the original (classic) and emerging (new) definitions of early repolarization (ER). A and B show the classic form of STE-type ER, which is the form identified by ECG software algorithms. Notice the presence of a J wave in (B), followed by an ascending/upsloping ST segment. Both forms are considered benign; C and D show the malignant form of ER demonstrated as slurring at the end of QRS complex (C) or a discrete notch/J wave (D) followed by a horizontal/downslopping ST segment (no ST elevation). Reproduced from ref.[49], with permission from the publisher. STE: ST elevation type ER; ECG: Electrocardiographic.
PREVALENCE
The ERS is commonly seen in athletes, cocaine users, hypertrophic obstructive cardiomyopathy and defects and/or hypertrophy of interventricular septal defects[21-24]. Prevalence of ERS varies between 3% and 24% in the general population, depending on the population studied and methods used for ECG interpretation. Young individuals, especially those predisposed to vagotonia, males, African Americans, and athletes are subpopulations known to have a higher prevalence of ERS[19,20]. Tikkanen et al[13] demonstrated that the location (inferior vs lateral leads) as well as J-point elevation of > 0.2 mV are linked to a significant risk of death from cardiac arrhythmias (adjusted relative risk, 2.98; 95%CI: 1.85-4.92; P < 0.001).
HISTORICAL PERSPECTIVE
The J-deflection presenting as either QRS slurring or notching was first described in 1936 by Shilpey et al[25] and was considered a normal ECG variant. In 1938, Tomaszewski[26] presented the case of an accidently frozen man whose ECG demonstrated a very slowly inscribed deflection between the QRS complex and the earliest part of the ST segment, representing a J wave. In 1953, Osborn[27] described a “current of injury” later named “the Osborn wave” in acidotic and hypothermic dogs at rectal temperatures < 25 °C.
In 1961, Wasserburger et al[28] further defined ER as a 1-4 mm takeoff of the ST-segment at the end of the QRS complex with a distinct notch or slur on the downslope of the R wave in the mid to left precordial leads.
In 1999, Gussak et al[29] suggested that ER may be malignant in some cases, based on observations that an ER pattern in arterial perfused wedge preparations can easily convert to one which gives rise to polymorphic ventricular tachycardia.
In 2000, evidence supporting above hypothesis was provided by Kalla et al[30] and Takagi et al[31]; they reported VF in patients with prominent J-wave and ST segment elevation in inferior leads without structural heart diseases and postulated that idiopathic VF with an ER pattern in inferior leads may represent a variant of the Brugada syndrome. In 2008, Haïssaguerre et al[8] and Nam et al[32] described a strong relationship between J-waves and many different forms of ventricular arrhythmias in the absence of known heart disease.
CELLULAR, MOLECULAR AND GENETIC CONSIDERATIONS
The pathophysiologic basis of the ER is currently not fully understood. The most discussed hypothesis incriminates that this may be related to either an increased susceptibility or vulnerability to cardiac arrest in critical ischemic conditions such as acute coronary syndromes[33], or to subtle changes in the cardiac action potential[34]. ER in its simplest form occurs in early phase of the cardiac action potential and is caused by the cardiac transient outward potassium current (Ito). If a situation arises where there is a reduced density of the Ito channels in the endocardium compared with epicardium or mid-myocardium[35], a large Ito current can occur that results in electrocardiographic ER and large voltage gradients that may generate J wave elevation (Figure 2) and have the propensity to initiate life threatening arrhythmias[34,35].
Figure 2.
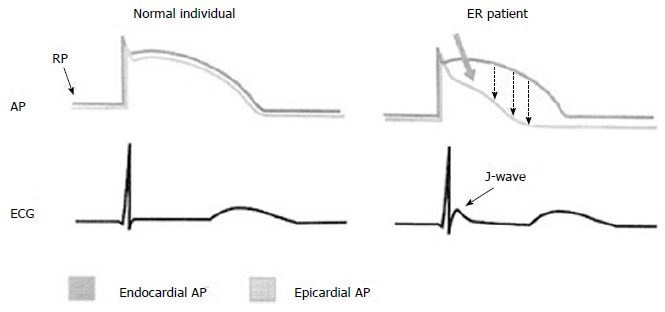
Schematic representation of the possible mechanisms underlying J-wave occurrence. Action potentials from epicardium and endocardium from normal individuals (left) and early repolarization (ER) patients (right) as well as the respective electrocardiograms are shown. A prominent phase I-notch and the loss of epicardial dome in phase - 2 (thick arrow) results in transmural dispersion of repolarization (dashed arrows) and appearance of the J-wave and ST-segment elevation on the surface ECG. AP: Action potential; ECG: Electrocardiogram; ER: Early repolarization; RP: Resting potention. Reproduced with permission, from ref.[67].
Another hypothesis regarding the mechanism causing ER suggests an association of localized depolarization abnormalities with repolarization anomalies, as it happens in type 1 Brugada syndrome[36-39].
The genetic basis of ER syndrome continues to be elucidated, with the evidence restricted to either case reports or preliminary studies that fall short of clearly identifying the genetic basis of ER[40,41]. The reported implicated gene mutations involve the KCNJ8 gene (responsible for the ATP sensitive potassium channel Kir6.1 - IKATP current), CACNA1C, CACNB2, CACNA2D1 genes (responsible for the cardiac L-type calcium channel - ICa.L current), and the SCN5A gene (responsible for the sodium channel - INa current)[40-44]. All of these might enhance the underlying inward – outward current imbalance responsible for accelerated epicardial repolarization.
CLINICAL MANIFESTATIONS OF ERS
The clinical presentation of patients with ERS can be subdivided into two main groups. The first includes those that manifest recognized symptoms of ERS, i.e., high risk patients with syncope and survivors of cardiac arrest[45]. A study by Abe et al[46] demonstrated that the ER was noticed in 18.5% in patients with syncope compared to 2% in healthy controls, this equates to almost 10 - fold increase risk of syncope in patients with ERS. Although very rare, this group is highly likely to have recurrent cardiac events. In his study, Haissaguerre demonstrated 41% risk of arrhythmia recurrences in this cohort, when he followed up 64 ERS patients for a median of 51 mo[8].
The second and the most common group are asymptomatic patients who are incidentally noted to have an ER pattern on their ECG[8]. Overall, this group is less likely to have adverse cardiac events, and the challenge here lies in distinguishing those with risk of sudden cardiac death from those that are likely to run a benign course of the condition[47,48].
ECG DIAGNOSIS OF ERS
The electrocardiographic hallmark of ERS is elevation (> 1 mm above baseline) of the QRS - ST junction manifested as either QRS slurring or notching, ST-segment elevation with upper concavity, and prominent T-waves in two or more contiguous inferior and/or lateral leads in a patient resuscitated from otherwise unexplained ventricular arrhythmia[20]. Recent studies omitted ST segment elevation from the definition of ERS, and state that the J point changes described above is sufficient to diagnose ERS[49]. The inclusion or exclusion of right precordial leads is also an area for debate. Haïssaguerre et al[8] argue that in order to differentiate ERS from Brugada Syndrome, right precordial leads should be excluded, while others state that, the distinction is less straightforward. The latter group are backed by recent data pointing out the similarities in mechanism, overlapping genetic predisposition and the clinical findings of both conditions. Indeed, the term “J-Wave Syndrome” has been suggested to describe ERS and Brugada Syndrome as a spectrum of a clinical condition[36].
Antzelevitch et al[36] described three subtypes of ERS, and highlighted a pattern of risk profile: (1) type 1: It shows ER in the lateral precordial leads that is seen in healthy male athletes and has the lowest risk of malignant arrhythmias (Figure 3); (2) type 2: It shows ER in the inferior and inferolateral leads and is associated with a greater risk of malignant arrhythmias; and (3) type 3: It shows ER pattern in all ECG leads (Figure 4) and has the highest risk of malignant arrhythmias and electrical storms.
Figure 3.
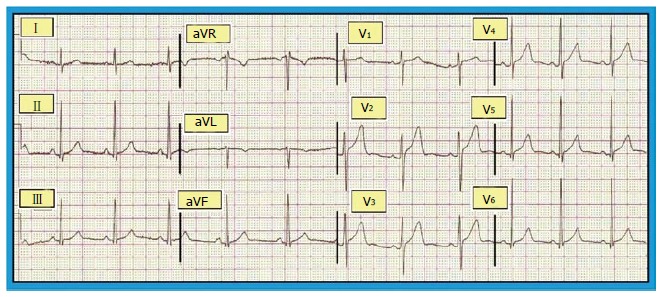
Benign early repolarization: Electrocardiogram showing ST segment elevation by at least 0.1 mV from the baseline. Reproduced with permission, from ref.[68].
Figure 4.
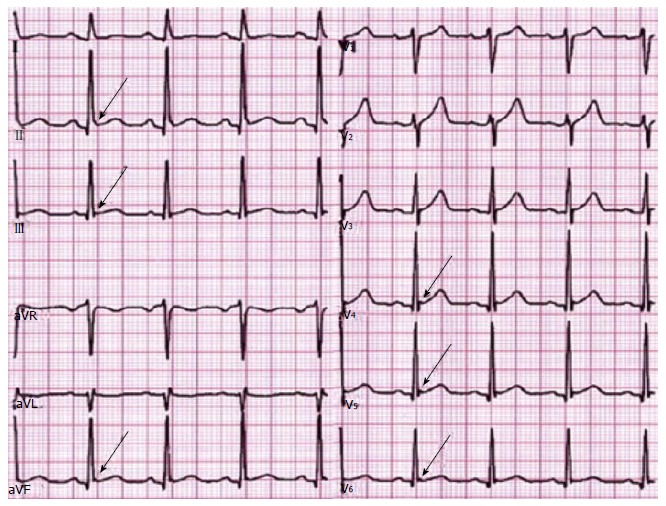
Malignant early repolarization: J-wave elevation (arrows) as slurring (lead II) and notching in the inferior and lateral leads and ascending ST segment in most leads. Reproduced with permission, from ref.[69].
The Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society (HRS/EHRA/APHRS) consensus statement on the diagnosis and management of primary inherited arrhythmia syndromes recommended criteria for the diagnosis of ER is shown in Table 1[50].
Table 1.
Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society consensus statement on the diagnosis and management of primary inherited arrhythmia syndromes recommended criteria for the diagnosis of early repolarization
| ER expert consensus recommendations on early repolarization diagnosis |
| ER syndrome is diagnosed in the presence of J-point elevation ≥ 1 mm in ≥ 2 contiguous inferior and/or lateral leads of a standard 12-lead ECG in a patient resuscitated from otherwise unexplained VF/polymorphic VT |
| ER syndrome can be diagnosed in an SCD victim with a negative autopsy and medical chart review with a previous ECG demonstrating J-point elevation ≥ 1 mm in ≥ 2 contiguous inferior and/or lateral leads of a standard 12-lead ECG |
| ER pattern can be diagnosed in the presence of J-point elevation ≥ 1 mm in ≥ 2 contiguous inferior and/or lateral leads of a standard 12-lead ECG |
ER: Early repolarization; ECG: Electrocardiogram; SCD: Sudden cardiac death. Reproduced from ref.[50], with permission from the publisher.
DIFFERENTIAL DIAGNOSIS
Early Repolarisation syndrome have a wide differential including Brugada Syndrome, short and long QT syndromes as well as other conditions causing ST segment elevation (ST segment elevation MI, acute pericarditis and idiopathic VF). Brugada sydnrome (BS), perhaps the closest clinical entity to ERS, is a primary repolarisation disorder characterized by a prominent J-wave causing a pattern of incomplete right bundle branch block and ST-segment elevation in the right precordial leads (V1-V3) (Figure 5) and significant risk of sudden cardiac death in individuals with no known structural heart disease[51]. BS, an autosomal dominant condition, is more common in males and has a variable penetrance[52,53]. Symptoms of BS include syncope with or without any warning signs, seizures and nocturnal agonal respiration; however, ECG remains the cornerstone of diagnosis of BS[54]. However, the Brugada ECG feature of provocation by sodium channel blocker is not observed in ER[55]. In fact, sodium channel blockers in most patients with ER attenuate the J-point, whereas the J-point is augmented by sodium-channel blockers in the right precordial leads in patients with a Brugada ECG.
Figure 5.
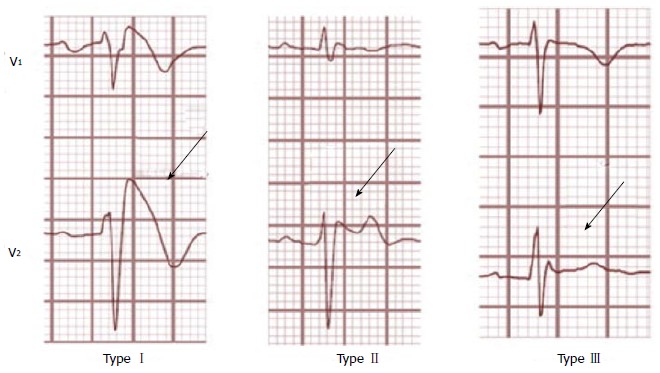
Brugada electrocardiogram-types. Type-1 is characterized by a complete or incomplete right bundle-branch block pattern with a coved morphology ST-segment elevation of ≥ 2 mm in the right precordial leads (V1-V3) followed by a negative T-wave. In type-2, ST-segment elevation has a saddleback appearance with a high takeoff ST-segment elevation of > 2 mm, a trough displaying > 1-mm ST-elevation followed by a positive or biphasic T-wave. Type-3 has an ST-segment morphology that is either saddleback or coved with an ST-segment elevation of < 1 mm. Reproduced with permission, from ref.[69].
In acute pericarditis, there is J-point elevation with resultant ST segment elevation, as seen in ER. Symptom presentation is distinctly different in the two conditions. Unlike ER, most patients with acute pericarditis have ST elevations diffusely in most or all limb and precordial leads. Additionally, patients with acute pericarditis often have deviation of the PR segment, which is not present in ER.
While patients with acute myocardial injury due to ST elevation myocardial infarction (STEMI) can initially have J-point elevation with concave ST segment elevation, the ST segment elevation typically becomes more pronounced and convex (rounded upward) as the infarction persists. However, the primary distinguishing factor between ER and acute myocardial injury is the presence of clinical symptoms such as chest pain or dyspnoea. ER and notching of the terminal QRS need to be considered in risk stratification for arrhythmias in patients with coronary artery disease and after coronary artery bypass grafting.
Table 2 gives a list of conditions with J-wave on the ECG.
Table 2.
Conditions with J-wave on the electrocardiogram
| Conditions with predominant J-waves |
| Hypothermia |
| Hypercalcaemia |
| Hyperkalaemia |
| Vasospastic angina |
| Brugada syndrome |
| Early repolarization syndrome |
| Short QT syndrome |
| Hypoxia |
| Acidosis |
| Pulmonary embolism |
| Arrhythmogenic right ventricular cardiomyopathy |
| Subarachnoid haemorrhage |
Reproduced from ref.[50], with permission from the publisher.
BENIGN OR MALIGNANT
The identification of high-risk patients with ERS remains challenging. Currently, surface ECG is the only available tool in order to differentiate between the benign and the malignant forms of ERS. A horizontal or descending ST-segment elevation has been associated with adverse outcomes (compared with a rapidly ascending ST-segment elevation) following J-point elevation[56,57]. The extent of the J-point elevation may also have prognostic implication: a slurred or notched J-point elevation ≥ 2 mm (0.2 mV) appears to be associated with a higher risk[13,57]. Other abnormalities, such as localization of the ER pattern in inferior or inferolateral (compared with lateral) leads[3] or extension of ER into a BrS pattern, may also represent a worse prognosis[19,58,59].
The benign type of ERS is commonly associated with young age group, left ventricular hypertrophy on ECG, lower blood pressure and lower heart rate, which are all features of healthy, physically active individuals. On the other hand, the malignant form of ERS, characterized by horizontal or descending ST-segment variation (Figure 6), is associated with older individuals and ECGs suggestive of ischaemic heart disease[60].
Figure 6.
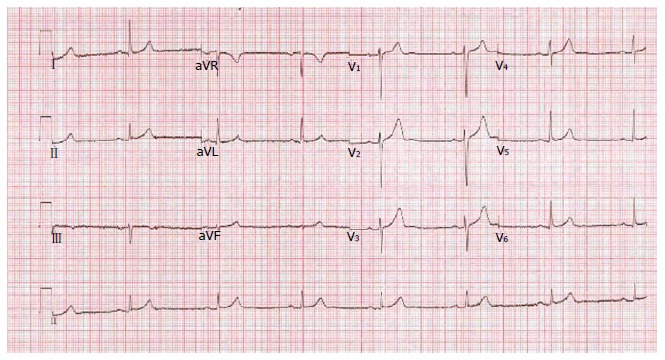
Malignant early repolarization: Horizontal ST-segment after early repolarization.
It appears that the morphology of the ST-segment could help in distinguishing “benign” from “malignant ER”[57]; nonetheless, there is no way to know who would be at considerable risk when presenting with slurring or notching of the QRS unless they have had a cardiac arrest[34].
TREATMENT
The ER pattern is a benign incidental finding, without any specific signs or symptoms attributed to it. There is no current risk stratification strategy for asymptomatic patients with ER pattern in general population and within families with ER pattern that would allow for identification of higher risk individuals with the ER pattern who might be candidates for treatment. The current consensus is that these patients do not require specific investigations or therapeutic interventions[28].
Among the survivors of SCD due to idiopathic VF, the reported rate of recurrent VF ranges between 22% and 37% at two to four years[6]. Because these patients have no structural heart disease, they have an excellent prognosis for long-term survival if VF is treated. As a result, such patients are best treated with an implantable cardioverter-defibrillator (ICD)[6,60-63]. HRS/EHRA/APHRS consensus statement on the diagnosis and management of primary inherited arrhythmia syndromes recommendations for therapeutic interventions in ERS are shown in Table 3[50].
Table 3.
Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society consensus statement on the diagnosis and management of primary inherited arrhythmia syndromes recommendations for therapeutic interventions in early repolarization syndrome
| Expert consensus recommendations on early repolarization therapeutic interventions | ||
| Class I | 1 | ICD implantation is recommended in patients with a diagnosis of ER syndrome who have survived a cardiac arrest |
| Class IIa | 2 | Isoproterenol infusion can be useful in suppressing electrical storms in patients with a diagnosis of ER syndrome |
| 3 | Quinidine in addition to an ICD can be useful for secondary prevention of VF in patients with a diagnosis of ER syndrome | |
| Class IIb | 4 | ICD implantation may be considered in symptomatic family members of ER syndrome patients with a history of syncope in the presence of ST-segment elevation > 1 mm in 2 or more inferior or lateral leads |
| 5 | ICD implantation may be considered in asymptomatic individuals who demonstrate a high-risk ER ECG pattern (high J-wave amplitude, horizontal/descending ST segment) in the presence of a strong family history of juvenile unexplained sudden death with or without a pathogenic mutation | |
| Class III | 6 | ICD implantation is not recommended in asymptomatic patients with an isolated ER ECG pattern |
ER: Early repolarization; ECG: Electrocardiogram; ICD: Implantable cardioverter-defibrillator.
It has been demonstrated that patients with VF and ER have a higher prevalence of recurrence of VF than VF patients without ER (43% vs 23%, P < 0.001) during a five years follow-up[8]. In a multicenter observational cohort study of 122 patients (90 males, mean age 37 ± 12 years) with ER in the inferolateral leads and more than three episodes of idiopathic VF (including those with electrical storm), isoproterenol was effective for the acute suppression of VF, immediately suppressing electrical storms in seven of seven patients[64]. In terms of long term therapy, VF recurrences have been demonstrated to be effectively suppressed by quinidine therapy[64]. Encouraging results recently emerged from a study by Gurabi et al[65], who demonstrated that in addition to quinidine, cilostazol, and milrinone suppress the hypothermia - induced VT/VF in a canine left ventricular model.
However, there exists a “gray area” in between the two ends of the spectrum, where no clear guidelines exist. Examples include patients with syncope who may have a “malignant” ER pattern and/or a significant family history of sudden cardiac death. The current guidelines suggest that ICD implantation may be considered in high-risk individuals with unexplained syncope[50].
SCREENING FAMILY MEMBERS
There are no current recommendations can be given to do ECG screening of the families of individuals with asymptomatic ER pattern or individuals with strong family history of ER or ER with VF. There are no recognized provocative tests that would help in diagnosing concealed ER in family members of patients with ERS, although preliminary observation advocate that concealed ER cases may be recognized by Valsalva maneurve[50,66].
CONCLUSION
In the recent years, ER syndrome has been associated with a significant risk of life - threatening arrhythmias and cardiac death. It is currently not possible to identify asymptomatic individual patients with ER who are at a higher risk of having cardiac arrhythmias with any clinically useful degree of accuracy. It is also not possible to identify asymptomatic individuals with a primary arrhythmogenic disorder attributable to ER. All patients with ER should continue to have modifiable cardiac risk factors addressed.
Until we have a better knowledge, physicians are left with the observation that in patients with ER in the inferolateral leads, life-threatening ventricular arrhythmias may occur and may lead to sudden cardiac death. Since there are a large number of patients who fit such a criteria but do not appear to have excess risk of arrhythmias, further data is needed to reveal how to identify the group of patients who would be at a significant risk and what measures can be taken to prevent it.
Footnotes
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 17, 2015
First decision: April 10, 2015
Article in press: June 2, 2015
P- Reviewer: Kirali K, Prosser HC, Rauch B S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97:457–460. doi: 10.1161/01.cir.97.5.457. [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Schwartz PJ, Crampton RS, Locati E, Carleen E. The long QT syndrome: a prospective international study. Circulation. 1985;71:17–21. doi: 10.1161/01.cir.71.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 6.Viskin S, Belhassen B. Idiopathic ventricular fibrillation. Am Heart J. 1990;120:661–671. doi: 10.1016/0002-8703(90)90025-s. [DOI] [PubMed] [Google Scholar]
- 7.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié JL, Nogami A, Babuty D, Yli-Mayry S, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 9.Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171–177. doi: 10.1016/s0002-9343(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 10.Mehta M, Jain AC, Mehta A. Early repolarization. Clin Cardiol. 1999;22:59–65. doi: 10.1002/clc.4960220203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RP, Estes EH, Doyle JT. Spatial vector electrocardiography; the clinical characteristics of S-T and T vectors. Circulation. 1951;3:182–197. doi: 10.1161/01.cir.3.2.182. [DOI] [PubMed] [Google Scholar]
- 12.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 13.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 14.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Sinner MF, Reinhard W, Müller M, Beckmann BM, Martens E, Perz S, Pfeufer A, Winogradow J, Stark K, Meisinger C, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7:e1000314. doi: 10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12-lead electrocardiogram. Circulation. 2011;123:2931–2937. doi: 10.1161/CIRCULATIONAHA.110.006460. [DOI] [PubMed] [Google Scholar]
- 17.Olson KA, Viera AJ, Soliman EZ, Crow RS, Rosamond WD. Long-term prognosis associated with J-point elevation in a large middle-aged biracial cohort: the ARIC study. Eur Heart J. 2011;32:3098–3106. doi: 10.1093/eurheartj/ehr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe A, Ikeda T, Tsukada T, Ishiguro H, Miwa Y, Miyakoshi M, Mera H, Yusu S, Yoshino H. Circadian variation of late potentials in idiopathic ventricular fibrillation associated with J waves: insights into alternative pathophysiology and risk stratification. Heart Rhythm. 2010;7:675–682. doi: 10.1016/j.hrthm.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, Kim YH, Antzelevitch C. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki S, Shah AJ, Haïssaguerre M. Early repolarization syndrome - a new electrical disorder associated with sudden cardiac death. Circ J. 2010;74:2039–2044. doi: 10.1253/circj.cj-10-0753. [DOI] [PubMed] [Google Scholar]
- 21.Serra-Grima R, Doñate M, Álvarez-García J, Barradas-Pires A, Ferrero A, Carballeira L, Puig T, Rodríguez E, Cinca J. Long-term follow-up of early repolarization pattern in elite athletes. Am J Med. 2015;128:192.e1–192.e9. doi: 10.1016/j.amjmed.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa EC, Bomfim Ade S, Benchimol-Barbosa PR, Ginefra P. Ionic mechanisms and vectorial model of early repolarization pattern in the surface electrocardiogram of the athlete. Ann Noninvasive Electrocardiol. 2008;13:301–307. doi: 10.1111/j.1542-474X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biasco L, Cristoforetti Y, Castagno D, Giustetto C, Astegiano P, Ganzit G, Gribaudo CG, Gaita F. Clinical, electrocardiographic, echocardiographic characteristics and long-term follow-up of elite soccer players with J-point elevation. Circ Arrhythm Electrophysiol. 2013;6:1178–1184. doi: 10.1161/CIRCEP.113.000434. [DOI] [PubMed] [Google Scholar]
- 24.Quattrini FM, Pelliccia A, Assorgi R, DiPaolo FM, Squeo MR, Culasso F, Castelli V, Link MS, Maron BJ. Benign clinical significance of J-wave pattern (early repolarization) in highly trained athletes. Heart Rhythm. 2014;11:1974–1982. doi: 10.1016/j.hrthm.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Shilpey R, Hallaran W. The four lead electrogram in 200 normal men and women. Am Heart J. 1936;11:325–345. [Google Scholar]
- 26.Tomaszewski W. Changement electrocardiographiques observes chez un home mort de froid. Arch Mal Coeur Vaiss. 1938;31:525–528. [Google Scholar]
- 27.Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953;175:389–398. doi: 10.1152/ajplegacy.1953.175.3.389. [DOI] [PubMed] [Google Scholar]
- 28.Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 29.Gussak I, Antzelevitch C, Bjerregaard P, Towbin JA, Chaitman BR. The Brugada syndrome: clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol. 1999;33:5–15. doi: 10.1016/s0735-1097(98)00528-2. [DOI] [PubMed] [Google Scholar]
- 30.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 31.Takagi M, Aihara N, Takaki H, Taguchi A, Shimizu W, Kurita T, Suyama K, Kamakura S. Clinical characteristics of patients with spontaneous or inducible ventricular fibrillation without apparent heart disease presenting with J wave and ST segment elevation in inferior leads. J Cardiovasc Electrophysiol. 2000;11:844–848. doi: 10.1111/j.1540-8167.2000.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 32.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tikkanen JT, Wichmann V, Junttila MJ, Rainio M, Hookana E, Lappi OP, Kortelainen ML, Anttonen O, Huikuri HV. Association of early repolarization and sudden cardiac death during an acute coronary event. Circ Arrhythm Electrophysiol. 2012;5:714–718. doi: 10.1161/CIRCEP.112.970863. [DOI] [PubMed] [Google Scholar]
- 34.Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol. 2010;56:1177–1186. doi: 10.1016/j.jacc.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 35.Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1031–H1041. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- 36.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–409. doi: 10.1016/s0735-1097(03)00713-7. [DOI] [PubMed] [Google Scholar]
- 38.Gussak I, George S, Bojovic B, Vajdic B. ECG phenomena of the early ventricular repolarization in the 21 century. Indian Pacing Electrophysiol J. 2008;8:149–157. [PMC free article] [PubMed] [Google Scholar]
- 39.Boineau JP. The early repolarization variant--an electrocardiographic enigma with both QRS and J-STT anomalies. J Electrocardiol. 2007;40:3.e1–3.10. doi: 10.1016/j.jelectrocard.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Haïssaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H, Nogami A, Ohkubo K, Kawata H, Hayashi Y, Ishikawa T, Makiyama T, Nagao S, Yagihara N, Takehara N, et al. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol. 2011;4:874–881. doi: 10.1161/CIRCEP.111.963983. [DOI] [PubMed] [Google Scholar]
- 42.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barajas-Martínez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, et al. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–555. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpón E, Hu D, Desai M, Borggrefe M, Häissaguerre M, Kanter R, Pollevick GD, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aizawa Y, Chinushi M, Hasegawa K, Naiki N, Horie M, Kaneko Y, Kurabayashi M, Ito S, Imaizumi T, Aizawa Y, et al. Electrical storm in idiopathic ventricular fibrillation is associated with early repolarization. J Am Coll Cardiol. 2013;62:1015–1019. doi: 10.1016/j.jacc.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 46.Abe A, Yoshino H, Ishiguro H, Tsukada T, Miwa Y, Sakaki K. Prevalence of J waves in 12-lead electrocardiogram in patients with syncope and no organic disorder. J Cardiovasc Electrophysiol. 2007;18:S88. [Google Scholar]
- 47.Rosso R, Halkin A, Viskin S. J waves and early repolarization: do not confuse me with the facts! Heart Rhythm. 2012;9:1603–1604. doi: 10.1016/j.hrthm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Viskin S, Rosso R, Halkin A. Making sense of early repolarization. Heart Rhythm. 2012;9:566–568. doi: 10.1016/j.hrthm.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 49.Perez MV, Friday K, Froelicher V. Semantic confusion: the case of early repolarization and the J point. Am J Med. 2012;125:843–844. doi: 10.1016/j.amjmed.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:e85–108. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 54.Sarkozy A, Chierchia GB, Paparella G, Boussy T, De Asmundis C, Roos M, Henkens S, Kaufman L, Buyl R, Brugada R, et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:154–161. doi: 10.1161/CIRCEP.108.795153. [DOI] [PubMed] [Google Scholar]
- 55.Kawata H, Noda T, Yamada Y, Okamura H, Satomi K, Aiba T, Takaki H, Aihara N, Isobe M, Kamakura S, et al. Effect of sodium-channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm. 2012;9:77–83. doi: 10.1016/j.hrthm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011;123:2666–2673. doi: 10.1161/CIRCULATIONAHA.110.014068. [DOI] [PubMed] [Google Scholar]
- 57.Rosso R, Glikson E, Belhassen B, Katz A, Halkin A, Steinvil A, Viskin S. Distinguishing “benign” from “malignant early repolarization”: the value of the ST-segment morphology. Heart Rhythm. 2012;9:225–229. doi: 10.1016/j.hrthm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Kawata H, Morita H, Yamada Y, Noda T, Satomi K, Aiba T, Isobe M, Nagase S, Nakamura K, Fukushima Kusano K, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–1168. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Tokioka K, Kusano KF, Morita H, Miura D, Nishii N, Nagase S, Nakamura K, Kohno K, Ito H, Ohe T. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014;63:2131–2138. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 60.Letsas KP, Charalampous C, Korantzopoulos P, Tsikrikas S, Bramos D, Kollias G, Efremidis M, Sideris A. Novel indexes of heterogeneity of ventricular repolarization in subjects with early repolarization pattern. Europace. 2012;14:877–881. doi: 10.1093/europace/eur390. [DOI] [PubMed] [Google Scholar]
- 61.Wever EF, Hauer RN, Oomen A, Peters RH, Bakker PF, Robles de Medina EO. Unfavorable outcome in patients with primary electrical disease who survived an episode of ventricular fibrillation. Circulation. 1993;88:1021–1029. doi: 10.1161/01.cir.88.3.1021. [DOI] [PubMed] [Google Scholar]
- 62.Marcus FI. Idiopathic ventricular fibrillation. J Cardiovasc Electrophysiol. 1997;8:1075–1083. doi: 10.1111/j.1540-8167.1997.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 63.Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States. Circulation. 1997;95:265–272. doi: 10.1161/01.cir.95.1.265. [DOI] [PubMed] [Google Scholar]
- 64.Haïssaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, Yli-Mayry S, Defaye P, Aizawa Y, Frank R, et al. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 65.Gurabi Z, Koncz I, Patocskai B, Nesterenko VV, Antzelevitch C. Cellular mechanism underlying hypothermia-induced ventricular tachycardia/ventricular fibrillation in the setting of early repolarization and the protective effect of quinidine, cilostazol, and milrinone. Circ Arrhythm Electrophysiol. 2014;7:134–142. doi: 10.1161/CIRCEP.113.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Bronis K, Kappas K, Manolis AS. Early repolarization: Not benign any more - The J-wave syndrome. Hospital Chronicles. 2012;7:215–228. [Google Scholar]
- 68.Grauer K. ECG Blog #47. Available from: http://tinyurl.com/KG-Blog-47.
- 69.Sethi KK, Sethi K, Chutani SK. Early repolarisation and J wave syndromes. Indian Heart J. 2014;66:443–452. doi: 10.1016/j.ihj.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


