Abstract
A novel paper-based Nanoceria Reducing Antioxidant Capacity (NanoCerac) assay for antioxidant detection (Sharpe, Frasco, Andreescu, & Andreescu, 2012), has been adapted for the first time as a high-throughput method, in order to measure the effect of brewing conditions and re-infusion on the antioxidant capacity of twenty-four commercial green teas. The oxygen radical absorbance capacity (ORAC) assay, frequently applied to complex foods and beverages, was used as a comparator measure of antioxidant capacity. A novel measure of sustained antioxidant capacity, the total inherent antioxidant capacity (TI-NanoCerac and TI-ORAC) was measured by infusing each tea six times. Effects of brewing conditions (temperature, brew time, etc.) were assessed using one popular tea as a standard. Both NanoCerac and ORAC assays correlated moderately (R2 0.80 ± 0.19). The average first-brew NanoCerac, TI-NanoCerac, first-brew ORAC and TI-ORAC were: 0.73 ± 0.1 GAE/g tea ; 2.4 ± 0.70 mmol GAE/g tea; 1.0 ± 0.3 mmol TE/g tea and 2.1 ± 0.71 mmol TE/g tea respectively. Brewing conditions including water temperature and infusion time significantly affected antioxidant capacity. The high-throughput adaptation of the original NanoCerac assay tested here offered advantages over ORAC, including portability and rapid analysis.
Keywords: Green tea, re-infusion, brewing conditions, Camellia sinensis, ORAC, NanoCerac, temperature, time, growing location, harvest season, loose leaf, bagged, catechin, antioxidant, polyphenol
Introduction
Tea, or Camellia sinensis is the most widely consumed beverage in the world aside from water (Tea Association of the USA, 2013) (Cooper, 2005), (Hsu, 2005), and exists in many varieties (black, oolong, green and white), defined by its degree of oxidation prior to drying (Alyabyev, 2004; Tsao, 2010).. Scientists have widely reported the benefits of consuming green tea (Hsu, 2005, 2010), (Dulloo, 2000; Elmets, 2001; Fassina, 2002; Kao, 2000; Yamamoto, 2003), in particular, due its high content of antioxidants called catechins (Henning, 2003). Interest in green tea consumption for improved health has sparked researchers to compare effects of brewing conditions (such as infusion time (Kyle, 2007) and water temperature (Perva-Uzunalic, 2006)) and tea varieties (Henning, 2003) on the antioxidant capacity of infusions. Although debate remains over correlation between in vitro and in vivo antioxidant capacity (Service, 2012), measuring the antioxidant capacity of green tea infusions is likely to reflect the total phenolic content in each infusion, and thus reflect possible health benefits.
The present state of the art in antioxidant analysis involves in vitro testing for concentration (employing HPLC (Kim, Lee, & Shin, 2013) (Gonzalez-Centeno et al., 2013), GC/ MS (D.A. Ananth, 2013), and Folin-Ciocalteu (Singleton, 1999)) as well as antioxidant capacity (using ORAC (Cao G, 1993), FRAP (Benzie IFF, 1996), and TEAC (Miller, 1993)). Antioxidant capacity directly correlates with total phenolic content (TP), as determined by the Folin- Ciocalteu method (Singleton, 1999). Here we applied the NanoCerac, a portable paper-based antioxidant assay, which has demonstrated ability to sensitively measure polyphenolics in field samples. (Sharpe et al., 2012) The principle of the NanoCerac assay is the formation of visually detectable charge transfer complexes formed by spontaneous binding of polyphenols to the surface of immobilized cerium oxide nanoparticles on the sensor. These charge transfer complexes create unique colorimetric responses to each polyphenol, with intensity dependent upon concentration. The visually detectable binding indicates the transfer of an electron from an antioxidant to the ceria nanoparticle and the resulting colorimetric response is used to indicate antioxidant capacity. Therefore we were interested in determining if the NanoCerac assay can be applied to determine polyphenol identity and concentration, e.g. the quantification of catechins in green tea, similar to other methods (R. B. E Sharpe, T Frascot, D Jayathilaka, A Marsh, S Andreescu, 2014) (S. A. E Sharpe, 2013).
The research reported here had three specific aims: 1. Perform a detailed comparative study of the antioxidant capacity of commercially available green tea as a whole botanical infusion; 2. Provide new data on the impact of brewing conditions, including re-brewing, on antioxidant capacity; and 3. Provide a proof of concept for the application of the NanoCerac assay in a scenario requiring high throughput due to a large number of samples. The results published here can be used as a resource for the optimal selection and preparation of commercially available green tea varieties, and provide a methodological example for the field application of an inexpensive high-throughput assay of antioxidant capacity, i.e., the NanoCerac.
Experimental
Materials & Equipment
Cerium (IV) oxide nanoparticles, or ceria: 20 wt. % colloidal dispersion in 2.5% acetic acid, 10–20 nm (289744) average particle size; sodium acetate and acetic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). The average particle size of the 10-20 nm ceria nanoparticles was verified by scanning electron microscopy (SEM) and particle size distribution (PSD). Filter paper (P5; medium porosity; slow flow rate) was purchased from Fisher Scientific (Waltham, MA, USA) and used as received. All reagents were used without further purification and all solutions were prepared with distilled, deionized water (Millipore, Direct-Q system) with a resistivity of 18.2 MΩ. Fluorescein sodium salt, and [2,2’-azobis (2-amidino-propane) dihydrochloride (AAPH) were from Fisher Scientific. The antioxidants 6-Hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid (Trolox), and epigallocatechin gallate (EGCG) were from Sigma Aldrich; L-ascorbic acid (AA) was from Acros.
Twenty-four varieties of green teas were selected for study based on availability and popularity in the areas where research was being conducted (Seattle, WA and upstate, NY). This selection process was carried out with the assumption that teas that are widely available and popular will represent an accurate cross-section of green teas being consumed by the general population. Commercial teas, Lipton, Lipton decaf, Salada, Salada decaf, Yogi, Yogi decaf, Tazo, Bigelow, Twinnings, Stash and Republic of Tea were attained from QFC Grocery Store, Bothell, WA; Takaokaya Sen-Cha Shizuoka was from Boo Han Oriental Market, Edmunds, WA; Zhena’s Gypsy Tea was from Tully’s Coffee Shop, Kenmore, WA; and Choice green tea was from Bastyr University. Loose-leaf teas, Gunpowder, and Hojicha were from the Potsdam, NY Co-op which sources their teas from Frontier Organic Teas; Gunpowder pinhead, Buddhist tea; Dragonwell; Mao Feng; Sencha Fukamushi, Guricha, and TeaBrew unbleached tea bags (100% natural, made from biodegradable paper from sustainably harvested US and Canadian wood pulp; made in Canada) were attained from the Perennial Tea Room, Pike Place Market, Seattle, WA; Hoji cha tea was from a tea shop in Kirkland, WA; gyokuro was from the Teavana store, Bellevue Mall, Bellevue, WA.
A fluorescence 96-well plate reader (Gemini EM fluorescence plate reader by Molecular Devices) was used to perform the ORAC assay for validation and inter-assay comparison purposes. Graphpad was used to normalize data and calculate area under the curve (AUC) values for the ORAC assay, and Microsoft Excel was used to compile, and compare final data for both the ORAC and the NanoCerac assays. An HP Scanjet 4800 series office scanner, model 4850 was used to record and export images of the NanoCerac sensors. MatLab was used for antioxidant capacity analysis of samples from the NanoCerac assay. Dropbox was used as a file sharing program, allowing the large amount of digital images to be immediately uploaded from a computer in Seattle, WA, onto a computer in Potsdam, NY, for analysis using MatLab.
Fabrication of cerium oxide sensors
Procedures reported previously (Sharpe et al., 2012) were applied for preparation of the ceria paper sensors. In brief, full 10cm diameter filter paper rounds were dipped into 4% Sigma cerium (IV) oxide (aq) in 2.5% acetic acid, and dried in the oven at 100C for 5 minutes, then allowed to dry completely on the bench top.
Commercial Tea Sampling
The mass of the tea bag used for each brand of commercial tea was determined by removing the loose tea from three bags of each tea type, and then weighing and averaging the mass of each bag alone. This value was used as a tare, and subtracted from the total mass of subsequent tea bags measured from each brand. The net mass of tea from each commercial brand was most commonly approximately two grams.
Loose-leaf Tea Sampling
Each tea was measured into three tared, biodegradable, unbleached tea bags, and the net mass of the tea was recorded (also approximately two grams per sample to mimic typical commercial quantities).
Brewing
Three tea bags of each of the twenty-four varieties of tea were brewed six times each under the same conditions. Teas were brewed in 200 mL of 80 °C water for five minutes. 1.5 mL of each tea was stored at 4°C and at −20°C for later testing. The antioxidant capacity of each resulting infusion was then measured in triplicate using both the ORAC and the NanoCerac assays.
Sample Analysis by ORAC assay
All teas were diluted twice (from 10g/L to 1g/L and then to 0.09g/L) to attain optimal concentrations for the ORAC assay. The final dilution was stored at 4°C and the ORAC value measured three times for each tea bag.
The ORAC assay was run using guidelines from ZenBio Laboratories (ZenBio, 2008) and Henning (Henning, 2003), who described the need to optimize experimental pH for testing the ORAC of dietary polyphenols (Henning, 2003) (Hsu, 2005). We therefore, adjusted the buffer to pH 5.44 for improved antioxidant stability. Due to the impact of pH on fluorescein light emissions (Doughty, 2010), for maintained sensitivity, we increased the concentration of fluorescein and AAPH as compared to previously published methods (Cao G, 1993). 75uL of 1.9uM fluorescein in a 75mM sodium acetate buffer (pH 5.44) were added to each well of the plate followed by 50uL of sample: diluted tea (0.09g/L), Trolox control (50uM) or buffer as a blank. The plate was then incubated at 37C for at least 10 minutes before addition of the peroxyl radical generator, 240mM AAPH ((2,2’-azobis (amidinopropane) dihydrochloride)), which initiates the reaction in which fluorescein is slowly oxidized, causing a decrease in fluorescence emission. The plate was promptly placed into the microplate reader and a kinetic assay was performed in which fluorescence readings were taken every 60 seconds for 90 minutes. Fluorescence quenching was monitored with excitation and emission wavelengths set to 485 nm and 538 nm respectively. Antioxidant capacity of each sample was assessed in terms of its ability to protect fluorescein from oxidation, through scavenging of peroxyl radicals. This antioxidant capacity was given an oxygen radical absorbance capacity (ORAC) value, which was calculated by comparing the net area under the fluorescence curve for each sample to a Trolox standard.. After correcting for the blank, the net AUC provided by each sample was compared to that of Trolox, resulting in a representation of antioxidant strength in terms of mmol Trolox per g sample. The equation used was (net AUC sample/ net AUC Trolox) × (mmol Trolox/ g sample). The second term refers to “in-well” concentrations of Trolox and sample, which are in turn µ of their initial concentrations (Trolox 12.5uM and tea 0.02g/L).
Sample analysis by NanoCerac
Tea samples were assembled in the first column of three 96-well plates, allowing twenty-four samples to be analyzed at once. Bags 1–3 of one brew for eight different teas at a time were analyzed side by side in this manner. All samples were diluted 11 times (3:1 ratio, sample: water), using a multichannel pipette, leaving the last of 12 columns filled with water only, as a blank. Using another multichannel pipette, 2.5uL of all samples were pipetted in triplicate, directly onto the 10cm ceria sensors. Two columns of six samples, pipetted in triplicate, were arranged on each sensing disk. Sample spots were circled using the large end of a pipette tip and a stamp pad. All sensors were scanned into a computer using default settings (200 dpi), saved as a .bmp image, and uploaded to a shared Dropbox® folder, which was accessed by a MatLab® user in another location. Images were then analyzed for antioxidant capacity with reference to a gallic acid standard.
A unique MatLab® algorithm was created that facilitated analysis of the ceria sensors. The program identifies stamped black circles on the page, and takes an average of RGB color intensities in the center area of the circle. The program then creates calibration curves using blue color intensity (BCI) vs. log of the concentration (g/L) of each tea. A series of rules was input into the program that allowed it to remove outliers and create a graph of the linear range for each calibration curve, with R-squared values nearing 0.99 for each. Outliers were removed using the standard Grubbs Method (Hodge, 2004) for removal of outliers from a calibration curve; and the Q-test (Lobato, 2001) for removal of outliers from a dataset. The slope of each of four hundred and thirty two calibration curves (three bags of 24 teas, brewed six times) were then exported to one excel file for comparison to the slope of a gallic acid standard. The equation (slope sample/ slope GA) = (mmol GAE/ g tea) was used to determine antioxidant capacity in terms of gallic acid equivalents, where slopes represent (BCI/ g tea/L) and (BCI/ mmol Trolox/L).
Determination of factors influencing antioxidant properties
After measuring the ORAC and NanoCerac of all teas, subgroup analyses were performed on all teas according to: growing location, harvest season, drying method, and whether the tea was loose leaf or bagged, price, caffeine content, and growing practice, i.e., organic vs. conventionally raised. All teas in this study were brewed six times facilitating analysis of a novel parameter, which we refer to as total inherent” (TI) antioxidant capacity. This refers to the summative antioxidant capacity extracted from one serving of tealeaves after a set number of infusions. Here, we compare TI AOX and first-brew AOX capacities of all teas. JMP software was used to perform one-way ANOVA, and Student T-Tests to determine statistically significant differences between each tea and each subgroup of tea with respect to first-brew and TI-AOX capacities. Subgroups that included only one representative tea were excluded from statistical analyses.
Results and Discussion
Effect of Brewing Conditions and Re-Infusion on the Antioxidant Capacity of Green Tea
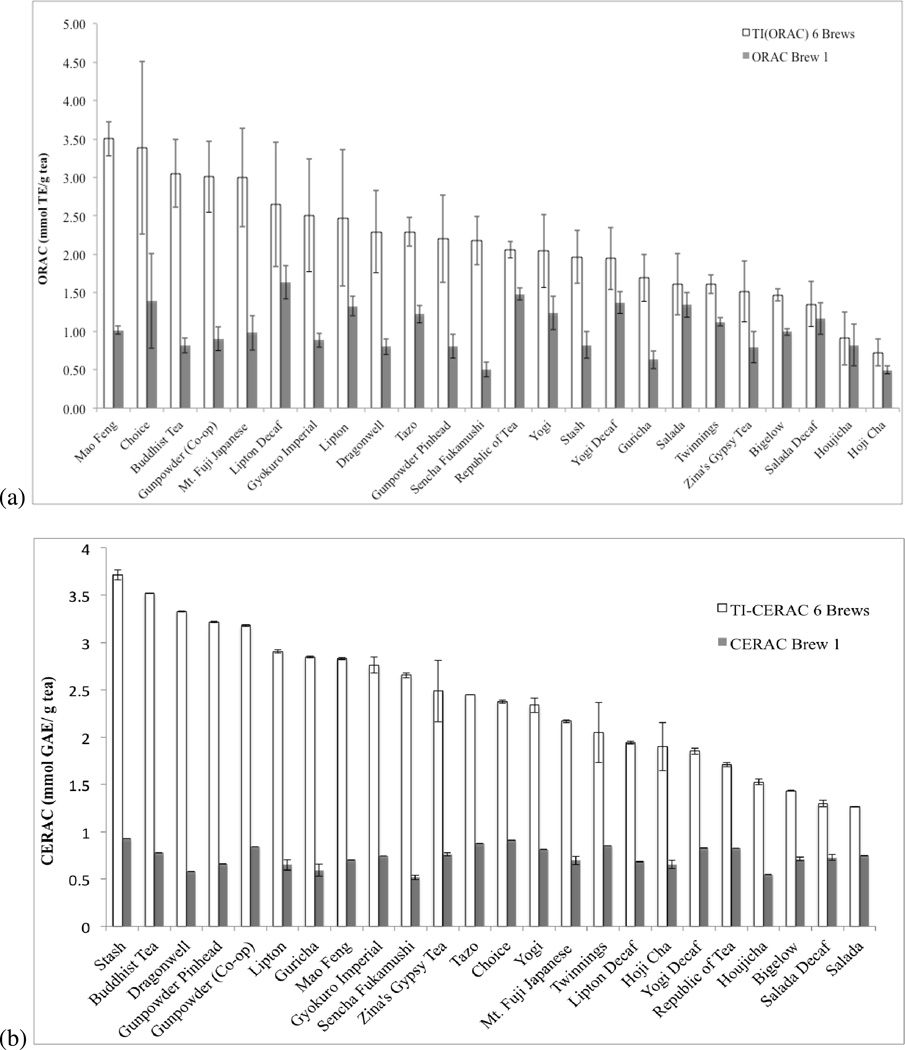
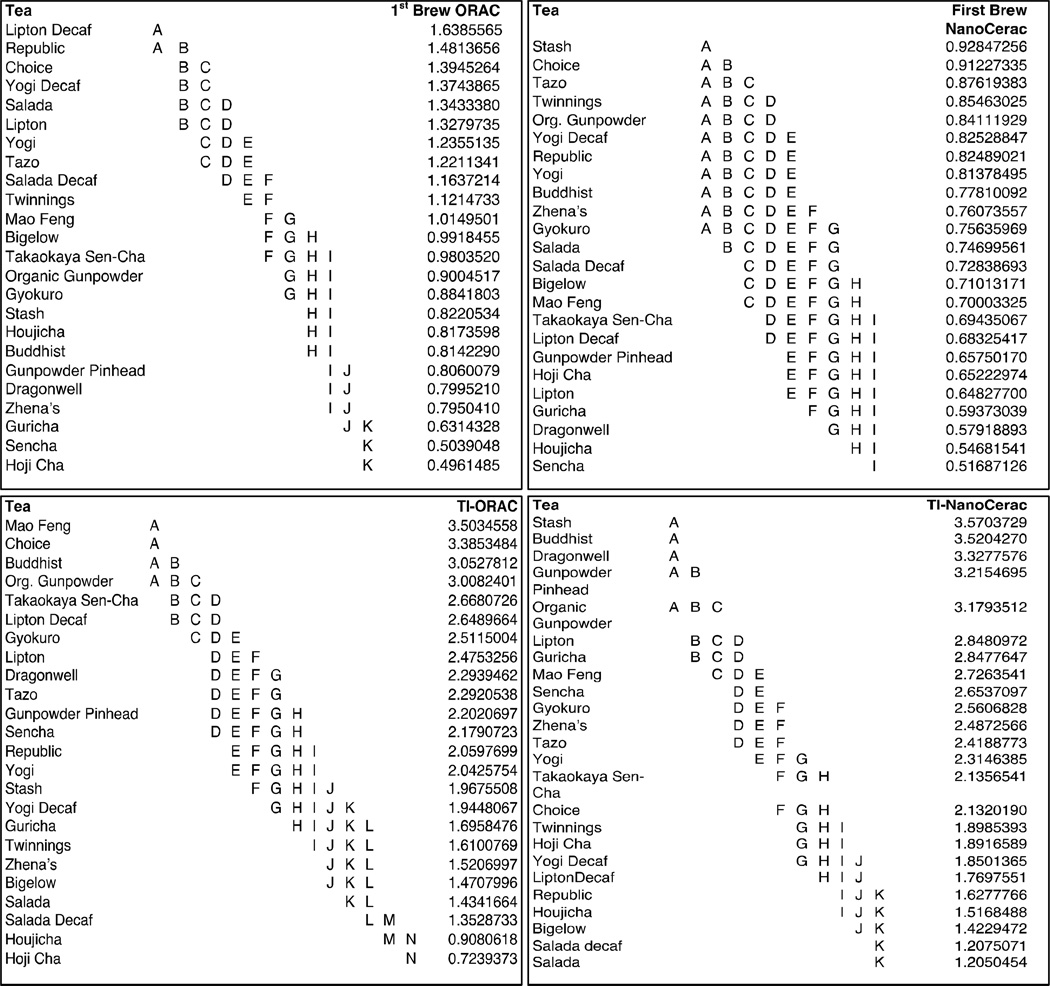
Figure 1 compares first-brew antioxidant capacity to total inherent (TI) antioxidant capacity for twenty-four varietal green teas, analyzed using the ORAC and NanoCerac assays. Significant variability was seen between teas for both first-brew and TI antioxidant capacity, as seen in Figure 2, depicting results of ANOVA and pairwise analyses. In many cases, more than twice the antioxidant capacity of the first brew can be attained through re-steeping the tea (Fig. 1). For example, the first brew of sencha fukamushi, with 0.5mmol TE/g (ORAC) and 0.52mmol GAE/g NanoCerac) comprises just 23% of the TI-ORAC and 19% of the TI-NanoCerac. On the other hand, the first brew of Salada® green tea, with 0.75mmol GAE/g and 1.34mmol TE/g, comprises 59% of the TI-NanoCerac and 83% of the TI-ORAC. It was found that the first-brew ORAC values ranged from 0.50 to 1.64 mmol TE/ g tea and averaged 1.02 mmol TE, CV 30%. The first-brew NanoCerac values ranged from 0.52 mmol GAE/g to 0.92 mmol GAE/g and averaged 0.73mmol GAE/g, CV 15%. TI-ORACs ranged from 0.72 to 3.5 mmol TEA and averaged 2.14 mmol TE (CV 34%). TI-NanoCeracs ranged from 1.3mmol GAE/g to 3.7 mmol GAE/g and averaged 2.4mmol GAE (CV 29%). The ORAC of the first brew of each tea constituted an average of 52% (CV 40%) of its TI-ORAC while the NanoCerac of the first brew of each tea constituted an average of 33% (CV 35%) of its TI-NanoCerac. Comparing the coefficient of variances, a greater difference is seen in the change in antioxidant capacity from initial brew to total capacity after serial brews (40% CV, ORAC; and 35% CV, NanoCerac), than is seen in the actual antioxidant capacity values of the first brew of each tea (30% CV, ORAC; 15% CV, NanoCerac), indicating that the reusability of teas vary much more than the antioxidant capacity of their first infusion.
Figure 1.
Antioxidant capacity of six brews (TI) as compared to one brew (Brew 1) for twenty-four varieties of green teas as (a) ORAC (mmol TE/ g tea) and (b) NanoCerac (mm GAE/ g tea). Values above are the average of nine trials for each type of tea; three tea bags analyzed in triplicate.
Figure 2. ANOVA and Student T-Test results demonstrating statistical similarity and differences in mean antioxidant capacity between tea types.
Groupings shown here indicate statistical similarity, with those that do not share a common letter being statistically different from one another.
Figure S1 in Supplemental Information shows how TI-AOX capacities are constituted for each tea, in terms of six successive infusions of each green tea, the sum of which determines TI-AOX values. Here, it can be observed that certain teas do not release significant levels of active antioxidants beyond their first brew. However, some teas continue to release active catechins throughout six or more brews. The factors that affect the ability to be reused were investigated in detail.
Understanding differences in antioxidant capacity between green tea varieties
The factors making each tea unique are outlined in Table S1 in the Supplemental Information. Factors that changed among the varieties studied were: growing location (eight teas from China; seven from Japan; four from Kenya; three blended from various countries including India, China, Sri Lanka, Belarus, Kenya, and the Ukraine; one from the US; and one from Brazil), initial drying (i.e. withering) method (ten steamed; two high temperature roasted; four fan dried; one pan-fried; and seven did not indicate), whether the tea was loose-leaf or bagged (ten loose leaf; fourteen bagged), and harvest season (six spring; two fall; two year round; and fourteen no indication). These are likely the factors that cause teas to have differing antioxidant capacities.
The Impact of Bags
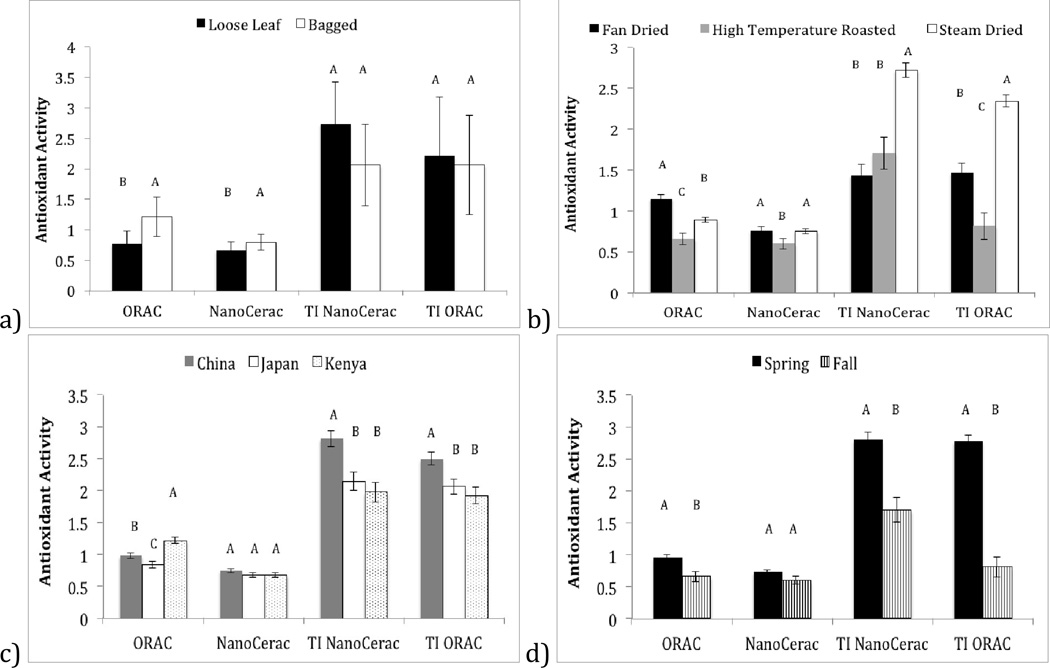
The impact of whether or not a tea is bagged depends on whether you brew the tea once, or several times. Figure S1 shows that eight of ten loose-leaf teas have measurable antioxidant activities even after six brews, while only three of fourteen bagged teas showed measurable antioxidant capacity after six brews. Figure 3a, however, indicates that the final summative antioxidant capacities (TI-AOX) of loose leaf vs. bagged teas does not significant differ, with a mean TI-ORAC of bagged vs. loose-leaf being 2.06 ± 0.81 and 2.21 ± 0.95, respectively (p=0.879), and a mean TI-NanoCerac of bagged vs. loose-leaf being 2.06 ± 0.67 and 2.74 ± 0.67, respectively (p=1). With loose-leaf teas showing greater reusability but the same TI-AOX capacity of bagged teas, it can be assumed that bagged teas must release increased catechins in their early brews. Figure 3a confirms this hypothesis, showing a statistically significant increase in the first-brew AOX capacity of bagged teas as compared to loose-leaf teas (ORAC: 1.21 ± 0.32, bagged; 0.77 ± 0.20, loose-leaf (p<0.0001); NanoCerac: 0.79 ± 0.13, bagged; 0.66 ± 0.13, loose-leaf, p<0.0001). Bagged teas often utilize very finely ground leaf particles, which have increased surface area as compared to loose-leaves, allowing more plant matter to come into direct contact with water during infusion. This increased surface area of ground leaves could cause most catechins to be released in the first-brew, while loose-leaf teas may release catechins in a more sustainable manner over the course of multiple infusions in which whole leaves would gradually open and become hydrated. Our results suggest that although bagged teas have advantages for the first brew, re-brewing loose tea enough times will have the same result.
Figure 3.
Analysis of teas by subcategories of growing location, harvest season, loose leaf vs. bagged, and drying method. Categories were determined using Table S1 in Supplementary Information, which describes teas in detail. One-way ANOVA was performed followed by student T tests, comparing all sample categories, using the JMP statistical analysis program. Differences and similarities of samples are indicated by letter groupings. Samples that do not share a common letter are statistically different.
The Impact of Drying Method
Drying method is quite important in determining final catechin concentration, as this initial drying effort (reducing moisture from 80% to 70% (Bigelow, 2012)) prevents oxidation of catechins by internal polyphenol oxidase (PPO) enzymes before further drying (reducing moisture to 3-4%) and packaging (Bigelow, 2012).
It was found that fan drying is correlated increased first-brew antioxidant capacity (Figure 3b), while steam-drying teas was correlated with increased TI-AOX capacity. A higher TI-AOX capacity in steamed teas indicates that the total amount of catechins preserved in the initial drying process of steam dried teas is significantly more than that of fan dried teas. This could be because of the extended amount of time required for fan drying (12–17 hours (America, 2009)). It is likely that during this time catechins inside the leaf are being oxidized by PPO, while catechins in plant cells closest to the exterior (in contact with the fan) are selectively preserved. Preserving catechins in exterior cells more efficiently than those in interior cells may explain why first-brew AOX capacity is increased for teas prepared using this drying process, as water will first contact these outer cells during infusion. In the case of steam drying, water vapor permeates the entire plant leaf inactivating PPO evenly and rapidly, lending itself to a more sustained catechin release across multiple brews, and a greater amount of catechin preservation, indicated by the higher summative TI-AOX capacity of steam-dried vs. fan-dried teas (TI ORAC: 2.34 ± 0.07, steamed; 1.47 ± 0.11, fan dried (p<0.0001); NanoCerac: 2.72 ± 0.09, steamed; 1.43 ± 0.13, fan dried, p<0.0001). High temperature roasting was found to result in decreased first-brew and TI-AOX capacity according to both ORAC and NanoCerac assays. The decrease in antioxidant capacity is likely because roasting destroys catechins and decreases caffeine content (Tea, 2002, 2007). Although this drying method decreases catechin content, it is preferred by some because of the decreased astringency (Tea, 2002, 2007).
The Impact of Growing Region
It was found that teas grown in China have significantly higher TI-AOX capacity than those grown in other regions (Figure 3c). Seven of eight teas grown in China were grown in the Zhejiang region, which is mountainous and misty. These conditions likely describe the ideal environment for growth and development of Camellia sinensis, creating tea plants with increased catechin content that can be released if infused repetitively.
The ORAC assay recognized, additionally, that teas from Kenya, showed higher first-brew antioxidant capacities. Fan drying is the method of choice for the majority of Kenyan teas studied here, likely explaining why Kenyan teas are also correlated with high first-brew AOX capacity.
The Impact of Harvest Season
A spring harvest was found, by the ORAC assay, to increase AOX capacity the first-brew; and was found by both ORAC and NanoCerac assays to increase TI-AOX capacity (Figure 3d), as compared to a fall harvest. This finding is intuitive as the leaves have only just appeared in spring, and thus have robust antioxidant stores for preservation.
The Impact of other Brewing Conditions
Results of analyses of brewing time, water temperature and quality, time between brews, as well as a comparison of organic vs. non-organic teas, and the effect of caffeinating on antioxidant capacity, have been included in the Supplemental Information section, along with analysis of storage conditions and stability (Figures S2 – S5). This data as a whole indicates that neither decaffeination nor use of organic practices effect antioxidant capacity, and that sale price does not reflect antioxidant content. It was, however, determined that the way in which tea is prepared and re-used can cause great variety in the antioxidant capacity of the resulting infusion. According to these findings, for optimal antioxidant extraction, brews of 5–10 minutes at temperatures 80–100°C will result in infusions with greater antioxidant capacity than teas created using a shorter brewing time and lower temperatures. Tealeaves can be re-used with a gap of at least 1 hour between re-brews without a strong effect on the relative change in antioxidant capacity. Additionally it was shown that tap water can be used to infuse tealeaves without interfering with the true antioxidant capacity of its contained catechins. This finding merits further investigation, however, due to time-dependent epimerization (H. H. K. Wang, 2000) (Cao, Alessio, & Cutler, 1993; Kumamoto, 2001) (Y. H. C.-T. Wang, 2009) (Chen, 2001) and decomposition (Henning, 2003), of catechins in tap water, likely prevented in our assay by the low pH buffer used and the preservation of tea at 4C. In practice one could likely preserve tea catechins through adjustment of pH or temperature; possibly through addition of lemon juice or storing tea in the refrigerator (Figure S3), where it is stable for at least two months.
High-Throughput Adaptation of the NanoCerac Assay
The NanoCerac assay is an innovative technology and this study is the first to utilize it in a high-throughput application including on-the-spot analysis of the antioxidant capacity of a wide variety of samples. The high346 throughput technique for the NanoCerac assay is depicted in Figure S6 in Supplemental Information. Compared to the original assay, we made the following changes: sample volume decreased from 20uL to 2.5uL; A multichannel pipette allowed simultaneous deposition of six samples; full sensing sheets (10cm diameter) were used instead of individual sensors (0.6cm diameter); full sheets were scanned for analysis, instead of manually reading each color spot using the CapSure® device; color spots were automatically analyzed and calibration curves assembled using a MatLab® algorithm that functioned without user operation, instead of inputting a RGB values and manually making calibration curves in Excel®. These modification provided the following functionality improvements and other benefits: the total analysis time was greatly decreased for multiple reasons including: significant decrease in sample drying time (from 90 minutes to 2 minutes); decrease in sensor handling time (no hole punching of sensors or placement on sticker paper necessary); and automated analysis (scanner and MatLab® instead of CapSure® and Excel®). In the assay, 2.5uL of multiple samples were deposited at a time, onto one full sensing sheet (10cm diameter), which was immediately scanned and analyzed using MatLab®. The original technique required the pipetting of 20uL of one sample at a time, onto a pre-cut 0.6cm sensor; followed by the placement of each individual sensor onto a sticker paper, the manual reading of each color response using the handheld CapSure® device, and then recording of values into excel, followed by manual creation of the calibration curve. The new high-throughput method greatly decreases the time required for analysis using this assay for multiple reasons including: significant decrease in drying time (from 90 minutes to 2 minutes); decrease in sensor handling time (no hole punching of sensors or placement on sticker paper necessary); and automated analysis (scanner and MatLab® instead of CapSure® and Excel®). This method allowed for high-throughput analysis of nearly fifty samples in two hours; less than the time required for complete analysis of just one sample using the previously published technique. Utilizing this high-throughput method, 1,296 samples were analyzed within a cumulative time period of less than twenty-four hours; a remarkable improvement from the original assay. This technique is especially recommended for any situation that requires the creation of a large number of calibration curves. We have recently demonstrated the identification of polyphenolic constituents using the NanoCerac assay with a large database of color reference standards, currently being created using a field portable color analysis device18. Using this high-throughput method to create calibration curves however, would greatly simplify the process of creating and utilizing such a database.
Comparison of the NanoCerac and ORAC Assays
Antioxidant capacity analyses for six infusions of twenty-four green teas agreed well between ORAC and NanoCerac assays (average coefficient of correlation R2 0.80 ± 0.19), offering confirmation that the NanoCerac assay evaluates antioxidant capacity with similar precision to commonly used methods. Table 1 discusses strengths and limitations of the NanoCerac assay compared to the ORAC assay, including discussion of reagent requirements, preparation and assay time, ease of data analysis, portability, storage stability, health risks and other features. In summary, the ORAC assay requires more time to prepare, run and analyze. It also requires more reagents, less stable reagents, and reagents that should be handled carefully. The advantage, however, is that the assay runs independently for 90 minutes.
Table 1.
Comparison of the ORAC vs. the NanoCerac assays for use in high throughput analysis.
| Assay | # Reagents |
Prep time |
Assay time |
Data analysis |
Portable | Storage of reagents |
Health risk |
Other Advantages |
|---|---|---|---|---|---|---|---|---|
| NanoCerac | Sample only | 5 min | 2 hours: 48 samples |
1 hour (scanner/MatLab) |
Yes | Highly stable | No | (1) Immediate visual analysis of color. (2) Field use. (3) Very inexpensive |
| ORAC | 3 + sample* | 1 hr | 8 hours: 48 samples |
1 hour (GraphPad/Excel) |
No | 3 months | AAPH | (1) Automated kinetic fluorescence analysis. (2) Increased precision |
Assay requires addition of three reagents: fluorescein, 576 AAPH and buffer.
The assay time was a major advantage of the NanoCerac assay, which required approximately one quarter of the time for the preparation and performance of the assay, however the lab investigator must be present the entire time. The total laboratory technician time required for the NanoCerac assay is equal to that of the ORAC assay (2 hours for 48 samples),with the additional 6 hours of ORAC kinetic assay time, for which the investigator need not be present. The NanoCerac assay is extremely inexpensive and portable, has no known health risks, and once prepared does note require additional reagents. These advantages make it ideal for use in the field for investigation of antioxidant containing plants. The visual color change produced on the sensor surface adds the additional dimension of immediate feedback, allowing for “screening”. The time delay in the application of the ORAC assay, i.e., 90-minutes, makes it less suitable for field and screening applications.
Conclusion
In summary, this study introduced a high-throughput adaptation of the novel NanoCerac assay, and has utilized it to describe the variability in antioxidant capacities of commercial green teas following multiple infusions. The ORAC and NanoCerac assays have been applied side-by-side to effectively monitor the release of catechins by various green tealeaves. This study validates the novel NanoCerac antioxidant capacity assay against the commonly accepted ORAC assay, showing acceptable correlation coefficients.
Analyses of results shown by both the ORAC and NanoCerac assay reveal that as a whole, the antioxidant capacity of green tea varied greatly depending on a wide variety of factors such as: leaf preparation (growing location, harvest season, drying method, and whether tea is loose leaf or bagged); as well as brewing techniques (re-infusions, water temperature, brewing time, and time between re-infusions). The differences in tea quality with regards to antioxidant capacity are not reflected in their prices, and for the teas studied herein, appear not to be affected by modification of tealeaves through decaffeination or pesticide use. Water temperature and brewing time were found to be directly related to antioxidant capacity, while time between re419 brews up to one hour, and use of tap vs. de-ionized water showed no effect. Re-infusion has a significant effect on antioxidant content of tea, and offers several advantages as well.
Those teas found to be best for single use, in terms of antioxidant capacity were largely teas that were bagged, fan dried, and harvested in the spring (i.e. Lipton®, Republic of Tea®, Stash®, Choice®, Tazo®, Twinnings ®, Gunpowder, Yogi®, Buddhist, Zhena’s®, and Gyokuro). Teas found by both the ORAC and NanoCerac antioxidant capacity assays to have the highest TI-AOX capacity largely included teas that were steam dried, grown in China, and/ or harvested in the spring (i.e. Buddhist, Choice® and Stash®, Dragonwell, Mao Feng and Gunpowder). Both assays found first-brew and TI-AOX capacities to decrease significantly when teas are dried using high-temperature roasting (Figure 3).
It was found that loose leaf teas can be re-infused more times than bagged teas (with eight of ten loose-leaf teas showing antioxidant capacity in the sixth brew), however there was no difference in the total inherent antioxidant capacity to be extracted from the loose tealeaves as compared to finely ground bagged tealeaves. There are proposed benefits to re-using tealeaves. These benefits are related to health including: decreased caffeine consumption (as most caffeine is removed in the fist brew) for avoidance of unwanted side-effects of caffeine (Rainy, 1985) (Dworzanki, 2009), environmental and economic savings and well as reduction of waste, as loose leaves can be re-used more times than bagged teas and typically involve less packaging and no commercial tea bags. Additionally, each successive brew will have a unique taste, possibly enhancing the tea drinking experience.
Individuals consuming green tea can use the information included herein to make more informed decisions about green tea selection and preparation for maximal health benefits. It is our intention that this comprehensive exploration of the effect of brewing conditions and re-steeping on the antioxidant capacities of twenty-four green tea varieties can help further understanding of the maximal health benefits to be attained through consumption of green tea.
Supplementary Material
Highlights.
Effects of re-infusion on antioxidant activity of twenty-four green teas are shown.
The impact of brewing conditions (temperature, time, etc.) is also studied.
Causes for varied re-infusion antioxidant activities of green teas are explored.
The portable NanoCerac assay was compared side-by-side, against the ORAC assay.
A comparison of the high-throughput NanoCerac method to the ORAC is included.
Acknowledgements
ES acknowledges the guidance of Dr. Leanna Standish, PhD, ND, LAc, FABNO, Dr. Kaleb Lund, PhD, Dr. Masa Sasagawa, ND, and Cindy Butler-Smith for their guidance; as well as the Bastyr University Tierney Research Laboratory for hosting this study.
This material is based upon work supported by the National Center for Complementary and Alternative Medicine at the National Institutes of Health under the Pre-doctoral Training Grant T32AT00815, as well as work supported by the National Science Foundation, Grant No. 0954919. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author (s) and do not necessarily reflect the views of the National Center for Complementary and Alternative Medicine or the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alyabyev AGL, Loseva N, Rachimova G, Tribunskih V, Estrina R, Sadunishvili T, Gulua L, Mchedlishvili N, Lopez-Rodriguez JN. The effect of a natural inhibitor isolated from the tealeaf on the energy processes in model systems. Thermochimica Acta. 2004;422:109–113. [Google Scholar]
- America, T. N. A World of Tea. [Retrieved February 2013];2009 from http://www.twiningsusa.com/template.php?id=26. [Google Scholar]
- Benzie IFF, S J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of "Antioxidant Power": The FRAP Assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bigelow Universitea: Tea descriptions. [Retrieved February 2013];2012 from http://www.bigelowtea.com/universitea/tea-descriptions.aspx. [Google Scholar]
- Cao G AH, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993;14(3):303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- Cao G, Alessio H, Cutler R. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- Chen ZZ, Q Y, Tsang D, Huang Y. Degradation of Green Tea Catechins in Tea Drinks. J. Agric. Food Chem. 2001;49(1):477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- Cooper RM, James, Morre Dorothy. Medicinal Benefits of Green Tea: Part I. Review of Noncancer Health Benefits. The Journal of Alternative and Complementary Medicine. 2005;11(3):521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- Ananth DA, S T, Rameshkumar A, Jeyadevi R, Aseervatham SB. Chemical constituents, in vitro antioxidant and antimicrobial potential of Caryota urens L. Free Radicals and Antioxidants. 2013;3:107–112. [Google Scholar]
- Doughty MJ. pH dependant spectral properties of sodium fluorescein opthalmis solutions revisited. Opthal. Physiol. Opt. 2010;30:167–174. doi: 10.1111/j.1475-1313.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Dulloo Aea. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine, and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- Dworzanki WOG, Burdan F. Side Effects of Caffeine. Pol Merkur Lekarski. 2009;161:357–361. [PubMed] [Google Scholar]
- E Sharpe RB, Frascot T, Jayathilaka D, Marsh A, Andreescu S. Metal oxide based multisensor array and portable database for field analysis of antioxidants. Sensors and Actuators B-Chemical. 2014;193:552–562. doi: 10.1016/j.snb.2013.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E Sharpe SA. Portable Nanoparticle Based Sensors for Antioxidant Analysis. In: Armstrong D, editor. Advanced Protocols in Oxidative Stress III. Humana Press; 2013. [Google Scholar]
- Elmets Cea. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44(425–432) doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- Fassina GBA, Benelli R, Varnier OE, et al. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS. 2002;16:939–941. doi: 10.1097/00002030-200204120-00020. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Centeno MR, Jourdes M, Femenia A, Simal S, Rossello C, Teissedre PL. Characterization of Polyphenols and Antioxidant Potential of White Grape Pomace Byproducts (Vitis vinifera L.) J Agric Food Chem. 2013 doi: 10.1021/jf403168k. [DOI] [PubMed] [Google Scholar]
- Henning S, Fajardo-Lira C, Lee HW, Youssefian AA, Go VLW, Heber D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates With the Antioxidant Capacity. Nutrition and Cancer. 2003;45(2):226–235. doi: 10.1207/S15327914NC4502_13. [DOI] [PubMed] [Google Scholar]
- Hodge VJAJ. A Survey of Outlier Detection Methodologies. Artificial Intelligence Review. 2004;22:85–126. [Google Scholar]
- Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005;52(6):1049–1059. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Hsu S. Green Tea and Beyond. Nova Biomedical Science Publishers; 2010. [Google Scholar]
- Kao Yea. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee HJ, Shin Y. Optimization and Validation of High-Performance Liquid Chromatography Method for Individual Curcuminoids in Turmeric by Heat-Refluxed Extraction. J Agric Food Chem. 2013 doi: 10.1021/jf402483c. [DOI] [PubMed] [Google Scholar]
- Kumamoto MSTNK, Tabata M. Effects of pH and Metal Ions on Antioxidative Activities of Catechins. Biosci. Biotechnol. Biochem. 2001;65(1):126–132. doi: 10.1271/bbb.65.126. [DOI] [PubMed] [Google Scholar]
- Kyle JAMM, Philip C, McNeill Geraldine, Duthie Garry G. Effects of Infusion Time and Addition of Milk on Content and Absorption of Polyphenols from Black Tea. J. Agric. Food Chem. 2007;55:4889–4894. doi: 10.1021/jf070351y. [DOI] [PubMed] [Google Scholar]
- Lobato IN, J C, Savin NE. Testing for Autocorrelation Using a Modified Box-Pierce Q Test. International Economic Review. 2001;42:187–205. [Google Scholar]
- Miller NR-EC, Davies MJ, Gopinathan V, Milnera A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Perva-Uzunalic AS, Mojca, Zeljko Knez, Bernd Weinreich, Frank Otto, Sabine Gruner. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chemistry. 2006;96:597–605. [Google Scholar]
- Rainy J. Headache related to chronic caffeine addiction. Tex Dent J. 1985;102(7):29–30. [PubMed] [Google Scholar]
- Service, U. A. R. Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010) [Press release] 2012 Retrieved from http://www.ars.usda.gov/News/docs.htm?docid=15866.
- Sharpe E, Frasco T, Andreescu D, Andreescu S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac) Analyst. 2012;138(1):249–262. doi: 10.1039/c2an36205h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V, Orthofer R, Lamuela-Raventos R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Tea Association of the USA, I. Tea Fact Sheet [Press release] 2013 Retrieved from http://www.teausa.com/14655/tea-fact-sheet. [Google Scholar]
- Tea Ro. Tea Ching: The Tea and Herb Companion. NY,NY: 2002, 2007. [Google Scholar]
- Tsao R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HHK. Epimerisation of catechins in green tea infusions. Food Chemistry. 2000;70:337–344. [Google Scholar]
- Wang YHC-T. Polyphenolic Chemistry of Tea and Coffee: A Century of Progress. J. Agric. Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- Yamamoto Tea. Green Tea Polyphenol Causes Differential Oxidative Environments in Tumor versus Normal Epithelial cells. The Journal of Pharmacology and Experimental Therapeautics. 2003;307(1):230–236. doi: 10.1124/jpet.103.054676. [DOI] [PubMed] [Google Scholar]
- ZenBio ORAC Antioxidant Assay Kit Cat # AOX-2 Instruction Manual ZBM0035.00: ZenBio Laboratories. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.