Abstract
Objective
The objective of this study was to compare immunohistochemical profile for the apoptosis regulators p53, C-MYC, bax, PUMA, and PTEN and the cell cycle regulatory proteins p21 and p27, as well as clinical factors between types I and II tumors.
Methods
In total, 131 patients in FIGO (International Federation of Gynecology and Obstetrics) stages I-II were divided into 2 groups of patients after type I tumors (n = 79) and type II tumors (n = 52). Differences in the immunohistochemical profile for the cell cycle–related proteins, detected by tissue microarrays and immune-histochemistry, were compared. For statistical tests, the Pearson χ2 test and the logistic regression model were used. All tests were 2-sided, and the level of statistical significance was P ≤ 0.05.
Results
In multivariate logistic regression analysis with recurrent disease as endpoint, FIGO stage (odds ratio [OR], 4.7), type I/II tumors (OR, 3.8), body mass index (BMI) (OR, 3.5), and p53 status (OR, 4.2) all were found to be independent predictive factors. In 2 different multivariate logistic regression analyses with type I/II tumors as endpoint, both p53+p21− (OR, 2.9) and p27 status (OR, 3.0) were associated with type II tumors. Differently, C-MYC status (OR, 0.4) was associated with type I tumors. Furthermore, age (OR, 1.04), BMI (OR, 0.4), and recurrent disease (OR, 4.3) all were associated to type II tumors. In survival analysis, there was a trend (P = 0.054) toward better disease-free survival for patients with type I tumors.
Conclusions
Concomitant positivity for p53 and negativity for p21, positivity for p27, and negativity for C-MYC in an epithelial ovarian tumor might strengthen the diagnostic option of type II tumor ovarian carcinoma. Patients with type II tumors were older, had lower BMI, and had more often recurrent disease than patients with type I tumors.
Key Words: Concomitant p53+p21−, FIGO stage I-II, Ovarian cancer; p27, Type I/II tumors
Epithelial ovarian cancer (EOC) is still the leading cause of death among women with gynaecological malignancies.1 Based on histopathology and molecular genetic alterations, ovarian carcinomas are divided into 5 main histological types.2 Furthermore, on the basis of morphologic and molecular genetic studies, the 5 types of ovarian cancer can be classified into 2 groups designated types I and II tumors. Thus, type I tumors are composed of low-grade serous (grade 1), low-grade endometrioid (grade 1 or 2), mucinous, and clear cell carcinomas. Type II tumors consist of high-grade serous (grade 2 or grade 3) and high-grade endometrioid (grade 3).3,4 Type I tumors often present in early stage and are slowly growing from benign cystic neoplasm through borderline tumor. They are relatively stable genetically and rarely harbor TP53 mutations with exception of concomitant KRAS mutations in low-grade mucinous tumors.5 In contrast, type II tumors are rapidly growing and more often present in advanced stage. They are genetically highly unstable and have a high frequency of TP53 mutations. There is mounting evidence that types I and II ovarian tumors develop along different molecular pathways.4,6 Some differences in clinical aspects between types I and II tumors have been noted.1 The classic clinicopathological factors have been proven insufficient to define prognostic subgroups.7 It is now widely accepted that cancer can arise as a cell cycle defect, but cellular homeostasis is regulated by proliferation, growth arrest, and apoptosis.8,9 Apoptosis is a biological process controlled by different regulators and potentially could be a goal for new cancer treatment approaches.10 As a transcription factor, wild-type p53 can limit cell proliferation after DNA damage by arresting the cell cycle or inducing apoptosis through the mitochondrial pathway by activating the expression of bax and PUMA.11 However, certain mutants of p53 can inhibit the proapoptotic function of p53 through the formation of heterotetramer with wild-type p53 and induce oncogenesis after up-regulation of C-MYC (Fig. 1).12 PTEN is a main tumor suppressor and induces apoptosis by up-regulation of wild-type p53.13 The cell cycle regulators p21 and p27 are belonging to 1 (the Cip/kip family) of the 2 major families regulating the cell cycle.14 The p21 gene is the primary mediator of p53-induced cell cycle arrest, and cells lacking functional p53 express only low levels of p21.10
FIGURE 1.
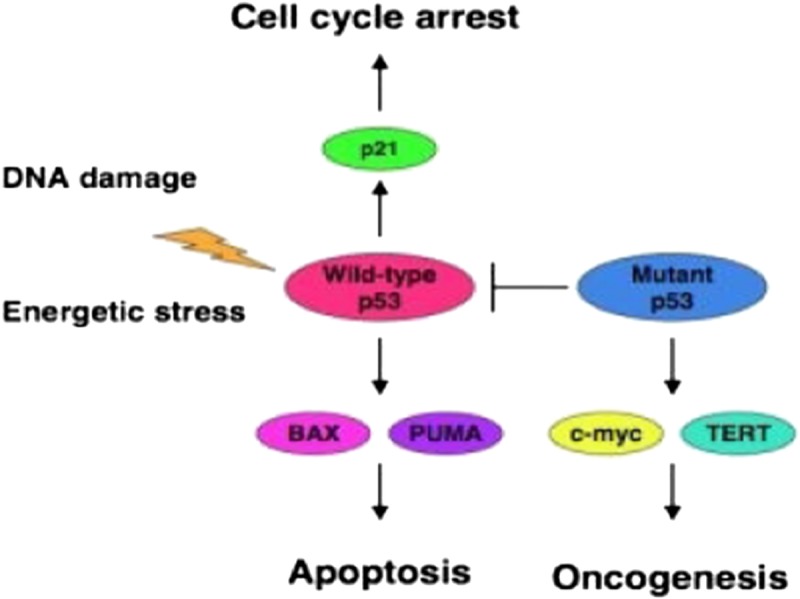
Wild p53 has important function for both cell cycle arrest and apoptosis. However, certain mutants of p53 can inhibit the proapoptotic function of p53 through the formation of heterotetramer with wild-type p53 and induce oncogenesis after up-regulation of C-MYC.
The primary aim of this study was to compare some clinicopathological features and differences in the immunohistochemical (IHC) profile for the apoptosis regulators p53, C-MYC, bax, PUMA, and PTEN and the cell cycle regulatory proteins p21 and p27, detected in previous studies,15–18 between the groups of types I and II tumors in patients with International Federation of Gynecology and Obstetrics (FIGO) stages I-II ovarian carcinoma.
MATERIALS AND METHODS
In total, 131 of 140 consecutive patients with FIGO stage (1988 FIGO staging) I-II EOC, who underwent primary surgery and postsurgical chemotherapy in the Uppsala-Örebro Medical Region during the 5-year period from January 1, 2000, to December 31, 2004, were entered into this study. All samples were collected with the patient’s informed consent (131 of 140 patients accepted) and were in compliance with the Helsinki Declaration19 and used in accordance with the Swedish Biobank Legislation and Ethical Review Act (approval by Uppsala Ethical Review Board, decision ref. UPS-03-477). There were 131 available tumors for analysis of p53 and p27, 130 tumors for PTEN and bax, and 129 tumors for analysis of p21, C-MYC, and PUMA (lower numbers because of technical issues in the staining process).
The staging procedure was done at the time of primary surgery. Modified surgical staging according to the European Organisation for Research and Treatment of Cancer surgical staging20 was undertaken in 34 (26%) of 131 cases, and in the remaining 77 patients (74%), surgical staging was regarded as minimal or inadequate. All patients had chemotherapy 4 to 6 weeks after primary surgery, usually (n = 105) paclitaxel 175 mg/m2 and carboplatin (area under the curve = 5) at 3-week intervals usually in 4 courses or single-drug carboplatin in 4 to 6 courses. The mean follow-up time was 65 months (range, 5–110 months). Survival was defined as date of confirmed histological diagnosis after primary surgery to date of recurrence, death, or last visit.
Tissue Microarray, Immunohistochemistry, and Interpretation
The specimens were obtained from the paraffin blocks containing the embedded tissue removed from the tumor at primary surgery, and after staining with hematoxylin-eosin, they were classified and graded by a single pathologist. The tissue microarrays were constructed as described previously.21 Two tissue core specimens (diameter, 0.6 mm) from all 131 ovarian carcinomas were arranged in 3 recipient paraffin blocks. The presence of tumor tissue on the arrayed samples was verified by hematoxylin-eosin–stained section by a pathologist. Five-micrometer-thick sections were cut from each multitissue block and were put on coated slides and dried overnight at 37°C. The sections were pretreated by heath-induced epitope retrieval in target-retrieval solution (Dako, Glostrup, Denmark), pH 6, or EDTA buffer, pH 9, for 7 + 7 minutes in microwave oven (99°C). Blocking with peroxidase was performed for 5 minutes. The slides were counterstained with hematoxylin for 2 minutes.
Information about the primary antibodies and IHC analyses can be found in previous studies.15–18 The IHC stainings were interpreted by 2 of the authors (I.S. and T.S.). At the time of evaluation, no information was available on the specific diagnosis and prognosis for the individual cases. A semiquantitative analysis22 was used, and the stainings were graded as negative, +, ++, and +++ for p53, p21, p27, C-MYC, PTEN, and bax, and all those markers were dichotomized into negative and positive (+, ++, +++) cases.23 However, for PUMA, weak positive staining (+) was graded as negative (−), and moderate/strong staining (++/+++) graded as positive.
The staining for p21 and p53 was considered to be positive when there was a strong, granular staining of the nuclei of the majority of tumor cells, but the staining for p27 and PTEN was considered to be positive when strong granular staining of the nuclei and cytoplasm of the tumor cells was found. Localization for C-MYC staining was nuclear, cytoplasmic, and membranous in the tumor cells. Staining for bax showed faint, nonspecific staining of the cytoplasm. However, the cases, which were interpreted as positive, showed strong granular and punctuate staining of the cytoplasm in most of the tumor cells. Weak cytoplasmic staining for PUMA was detected in tumor cells in almost all tumors evaluated. As negative staining of cytoplasm hardly was detected in this series of patients, our findings were limited to weak or moderate/strong staining of cytoplasm.
Statistical Analysis
The Pearson χ2 test was used for testing proportional differences in univariate analyses. The survival curve was generated by using the Kaplan-Meier technique, and differences between these curves were tested by the log-rank test. The logistic regression model was used for both univariate and multivariate analysis with recurrent disease and tumor type (I/II) as the endpoint, respectively. All tests were 2-sided, and the level of statistical significance was P ≤ 0.05. The STATISTICA 12.0 (StatSoft) statistical package for personal computers was used for the analyses.
RESULTS
Patients
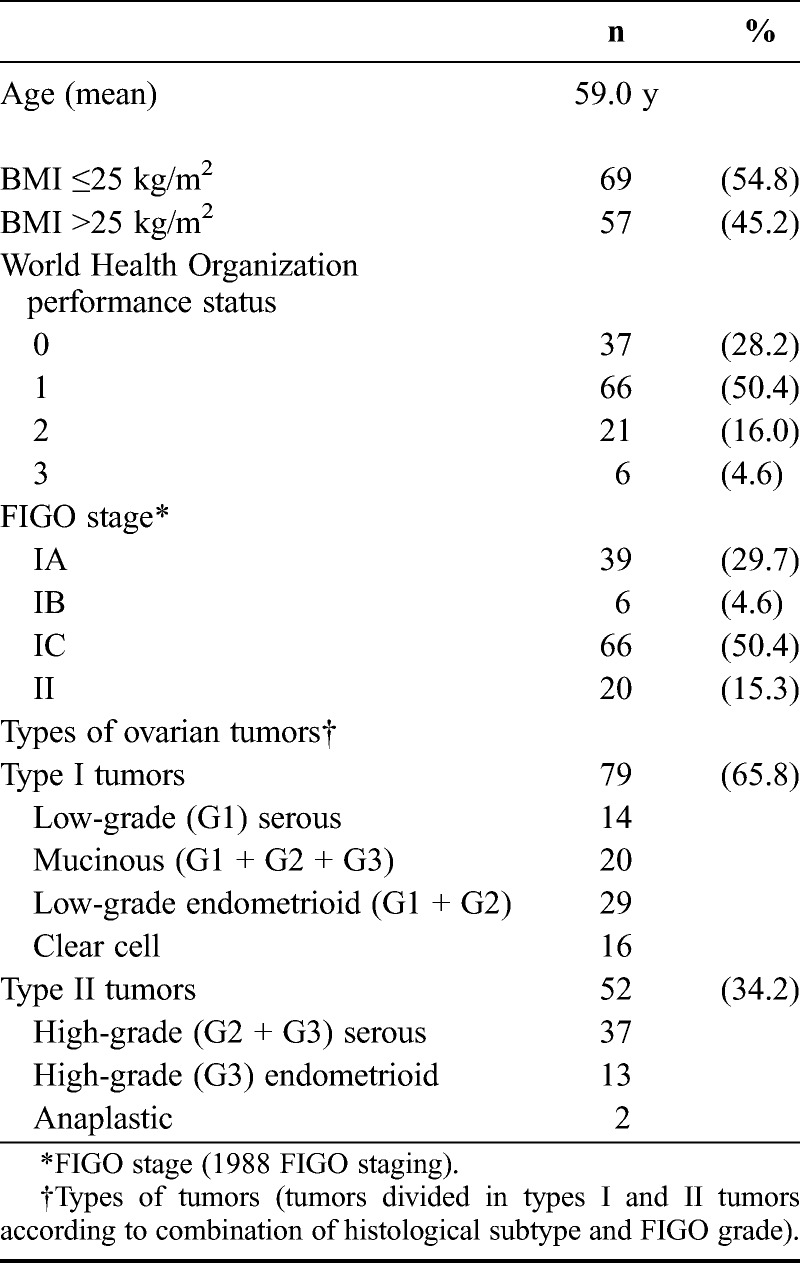
Patients’ characteristics are demonstrated in Table 1. The study population included 79 type I tumors (65.8%) and 52 type II tumors (34.2%). Primary cure was achieved in all 131 patients. The number of recurrences in the complete series was 34 (26%) of 131, and 22 of these patients died because of disease. In the complete series, recurrent disease was associated with FIGO substages (P = 0.0005), FIGO grade (P = 0.030), adequate surgical staging (P = 0.033), and residual disease (P = 0.001). The 5-year disease-free survival rate was 68%, the disease-specific survival rate was 76%, and the overall survival rate was 71%.
TABLE 1.
Patients’ characteristics (n = 131)
Clinical Features Compared Between Type I and Type II Tumors
The mean age of patients with type I tumors (57.2 years) did not differ from that in type II group (60.7 years) (Table 2). However, the group of patients with type I tumors had significantly (P = 0.039) higher body mass index (BMI) compared with the type II group. Residual tumor after primary surgery (all 6 patients in FIGO stage IIC) occurred more frequently (P = 0.025) in type II tumors. Recurrent disease was more frequently (P = 0.025) found among patients with type II tumors, and it was a trend (P = 0.054) toward better disease-free survival among patients of type I tumors (Fig. 2).
TABLE 2.
Clinical features compared between types I and II tumors
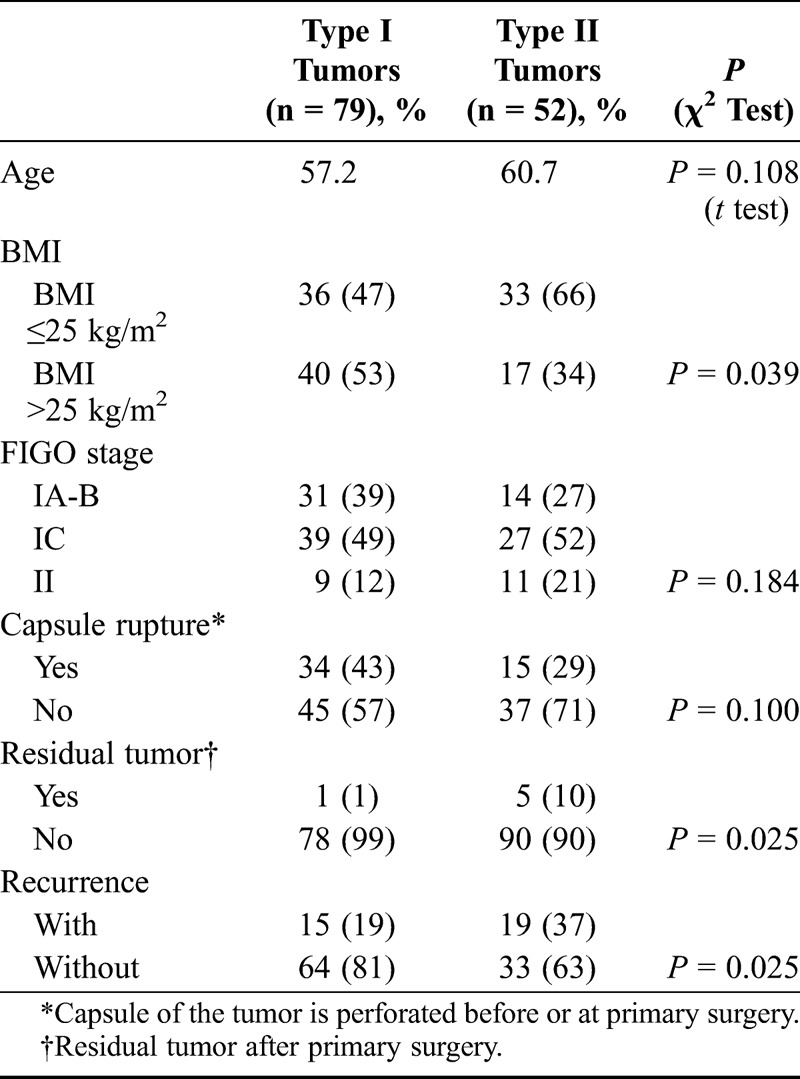
FIGURE 2.
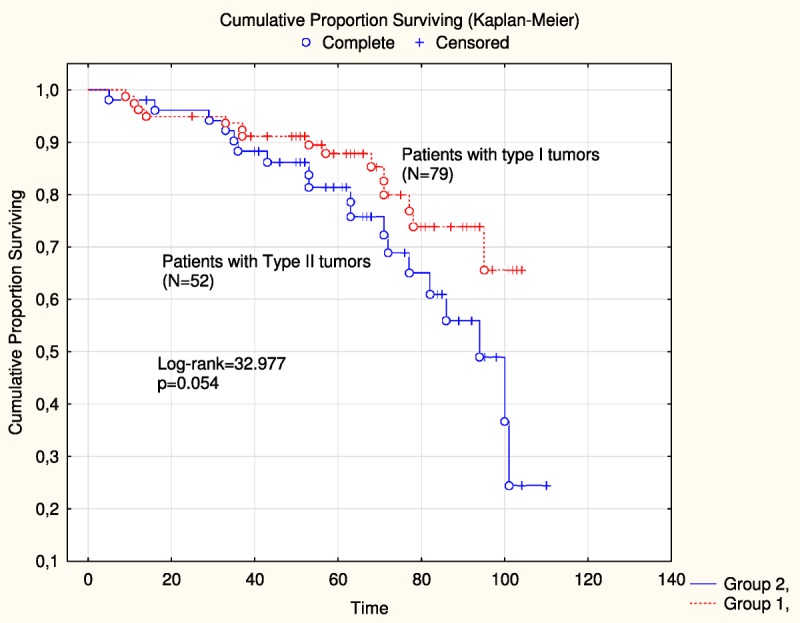
Disease-free survival was compared between patients with type I tumors (n = 79) and type II (n = 52) tumors in the study.
Cell Cycle–Associated Proteins Compared Between Type I and Type II Tumors
In previous studies15–17 including the total series of patients (n = 131) and18 including 105 (adjuvant taxane chemotherapy) of 131 patients, respectively, results from IHC for some cell cycle–associated proteins and several combinations of those have already been presented. The status of protein expression (positivity/negativity) for the apoptosis regulators, p53, C-MYC, bax, PUMA, and PTEN and the cell cycle regulatory proteins p21 and p27 and some combinations of those is presented (Table 3) and compared between types I and II tumors. Positive staining for p53 and p27 alone was more frequently detected in type II tumors (Fig. 3). Concomitant positivity for p53 and negativity for p21 (p53+p21−) and concomitant positivity for bax and negativity for p53 (bax+p53−) also were more frequently found in type II tumors compared with expression status for alternative combinations of biomarkers detected in type I tumors. Differently, concomitant positivity for p21 and p27 (p21+p27+), concomitant positivity for C-MYC and negativity for p53 (C-MYC+p53−), concomitant positivity for C-MYC and p21 (C-MYC+p21+), and concomitant negativity for PUMA and p53 (PUMA−p53−) were more frequently detected in type I tumors compared with expression status for alternative combinations of those biomarkers found in type II tumors.
TABLE 3.
Protein expression of apoptosis regulators versus types I and II ovarian tumors (n = 131)
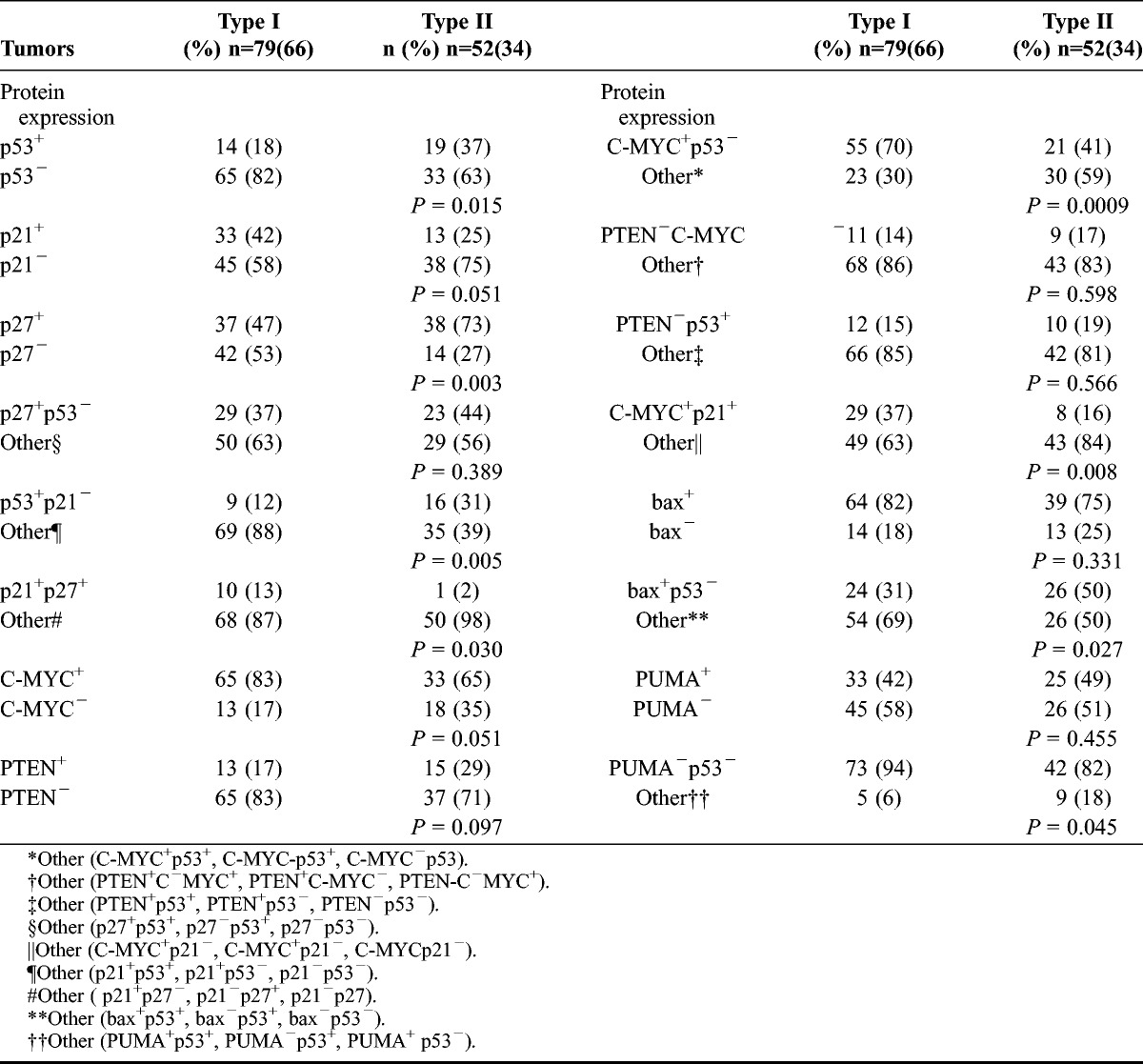
FIGURE 3.
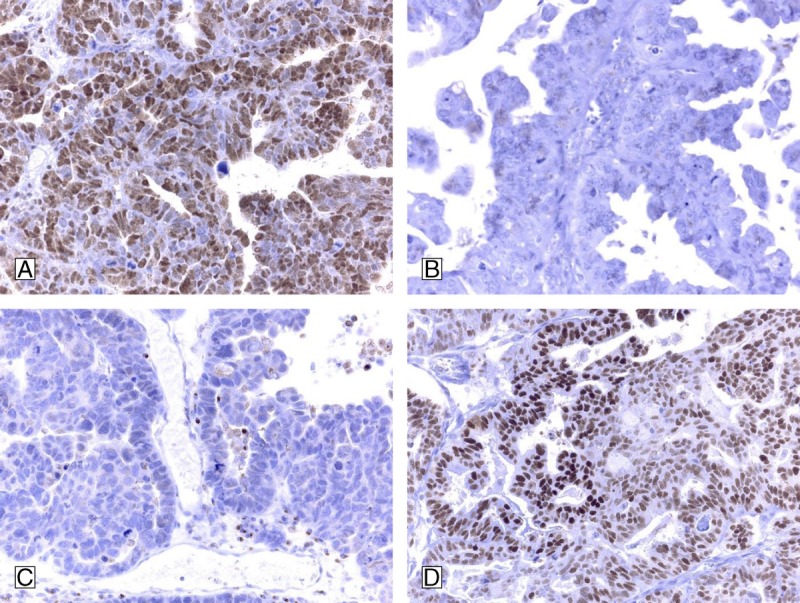
Immunohistochemical results for type II tumor (high grade serous) are demonstrated: A, High grade serous carcinoma with strong positivity for p27. B, High grade serous carcinoma, negative for p21. C, High grade serous carcinoma, negative for c-myc. D, High grade serous carcinoma with p53-expression. Concomitant positivity for p53 and negativity for p21, positivity for p27, and negativity for C-MYC in an epithelial ovarian tumor might strengthen the diagnostic option of type II tumor ovarian carcinoma.
Multivariate Analysis
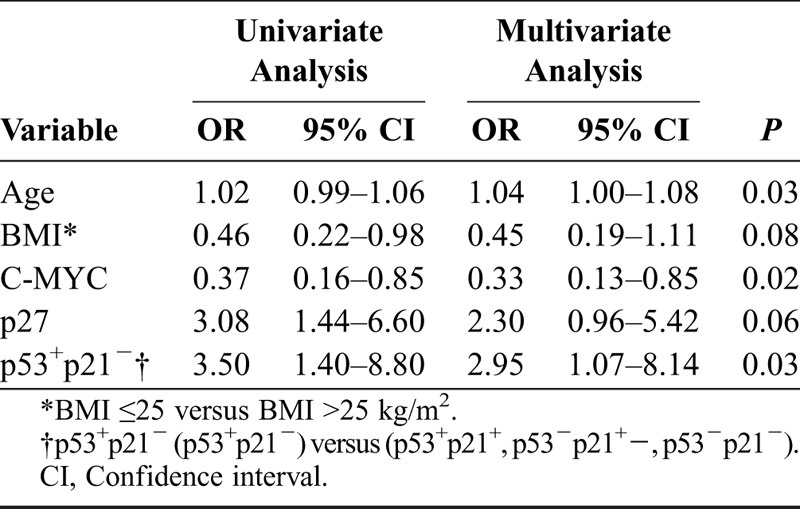
Results are shown in Tables 4A-C for univariate and multivariate logistic regression analyses. In Table 4A, with recurrent disease as endpoint, FIGO stage, tumor type (I vs II), BMI in the 2 groups, and p53 status all were independent predictive factors for recurrent disease. In a multivariate logistic regression analysis with type I/II tumors as endpoint, recurrent disease and status of p53 or p53+p21− were not introduced in the multivariate model together because of strong correlation between them. In Table 4B, age, status of C-MYC, and concomitant positivity for p53 and negativity for p21 all were independent predictive factors. In a separate multivariate logistic regression analysis with type I/II tumors also as endpoint (Table 4C), age, BMI, p27 status, and recurrent disease all were independent predictive factors.
TABLE 4.
A. Predictive factors for recurrent disease (univariate and multivariate logistic regression analysis)
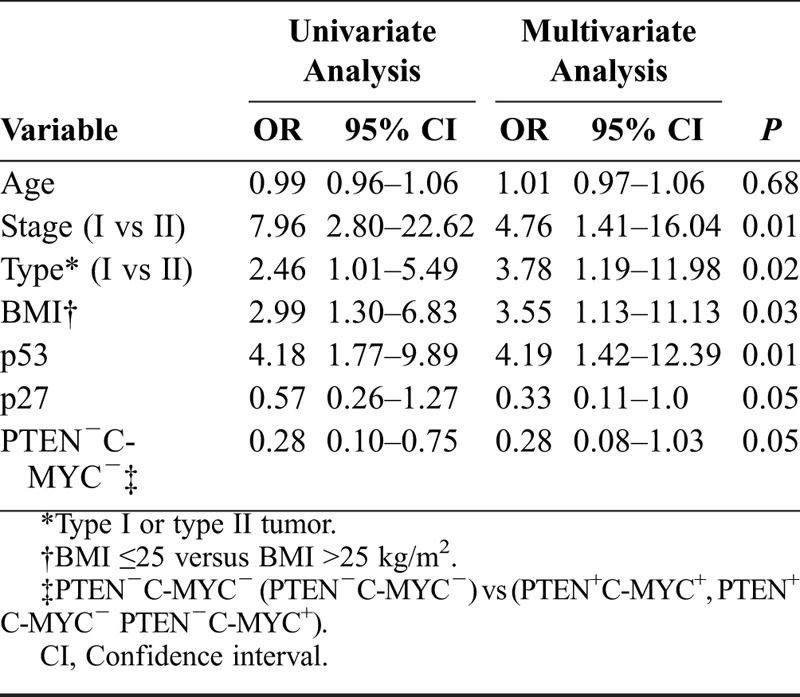
TABLE 4.
C. Possible predictive factors that distinguish types I and II tumors EOC in FIGO stages I-II (univariate and multivariate logistic regression analysis)
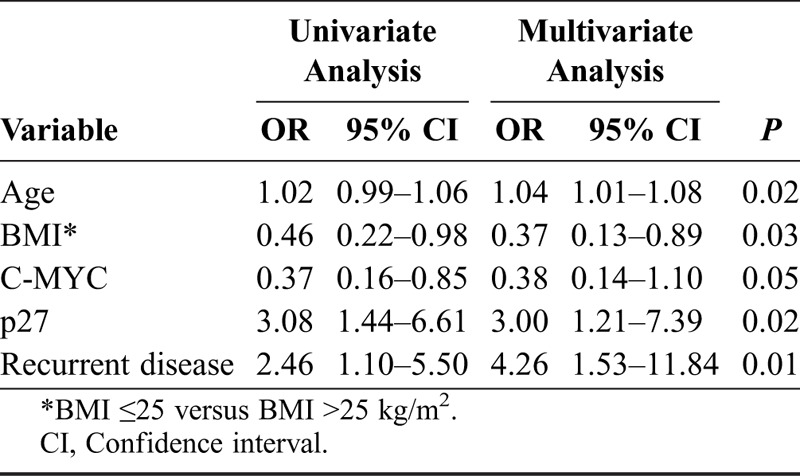
TABLE 4.
B. Possible predictive factors that distinguish types I and II tumors EOC in FIGO stages I-II (univariate and multivariate logistic regression analysis)
DISCUSSION
Findings from the present study have confirmed that types I and II ovarian tumors exhibit different clinicopathological and IHC features. This is confirmed by previous studies on the basis of morphologic and molecular genetics, showing, that the 5 main histological types of ovarian cancer can be classified into types I and II.2,7 Positive staining for the apoptosis regulator p53, concomitant positivity for p53 and negativity for the cell cycle regulator p21 (vs other combinations in one group), and positive staining for the cell cycle regulator p27 were more frequently detected in type II tumors. Differently, positive staining for the apoptosis regulator C-MYC was more frequently seen in type I tumors. Our findings related to the IHC differences between types I and II tumors are supported by earlier studies.4,10,24
Differences in clinical aspects between types I and II tumors have also been noted. Thus, patients with type II tumors were of higher age, had lower BMI, and had more often recurrent disease than patients with type I tumors. Previous studies have reported that patients with low-grade serous carcinoma are younger than those with high-grade serous tumors and that there is an elevated risk for EOC in women with higher BMI for type I tumors.24,25 Furthermore, recurrent disease occurred more frequently among patients in the subgroup of type II tumors, which had 3.8 times increased risk of recurrent disease compared with women with type I tumor. In our series, there was a trend (P = 0.054) for better disease-free survival for patients with type I tumors compared with patients with type II tumors (Fig. 2).
Nearly 30% of the EOC patients will present at diagnosis with stage I-II disease with a favorable survival. However, about 30% of those patients will have recurrent disease and survival that is comparable with survival after recurrent disease for patients with stage III-IV disease.1 In our study limited to FIGO stages I-II, the recurrence rate was 26% for all patients with types I and II tumors in 1 group, and in multivariate analysis, the FIGO stage was the strongest (odds ratio [OR], 4.7) clinical predictive factor for recurrent disease. In a study from Canada26 on 605 patients in FIGO stages I-II, the FIGO stage was a strong (OR, 8.0) predictive factor for tumor recurrence. The p53 status was also an independent predictive factor for recurrent disease in the present study with an OR of 4.2. This has been confirmed in other studies.27,28 In a meta-analysis including 62 studies, a hazard ratio of 1.47 for p53 status was found with overall survival as endpoint.29
There is mounting evidence that types I and II ovarian tumors develop along different molecular pathways,4,6 and tumor type is considered to reflect different diseases with different clinical behavior, response to chemotherapy, and prognosis.26 The apoptosis regulator p53 was an independent predictive factor for type II tumor only in combination with the cell cycle regulator p21. Thus, the IHC profile of p53 positivity and concomitant negativity of the cell cycle regulator p21 was unique for type II tumors. The p21 gene is the primary mediator of p53-induced cell cycle arrest, and cells lacking functional p53 express only low levels of p21.10 Presence of the cell cycle regulator p27 alone was mostly (OR, 3) detected in type II tumors, and an OR of 0.3 for p27 status with recurrent disease as endpoint meant 70% decreased risk for a patient with p27 positivity of the tumor to have recurrent disease. In 1 study,30 the p27 status alone was related to better survival or in combination with p53 in another study.15 Differently, presence of the apoptosis regulator C-MYC was a predictive factor for type I tumor in a logistic regression analysis. The proposed model by assigning different ovarian tumors into 2 categories (types I and II) based on clinical, morphological, and molecular genetic characteristics facilitates understanding the pathogenesis of ovarian cancer.31 Type I tumors are considered as slowly growing neoplasm through borderline tumors, but according to 1 study,32 it is possible that some serous type I tumors can progress to serous type II tumors. How could this new proposed model, which divides ovarian tumors into 2 different artificial types of tumors, become helpful in the clinics? At present, adjuvant therapy for ovarian cancer is mainly dependent on FIGO stage and grade rather than type, and many hospitals have recommended that patients with ovarian carcinoma in FIGO stage IA of tumor grade 1 should not receive adjuvant therapy.33 Type I tumors, which are considered resistant to traditional chemotherapeutic agents, may in the future have treatment guidelines, which are different from type II tumors.24 The grade assignment lacks reproducibility depending on the grading system used, and tumor grade does not alone reflect the biological differences of the different types. Based on improvements in how many types are assigned by pathologists, the reproducibility of typing is much better than that of grading.34,35
Differences in the IHC profile for p53 p21 status, p27 status, and C-MYC status between types I and II tumors were detected in the present study. Concomitant positivity for p53 and negativity for p21, positivity for p27, and negativity for C-MYC in an epithelial ovarian tumor might strengthen the pathological diagnosis of type II tumor ovarian carcinoma. Thus, our findings might be useful in the histopathologic distinction of types I and II ovarian carcinoma, and the use of designed panels, based on observations in a defined tumor material, as in this series, may increase the diagnostic options. Tumor type has more reproducibly assigned than grade and identifies a larger cohort of women with stage I/II ovarian carcinoma with favorable outcomes and therefore is superior to grade in estimating risk of death from ovarian carcinoma.26
However, some authorities in the field are in some disagreement with the new classification into 2 groups, designed as types I and II tumors. Thus, clear cell carcinoma exhibits morphologic, molecular, and clinical features that do not entirely resemble either type I or type II tumors, and unlike other type I tumors, clear cell carcinoma is designed as high grade at presentation.36,37 This is in agreement with recent findings from Zannoni et al,36 where they show that clear cell ovarian carcinoma should be studied separately, but still in comparison with the groups of types I and II tumors. It is likely, that serous type II carcinomas containing cells with clear cytoplasm have erroneously been classified as clear cell type I carcinoma in different studies.38 According to Prat,2 the classification into just 2 types (I and II) is artificial and limits progress in understanding the biology of the less common types of ovarian carcinoma. Limitations of this study correspond to the relative limited number of patients included and the tissue microarray technology, where only 2 0.6-mm core biopsies were obtained from each specimen for analysis as ovarian carcinomas can be very heterogeneous, and such specimens may not be representative of the tumor in some cases. Finally, the method of semiquantitative analysis22 was used for the interpretation, where all markers were dichotomized into negative and positive groups.23 However, our findings might be useful in the pathologic diagnosis of distinction of types I and II epithelial ovarian carcinoma.
Footnotes
No funding was received for this work.
The authors declare no conflicts of interest.
REFERENCES
- 1. Chan JK, Tian C, Teoh D, et al. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010; 116: 307– 311. [DOI] [PubMed] [Google Scholar]
- 2. Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012; 23 (suppl 10): x111– x117. [DOI] [PubMed] [Google Scholar]
- 3.McCluggage: Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogene. Pathology. 2011; 420– 432. [DOI] [PubMed] [Google Scholar]
- 4. Kurman RJ, Shih I-M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010; 34: 433– 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rechsteiner M, Zimmermann A-K, Wild PJ, et al. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol. 2013; 95: 235– 241. [DOI] [PubMed] [Google Scholar]
- 6. Kurman RJ, Shih I-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol. 2011; 42: 918– 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crijns APG, Duiker EW, de Jong S, et al. Int J Gynecol Cancer. 2006; 16 (suppl 1): 152– 165. [DOI] [PubMed] [Google Scholar]
- 8. Nam EJ, Kim YT. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer. 2008; 18: 1169– 1182. [DOI] [PubMed] [Google Scholar]
- 9. Foster I. Cancer: A cell cycle defect. Radiography. 2008; 14: 144– 149. [Google Scholar]
- 10. Lee YH, Heo JH, Kim TH, et al. Significance of cell cycle regulatory proteins as malignant and prognostic biomarkers in ovarian epithelial tumors. Int J Gynecol Pathol. 2011; 30: 205– 217. [DOI] [PubMed] [Google Scholar]
- 11. Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005; 24: 2899– 2908. [DOI] [PubMed] [Google Scholar]
- 12. Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel). 2011; 3: 994– 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YK, Park NH. Prognostic value and clinicopathological significance of p53 and PTEN in epithelial ovarian cancers. Gynecol Oncol. 2009; 112: 475– 480. [DOI] [PubMed] [Google Scholar]
- 14. Bali A, O’Brien PM, Edwards LS, et al. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004; 10: 5168– 5177. [DOI] [PubMed] [Google Scholar]
- 15. Skirnisdottir IA, Sorbe B, Lindborg K, et al. Prognostic impact of p53, p27, and C-MYC on clinicopathological features and outcome in early-stage (FIGO I-II) epithelial ovarian cancer. Int J Gynecol Cancer. 2011; 21: 236– 244. [DOI] [PubMed] [Google Scholar]
- 16. Skirnisdottir I, Seidal T. Prognostic impact of concomitant p53 and PTEN on outcome in early stage (FIGO I-II) epithelial ovarian cancer. Int J Gynecol Cancer. 2011; 21: 1024– 1031. [DOI] [PubMed] [Google Scholar]
- 17. Skirnisdottir I, Seidal T. Association of p21, p21 p27 and p21 p53 status to histological subtypes and prognosis in low-stage epithelial ovarian cancer. Cancer Genomics Proteomics. 2013; 10: 27– 34. [PubMed] [Google Scholar]
- 18. Skirnisdottir I, Seidal T. The apoptosis regulators p53, bax and PUMA: Relationship and impact on outcome in early stage (FIGO I-II) ovarian carcinoma after post-surgical taxane-based treatment. Oncol Rep. 2012; 27: 741– 747. [DOI] [PubMed] [Google Scholar]
- 19.WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects (http://www.wma.net/en/30publications/10policies/b3/index.html). [PubMed]
- 20. Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer–Adjuvant ChemoTherapy in Ovarian Neoplasm Trial. J Natl Cancer Inst. 2003; 95: 113– 125. [PubMed] [Google Scholar]
- 21. Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998; 4: 844– 847. [DOI] [PubMed] [Google Scholar]
- 22. Seidal T, Balaton AJ, Battifora H. Interpretation and quantification of immunostains. Am J Surg Pathol. 2001; 25: 1204– 1207. [DOI] [PubMed] [Google Scholar]
- 23. Köbel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008; 5: 1749– 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vang R, Shih I-M,, Kurman R. Ovarian low grade and high grade serous carcinoma. Adv Anat Pathol. 2009; 16: 267– 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olsen CM, Nagle CM, Whiteman DC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013; 20: 251– 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Köbel M, Kalloger SE, Santos JL, et al. Tumor type and substage predict survival in stage I and II ovarian carcinoma: insights and implications. Gynecol Oncol. 2010; 116: 50– 56. [DOI] [PubMed] [Google Scholar]
- 27. Skirnisdottir I, Seidal T, Sorbe B. A new prognostic model comprising p53, EGFR, and tumor grade in early stage epithelial ovariancarcinoma and avoiding the problem of inaccurate surgical staging. Int J Gynecol Cancer. 2004; 14: 259– 270. [DOI] [PubMed] [Google Scholar]
- 28. Heini Lassus AL, Lundin Johan, Pentti Lehtovirta aRB. Distinct subtypes of serous ovarian carcinoma identified by p53 determination. Gynecol Oncol. 2003; 91: 504– 512. [DOI] [PubMed] [Google Scholar]
- 29. de Graeff P, Crijns AP, de Jong S, et al. Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer. 2009; 101: 149– 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newcomb E, Sosnow M, Demopoulos R, et al. Expression of the cell cycle inhibitor p27KIP1 is a new prognostic marker associated with survival in epithelial ovarian tumors. Am J Pathol. 1999; 154: 19– 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurman RJ, Visvanathan K, Roden R, et al. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008; 198: 351– 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shih I-M, Kurman RJ. Ovarian tumorigenesis. Am J Pathol. 2004; 164: 1511– 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trope C, Kaern J. Adjuvant chemotherapy for early-stage ovarian cancer: review of the literature. J Clin Oncol. 2007; 25: 2909– 2920. [DOI] [PubMed] [Google Scholar]
- 34. Gilks CB, Ionescu DN, Kalloger SE, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008; 39: 1239– 1251. [DOI] [PubMed] [Google Scholar]
- 35. Singh N, Ayhan A, Menon U, et al. Grading of serous ovarian carcinoma: further evidence of a lack of agreement between conventional grading systems. Histopathology. 2008; 52: 393– 395. [DOI] [PubMed] [Google Scholar]
- 36. Zannoni GF, Morassi F, Prisco MG, et al. Clinicopathologic and immunohistochemical features of ovarian clear cell carcinomas in comparison with type I and type II tumors. Int J Gynecol Pathol. 2012; 31: 507– 516. [DOI] [PubMed] [Google Scholar]
- 37. Zhao C, Wu LS, Barner R. Pathogenesis of ovarian clear cell adenofibroma, atypical proliferative (borderline) tumor, and carcinoma: clinicopathologic features of tumor with endometriosis or adenofibromatous components support two related pathways of tumor development. J Cancer. 2011; 2: 94– 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Köbel M, Kalloger SE, Huntsman DG, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009; 33: 14– 21. [DOI] [PubMed] [Google Scholar]


