Abstract
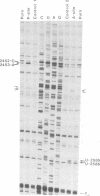
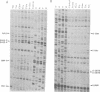
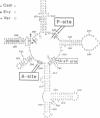
Photo-reactive 3-(4'-benzoylphenyl)propionyl-Phe-tRNA bound to the A- or the P-site was crosslinked to 23S RNA upon irradiation at 320 nm. The sites of reaction were identified as U-2584 and U-2585 at the A-site and A-2451 and C-2452 at the P-site. Minor crosslinks from both sites were observed at nucleotides A-2503 to U-2506. All sites identified lie in close proximity according to the secondary structure model and constitute part of the highly conserved loop region of domain V. Antibiotics known to inhibit peptidyl transferase activity had a pronounced effect on photo-crosslinking. In addition, tetracycline was also shown to photo-crosslink to this region. These experiments permit a dissection of the peptidyl transferase region on the 23S RNA into two distinct areas for the A- and P-site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Kurland C. G. Elongating ribosomes in vivo are refractory to erythromycin. Biochimie. 1987 Aug;69(8):901–904. doi: 10.1016/0300-9084(87)90218-5. [DOI] [PubMed] [Google Scholar]
- Barta A., Kuechler E., Branlant C., Sri Widada J., Krol A., Ebel J. P. Photoaffinity labelling of 23 S RNA at the donor-site of the Escherichia coli ribosome. FEBS Lett. 1975 Aug 1;56(1):170–174. doi: 10.1016/0014-5793(75)80134-7. [DOI] [PubMed] [Google Scholar]
- Barta A., Kuechler E. Part of the 23S RNA located in the 11S RNA fragment is a constituent of the ribosomal peptidyltransferase centre. FEBS Lett. 1983 Nov 14;163(2):319–323. doi: 10.1016/0014-5793(83)80844-8. [DOI] [PubMed] [Google Scholar]
- Barta A., Steiner G., Brosius J., Noller H. F., Kuechler E. Identification of a site on 23S ribosomal RNA located at the peptidyl transferase center. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3607–3611. doi: 10.1073/pnas.81.12.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Stiege W. Structure and function of ribosomal RNA. Biochem J. 1985 Jul 1;229(1):1–17. doi: 10.1042/bj2290001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Monro R. E., Vazquez D. Substrate and antibiotic binding sites at the peptidyl transferase centre of E. coli ribosomes. FEBS Lett. 1970 Feb 16;6(3):273–277. doi: 10.1016/0014-5793(70)80076-x. [DOI] [PubMed] [Google Scholar]
- Douthwaite S., Prince J. B., Noller H. F. Evidence for functional interaction between domains II and V of 23S ribosomal RNA from an erythromycin-resistant mutant. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8330–8334. doi: 10.1073/pnas.82.24.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi M., Prasad S. M., Morgan E. A. Chloramphenicol-erythromycin resistance mutations in a 23S rRNA gene of Escherichia coli. J Bacteriol. 1985 May;162(2):551–557. doi: 10.1128/jb.162.2.551-557.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Monro R. E., Torres-Pinedo R., Vazquez D. Substrate- and antibiotic-binding sites at the peptidyl-transferase centre of Escherichia coli ribosomes. Studies on the chloramphenicol. lincomycin and erythromycin sites. Eur J Biochem. 1971 Nov 11;23(1):185–193. doi: 10.1111/j.1432-1033.1971.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Printz M. P. Benzophenone triplet: a new photochemical probe of biological ligand-receptor interactions. Nat New Biol. 1973 Mar 28;242(117):127–128. doi: 10.1038/newbio242127a0. [DOI] [PubMed] [Google Scholar]
- Geigenmüller U., Nierhaus K. H. Tetracycline can inhibit tRNA binding to the ribosomal P site as well as to the A site. Eur J Biochem. 1986 Dec 15;161(3):723–726. doi: 10.1111/j.1432-1033.1986.tb10499.x. [DOI] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. A., Hasan T., Hall C. C., Strycharz W. A., Cooperman B. S. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry. 1983 Jan 18;22(2):359–368. doi: 10.1021/bi00271a020. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Hall C. C., Johnson D., Cooperman B. S. [3H]-p-azidopuromycin photoaffinity labeling of Escherichia coli ribosomes: evidence for site-specific interaction at U-2504 and G-2502 in domain V of 23S ribosomal RNA. Biochemistry. 1988 May 31;27(11):3983–3990. doi: 10.1021/bi00411a014. [DOI] [PubMed] [Google Scholar]
- Hodgin L. A., Högenauer G. The mode of action of pleuromutilin derivatives. Effect on cell-free polypeptide synthesis. Eur J Biochem. 1974 Sep 16;47(3):527–533. doi: 10.1111/j.1432-1033.1974.tb03721.x. [DOI] [PubMed] [Google Scholar]
- Högenauer G. The mode of action of pleuromutilin derivatives. Location and properties of the pleuromutilin binding site on Escherichia coli ribosomes. Eur J Biochem. 1975 Mar 3;52(1):93–98. doi: 10.1111/j.1432-1033.1975.tb03976.x. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Barta A. Aromatic ketone derivatives of aminoacyl-tRNA as photoaffinity labels for ribosomes. Methods Enzymol. 1977;46:676–683. doi: 10.1016/s0076-6879(77)46085-3. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Steiner G., Barta A. Photoaffinity labeling of peptidyltransferase. Methods Enzymol. 1988;164:361–372. doi: 10.1016/s0076-6879(88)64055-9. [DOI] [PubMed] [Google Scholar]
- Menninger J. R., Otto D. P. Erythromycin, carbomycin, and spiramycin inhibit protein synthesis by stimulating the dissociation of peptidyl-tRNA from ribosomes. Antimicrob Agents Chemother. 1982 May;21(5):811–818. doi: 10.1128/aac.21.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Chloramphenicol, erythromycin, carbomycin and vernamycin B protect overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie. 1987 Aug;69(8):879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze H., Nierhaus K. H. Minimal set of ribosomal components for reconstitution of the peptidyltransferase activity. EMBO J. 1982;1(5):609–613. doi: 10.1002/j.1460-2075.1982.tb01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Eisenberg D. The evolution of catalytic function. Science. 1987 Nov 6;238(4828):729-30, 807. doi: 10.1126/science.2445035. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Erythromycin and spiramycin resistance mutations of yeast mitochondria: nature of the rib2 locus in the large ribosomal RNA gene. Nucleic Acids Res. 1984 Nov 26;12(22):8313–8318. doi: 10.1093/nar/12.22.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. A plasmid-coded and site-directed mutation in Escherichia coli 23S RNA that confers resistance to erythromycin: implications for the mechanism of action of erythromycin. Biochimie. 1987 Aug;69(8):891–900. doi: 10.1016/0300-9084(87)90217-3. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Reverse transcriptase pauses at N2-methylguanine during in vitro transcription of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3751–3754. doi: 10.1073/pnas.76.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986 Jan 31;231(4737):470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]