Abstract
It is well established that bone responds to mechanical stimuli whereby physical forces are translated into chemical signals between cells, via mechanotransduction. It is difficult however to study the precise cellular and molecular responses using in vivo systems. In vitro loading models, which aim to replicate forces found within the bone microenvironment, make the underlying processes of mechanotransduction accessible to the researcher. Direct measurements in vivo and predictive modeling have been used to define these forces in normal physiological and pathological states. The types of mechanical stimuli present in the bone include vibration, fluid shear, substrate deformation and compressive loading, which can all be applied in vitro to monolayer and three-dimensional (3D) cultures. In monolayer, vibration can be readily applied to cultures via a low-magnitude, high-frequency loading rig. Fluid shear can be applied to cultures in multiwell plates via a simple rocking platform to engender gravitational fluid movement or via a pump to cells attached to a slide within a parallel-plate flow chamber, which may be micropatterned for use with osteocytes. Substrate strain can be applied via the vacuum-driven FlexCell system or via a four-point loading jig. 3D cultures better replicate the bone microenvironment and can also be subjected to the same forms of mechanical stimuli as monolayer, including vibration, fluid shear via perfusion flow, strain or compression. 3D cocultures that more closely replicate the bone microenvironment can be used to study the collective response of several cell types to loading. This technical review summarizes the methods for applying mechanical stimuli to bone cells in vitro.
Introduction
The human skeleton is constantly subjected to mechanical loading. In the absence of bodily movement, vibrations from the local environment cause low-magnitude mechanical signals in the bone microenvironment. Physical activities such as walking or exercise increase loading by the movement of interstitial fluid within the bone to create shearing forces against cells and deformation of the bone matrix, which leads to both strain and compressive forces.1 All of these mechanical stimuli are important in regulating the activity of bone cells via mechanotransduction.
Mechanotransduction is the process whereby mechanical stimuli are detected by cells and converted into chemical signals.2 Localized signaling molecules such as ATP,3 prostaglandins4,5 and nitric oxide,6 to name just a few, are released to regulate and coordinate the response of nearby cells. Osteocytes are regarded as the key mechanosensing cells of the bone that regulate the activity of osteoblasts and osteoclasts.7,8 However, in vitro studies have shown that osteogenic progenitors,9,10 osteoblasts3,11,12 and osteoclasts13,14 are capable of responding to loading. The changes in bone formation induced by mechanotransduction are termed adaptive remodeling.8
The aim of an in vitro loading model is to recreate the conditions required to engender mechanotransduction in a controlled cell culture environment. The specific objectives may vary from applying physiologically relevant levels of particular mechanical stimulation to exceeding the normal physiological conditions, which may represent pathological or induced states. Vibration is a ubiquitous low-level mechanical stimulus that can be applied at increased levels to human patients using vibrating platforms and this stimulus can be replicated in vitro.15,16 Physiologically relevant levels of fluid shear stress (FSS) around osteocyte processes in mature bone are predicted to be 8–30 dyn cm2,17,18 making anything outside this range potentially pathological. Physiologically relevant levels of substrate strain have been measured as 2000–4000 μɛ,19,20,21 with anything above 5000 μɛ causing periosteal woven bone formation and anything below 200 μɛ (i.e. from microgravity conditions) causing bone loss,22,23 although lower strain thresholds may be required for wound healing. The flexibility of in vitro studies allows for entire ranges of physiological and pathophysiological loading states such as these to be investigated.
In vitro studies allow for the investigation of isolated forms of mechanical stimuli, thus comparisons between different types of loading can be made.3,24 Also, while bone contains multiple cell types at different stages of differentiation, in vitro models allow for the effects of loading to be studied on individual cell types at specific stages of the differentiation process.25 A common criticism of in vitro studies is that the substrate and the surroundings of the cell are too different to the bone microenvironment for findings obtained to be relevant. However, advances in cell culture techniques now make it possible to apply loading not just in monolayer but also in 3D cell cultures on scaffold materials that more closely resemble the bone microenvironment. Furthermore, coculture models of osteoblasts and osteocytes have been developed, which will allow for the interaction between these important bone cell types to be studied in response to loading.26
This technical review will describe optimized techniques for loading bone-derived cells by vibration, fluid flow, substrate strain or compression in monolayer and 3D cultures to provide a concise yet comprehensive guide to bone cell loading in vitro.
Vibration
Vibration is by far and away the most common mechanical stimulus experienced by bone cells on a daily basis. Low-magnitude, high-frequency vibration is a distinct force event that induces no or minimal membrane strain or fluid shear.15 Cells are typically seeded into a multiwell plate (precoated with collagen if using osteocyte like cells) and grown to 80% confluence16 (Figure 1a). The plate is then attached to a loading rig, which may be commercially available (e.g. Electroforce 3200, BOSE) or adapted in-house (e.g. a vibration platform attached to a shaker, ET-127; Labworks Inc.,Costa Mesa, California, USA16) (Figure 1b). Horizontal vibration is applied at 30–90 Hz frequency15,16 and no more than 0.3 g, which has been shown to deliver shear stress five orders of magnitude lower than those found in vivo.16 End points from previous studies have focused on transcriptional changes and secretion of signaling molecules.16
Figure 1.
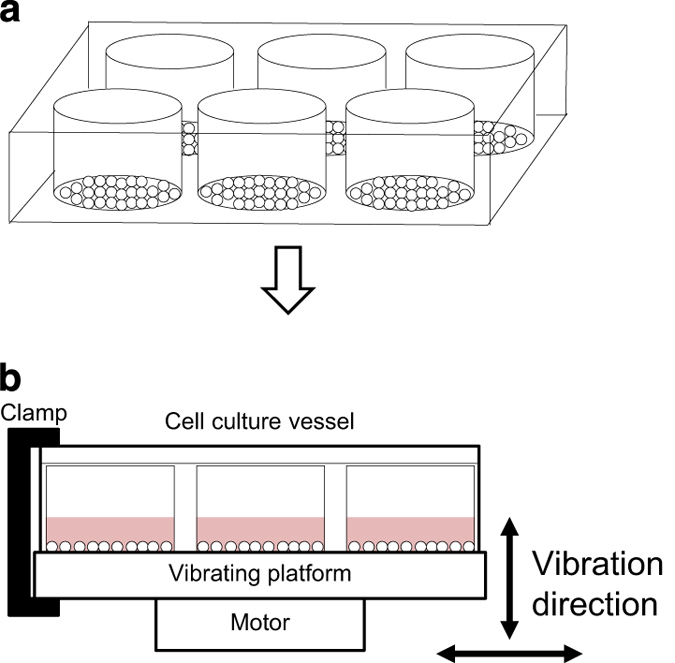
Vibration in monolayer. (a) Cells are seeded into a multiwell plate and cultured to 80% confluence upon which the plate is clamped into a rig (b) for vibration at 0.3 g and up to 100 Hz, which is sufficient to engender shearing forces without creating fluid shear.15,16
Specific materials: Commercially bought or custom-adapted loading rig with low-magnitude, high-frequency vibration capabilities.
Fluid flow
Fluid shear stresses are a potent stimulator of bone cells in vitro. Fluid-flow profiles may be steady (continuous flow) or dynamic (either pulsatile or oscillatory), and such oscillatory/dynamic flow profiles mimic the behavior of interstitial fluid flow in the bone.27
Herein, two methods that provide tightly regulated fluid shear stress (FSS) stimulation are described: a simple ‘see-saw' rocking method and the well-established parallel-plate flow system (which includes a brief description of micropatterning for osteocytic cell lines under flow). Both techniques produce oscillatory (dynamic) flow profiles mimicking the behavior of interstitial fluid flow in the bone; however, the range of obtainable shear stresses is lower in the rocking system (potentially relevant to a healing wound or progenitors in the bone marrow27) compared with the parallel-plate system (relevant to mature bone18).
Rocking ‘see-saw' culture
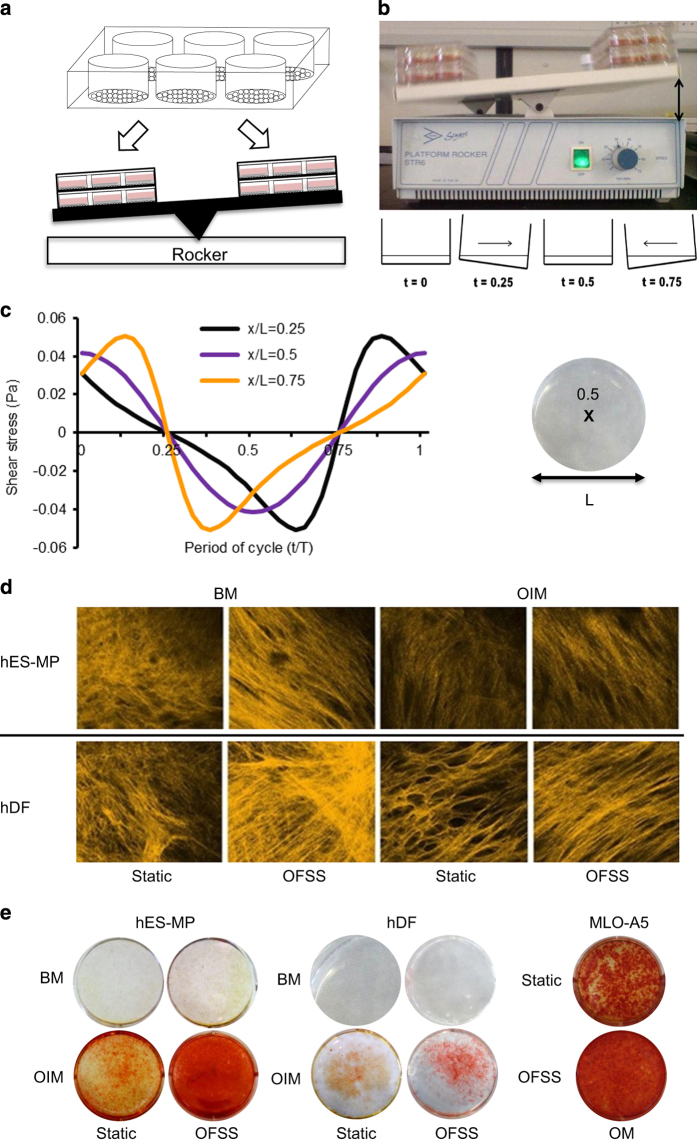
The simple rocking ‘see-saw' system can produce low-magnitude (e.g. 0.001–0.25 Pa) oscillatory FSS (OFSS) in standard culture dishes. It is a high throughput method allowing testing of large numbers of mechanically relevant parameters simultaneously.
Cells are seeded into the tissue culture vessel (e.g. six-well plate) with sufficient time to attach and proliferate (to subconfluence) before being placed on a rocking ‘see-saw' platform with the well plate long axis perpendicular to the direction of rocking (Figures 2a and b). Precoating culture dish with gelatin (1 mg ml−1) before seeding will greatly reduce detachment of cells because of fluid flow. Activating ‘see-saw' rocking motion results in gravitational media movement back and forth across the cells subjecting them to OFSS (Figure 2c). Media volume should be large enough so as not to expose the cells to air during any part of the cycle. If platform rocker is not high humidity resistant, then rocking can be performed for short bouts (⩽2 h) outside an incubator. Short bouts of loading (5–120 min) can be applied to measure immediate-early responses, for example, prostaglandin-2 (PGE2) release and osteogenic gene expression. Long-term culture up to 21 days of intermittent rocking fluid exposure (0.5–2 h per day) can enhance osteogenesis and matrix deposition9 in a range of mature and progenitor cells (Figure 2d). For osteogenic progenitor cells, increases in mineral deposition have only been observed when cells were cultured in media containing dexamethasone (Figure 2e). The characteristic shear stress at the bottom of the dish can be calculated using a lubrication-based model described by Zhou et al.28 for a rectangular well (Equation (1)) or a circular well (Equation (2)):
Figure 2.
Rocking ‘see-saw' platform with media-filled six-well culture plates and shear stress profile. (a) Cells are seeded into multiwell plates and cultured to 80% confluence. (b) Platform tilts at a predetermined angle (indicated by double-headed arrow) and fluid flow-induced shear stress (calculated from Equation (2)) that is oscillatory in nature (c) is produced across the well bottom (x/L is a point in space along the well bottom, where x/L=0.5 is the center) at different points in the cycle.26,27,28 Matrix production is enhanced by ‘see-saw' rocking in osteogenic progenitor cells. (d) Second harmonic generation imaging shows increased collagen deposition/organization by human dermal fibroblasts (hDFs) and human embryonic stem mesenchymal progenitors (hES-MPs) subjected to OFSS versus static (no-flow) conditions. (e) Mineral deposition is increased by OFSS stimulation compared with static culture, but only in osteoinduction groups. BM, basal media; OIM, osteoinduction media; OM, osteogenic media.9,28,29
 |
 |
where μ is the fluid viscosity, θmax the maximal flip angle, δ the ratio of fluid depth to well length and T the time for one cycle. The magnitude of FSS can be adjusted by altering these four parameters. Computational fluid mechanics have also been used to validate shear stresses in a rocking dish,29 showing good agreement with the lubrication model. Rocking speed should be controlled to avoid waves.
Specific materials: Platform rocker with adjustable speed and angle (optional) (e.g. Stuart), standard culture plates (e.g. six-well plates), gelatin (1 mg ml−1) solution (e.g. porcine gelatin in distilled water H2O).
Parallel-plate flow chamber
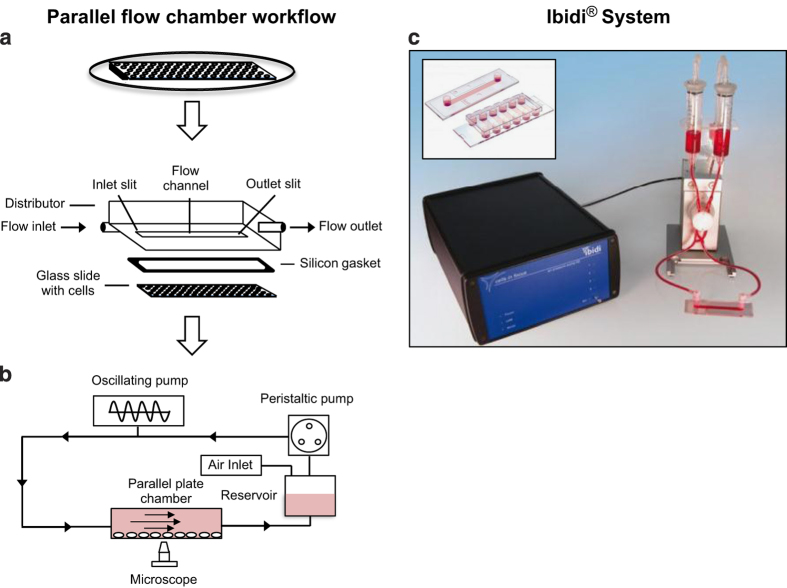
The parallel-plate flow system is capable of producing well-defined physiologically relevant wall shear stresses in the range of 0.01–3 Pa1 and therefore has been widely used for studying bone cell responses to fluid flow. The system is capable of applying different fluid-flow profiles, including steady (continuous), pulsatile and oscillatory to a monolayer of cells. Chambers can be made in-house10 or bought commercially30,31 (unassembled or preassembled) (Figure 3).
Figure 3.
Schematic of parallel-plate fluid flow system and chamber setup. (a) Cells are seeded onto a slide and allowed to attach. Typically, a silicon gasket is sandwiched between a quartz glass slide (with cells attached) and a polycarbonate distributor held together by vacuum or stainless-steel and Teflon plates screwed together. Inlet and outlet slots on the distributor or plates allow fluid flow from a media reservoir into the chamber driven by a pulsatile pump. (b) Oscillatory fluid flow is provided by attaching an oscillatory pump system. (c) Commercially available Ibidi pump system with parallel-plate chamber, the pump can produce static, pulsatile and oscillatory flow profiles. Inset shows disposable single and multichannel chambers.10,30,31
Cells are seeded onto precoated glass slides and allowed to attach in an incubator before sealing with a silicon gasket using either a polycarbonate distributor (under vacuum) or stainless-steel/Teflon plates (screws) placed on the top containing inlet and outlet ports (Figure 3a). Cells can also be injected or flushed (using low oscillatory flow rates and frequencies) into preassembled chambers. Quartz slides or a quartz chamber window are used to enable sample visualization.32,33 Slides are generally precoated with collagen I (∼0.1 mg ml−1), fibronectin (∼0.05–0.1 mg ml−1) or poly-L-lysine (∼0.5–1 mg ml−1) to prevent cell detachment that may result from high peak shear stress. After 24–72 h of no-flow culture (∼80% confluence), cells are subjected to fluid flow (normally recirculating) from a media reservoir, typically delivered by a peristaltic (steady) or oscillatory (oscillatory and pulsatile) pump (Figure 3b). Laminar flow will cover the chamber, except at the entrances (∼1 mm range) where turbulence occurs, and thus analysis of these cells is best avoided. Flow exposure can induce early signaling events in osteoblasts and osteocytes within seconds (e.g. intracellular calcium release10,32), minutes (extracellular ATP release3) or in the first hour (e.g. nitric oxide and PGE2 release24,30). Flow regimens are rarely performed above 24 h (continuous and intermittent). Wall shear stress can be calculated (Equation (3)), where μ is the fluid viscosity, Q is the flow rate, b is the flow channel width and h the flow channel height:
 |
To better mimic the in vivo bone cell network topology, 2D micropatterns can be fabricated using microcontact printing and self-assembled monolayer techniques.34 This is particularly useful for obtaining spatially controlled networks of osteocytic or osteoblastic cells in vitro, unlike the conventional monolayer cultures. These micropatterned surfaces can then be used in a parallel-plate flow chamber to enable the study of fluid-flow-mediated signaling among individual cells and the cell network interaction.35
Specific materials: Peristaltic pump (e.g. 200 series multichannel cassette pump; Watson Marlow Pumps Group, Falmouth, UK), oscillatory pump (e.g. Ibidi pump system; Ibidi, Munich, Germany), silicon tubing (⩾0.5 m), peristaltic pump tubing, polypropylene connectors for connecting silicon tubing to inlet/outlet and pump tubing (e.g. elbow and female Luer), media bottle with holes for tubing and air filter, self-assembled chamber, glass slides, for example, quartz, silicon gasket, polycarbonate distributor or stainless-steel/Teflon plates (with inlet and outlet ports), vacuum pump for polycarbonate distributor; screws for plates, commercially available disposable chambers (e.g. 0.4 × 3.8 × 17 mm3 flow chamber; Ibidi (Figure 3c)), collagen I solution 0.1 mg ml−1 (e.g. rat tail in 0.1% acetic acid); fibronectin solution 0.5–50 μg ml−1 (e.g. fibronectin powder from bovine plasma in diH2O); poly-L-lysine (0.5–1 mg ml−1) solution.
General points
In both systems, differentiation media are typically added 24 h after seeding or at the onset of OFSS. Cell type, seeding density, number of focal adhesion points and length of attachment time before application of oscillatory fluid flow all influence detachment strength. Chemotransport is also likely to influence cell response to fluid flow.36 Continuous application of shear stress may lead to cellular desensitization; therefore, periods of rest or low flow may be beneficial.25
Substrate strain
As in other models, the investigation of bone cells' adaptive response to mechanical strain requires quantification of mechanical inputs received by bone in vivo and bone cells in vitro. To ensure that the strain applied to bone cells in vitro is physiologically relevant and comparable to strain received in vivo, electrical strain gauges have been used to validate the direct measurement of surface bone strain in vivo and ex vivo between 2000–4000 μɛ.19,20,21 Described herein are two of the most widely used models, the commercially available FlexCell tension system and the four-point bending model.
FlexCell tension system
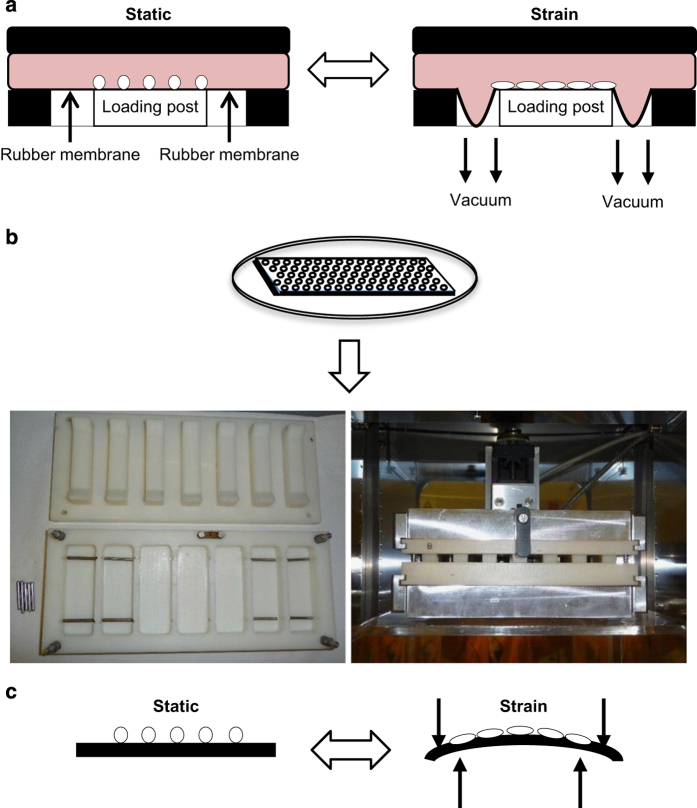
A widely used commercially available device to apply strain to cells growing in vitro is the Flexcell FX-5000 Tension System (Flexcell International Corporation, Hillsborough, NC, USA). This device was originally developed by Banes et al.37 and consists of a computer-controlled vacuum unit and a baseplate to hold flexible silicone rubber-bottomed culture dishes (Figure 4a). Briefly, bone primary cells or cell lines are cultured onto culture plates (compatible plates: BioFlex, Tissue Train, UniFlex, HT BioFlex; Flexcell International Corporation) with appropriate culture media and serum. Upon confluence, plates are placed in each baseplate well and a defined static or variable duration cyclic tension is applied using the FlexSoft FX-5000 software (Hillsborough, NC, USA), supplied with the system. This is achieved as a vacuum is repeatedly applied to the dishes via the baseplate. The membrane stretches downwards and deforms into a concave shell, and upon relaxation of the pressure, the membrane returns to its original flat shape. Typical parameters vary from 0.05 to 1 Hz and 1 to 12% uniaxial strain (up to 120 000 μɛ).38,39,40 The concave nature of the FlexCell engenders heterogeneous strain38,41 and the viscoelastic properties mean that specific calibration curves must be determined for each test.42
Figure 4.
Schematic of the FlexCell system and an in vitro four-point bending model. (a) For the FlexCell system, osteoblasts are seeded as a monolayer onto FlexCell compatible bases and plates. A vacuum underneath the well draws down the rubber seal beneath the FlexCell and in doing so causes stretch to occur beneath the cells.37,38,39,40,41,42 For the in vitro four-point bending jig, (b) osteoblasts were seeded as a monolayer onto the custom-made plastic slides and bathed in media in the jig apparatus housed in a 37 °C incubator with 5% CO2. (c) The cells experience a strain of 3400 μɛ over a period of 10 min during which the slide is deformed 600 times.6,12,43,86,87
Specific materials: Flexcell FX-5000 Tension System (Flexcell International Corporation); BioFlex, Tissue Train, UniFlex or HT BioFlex culture plates (Flexcell International Corporation).
Four-point bending model
Four-point bending rigs have the advantage of providing uniform strain.11 Previous studies reported that 3400 μɛ was required to trigger cellular responses in a four-point bending model, and, more recently, a dose–response study using the same model reported that strain magnitudes <3400 μɛ were not sufficient to increase proliferation in response to the application of strain in vitro.12
Four-point bending devices can be purchased commercially or made in-house. In both cases, they usually consist of a tray of wells, each containing steel bars, which lay towards the end of the well (Figures 4b and c; custom-made four-point bending system designed by Professor Lennart Stromberg (Karolinska Institute, Solna, Sweden)). Briefly, primary bone cells or cell lines are seeded onto sterile tissue culture-treated plastic slides (Nunc, Dossel, Germany) and maintained for 24 h before loading and before being transferred to a loading tray with 11 ml of culture media. The tray is inserted into a custom-made metal jig that is under the control of a motor that moves the lid up and down (Figure 4b). The structure of the lid includes protrusions that apply pressure on the extreme ends of the slide when the motor is turned on. As the contact points of the lid lie distal to the contact points of the metal bars in the trays upon which the slides rest, downward pressure of the lid results in four-point bending of the slide (Figure 4c). The jig is controlled by computer software (Servo Systems Co., Montville, NJ, USA), which regulates both the timing of load and the load parameters (strain cycle frequency and number; the extent of movement of the lid), thus controlling the deformation of the slide, and subsequently the strain imparted to the slide containing the bone cells. The jig apparatus is maintained in an incubator at 37 °C and 5% CO2, so that the cells are not stressed by fluctuations in temperature or pH during four-point bending.
The protocol for loading osteoblasts has been optimized so that physiological levels of strain19,20,21 can be applied to cultured cells in vitro. The optimized protocol for loading osteoblasts has a waveform in each strain cycle that consists of a tamped 1 Hz square wave, with strain rates on and off of 23 000 μɛ s−1 generating a peak strain of 3400 μɛ. The on dwell time for each cycle is maintained at 0.4 s, whereas the cycle number per second is controlled by off dwell time, which was maintained at 0.75 s. The strain treatment period lasts for 10 min, following which slides are transferred to a four-well dish and incubated in the media from the loading tray. Osteoblast cultures subjected to substrate strain in this model exhibit increased proliferation,43 PGE2 release,4 ATP release and changes in immediate-early gene expression.3 In contrast to the effects on osteoblasts, substrate strain decreases proliferation in RAW cells13 and in primary osteoclast cultures.14
Specific materials: Custom-made four-point bending system designed by Professor Lennart Stromberg (Karolinska Institute); sterile tissue culture-treated plastic slides (Nunc); CELLSTAR four-well plates (Greiner, Stroudwater, UK).
3D Models for loading single-cell cultures of osteoblasts
3D cell culture aims to provide a more realistic spatial environment for cells to provide behavioral queues and form complex tissues with structures similar to those in vivo. Mechanical loading can be applied to these by different methods, by introducing fluid flow through the constructs (perfusion) or by applying tensional forces/substrate strain or compressive forces to the construct. Mechanical loading is often used in bone tissue engineering to improve the quantity or quality of the extracellular matrix deposited and studies using osteoblasts or their precursors will be covered in this section.
Choice of scaffolding material
When applying mechanical loading to cells in 3D, cells are seeded into a scaffold for culture and subsequent stimulation. The scaffold itself should encourage cell attachment and proliferation, be of a suitable stiffness for the required tissue and have porosity and pore interconnectivity, which allow the flow of nutrients and waste through the scaffold.44,45,46,47 The stiffness, porosity and interconnectivity also affect the transmission of mechanical stimulation to the cells. For an open porous structure, fluid flow can pass through the scaffold and cause shear stress on the cells. For compressive loading, as well as transmission of forces through the scaffold itself, fluid may also move within the scaffold pores because of the applied loading. For bone constructs, materials including hydroxyapatite (HA)48 or tricalcium phosphate (TCP) scaffolds,49 polyurethane (PU) sponges,50 collagen-based scaffolds51 and electrospun matrices have been commonly used.
Electrospinning is a simple technique capable of fabricating nano- and microfibrous scaffolds from polymeric solutions that can mimic isotropic and anisotropic ECM fibrous 3D architectures with interconnecting pores and high surface area to volume ratios.52 Many polymers can be electrospun once dissolved in an appropriate solvent, such as synthetic polyesters and polyethers, or naturally occurring materials, such as chitosan or gelatin; the main criterion is that chain entanglement must be obtained in the solution. Polymer solutions are dispensed using a programmable syringe pump through a hypodermic syringe with a blunt tip needle connected to a variable high-voltage power supply (0–30 kV). At critical voltage (voltage required to exceed solution surface tension), solutions project horizontally towards an earthed, variable speed steel rotating drum (10–30 cm away) where continuous non-woven fibers are deposited. To generate scaffolds with no preferred angle of fiber orientation, the collector surface speed should be <1 m s−1, and to collect highly aligned fibers, the collector surface speed should be >5 m s−1. These fibrous matrices support progenitor and mature bone cell growth and are suitable for dynamic tensile stimulation of cells or perfusion flow cultures.53
Specific materials: HA—macroporous HA scaffolds with 500 or 300 μm pore size, 4 mm high and 10 mm diameter (BIOCETIS, Boulogne-sur-Mer, France);48 TCP—cylindrical, porous calcium phosphate scaffold with a 5 mm diameter and a 3.5 mm height (Skelite; Stellar Pharmaceuticals, London, ON, Canada);49 PU—a polyether PU foam was obtained as a large industrial grade block from Caligen Foam Ltd. (Lancashire, UK);50 collagen—fabricated in-house, the collagen solution consisted of 70% volume/volume (v/v) vitrogen (3 mg ml−1 type I collagen; Angiotech BioMaterials Corp, Palo Alto, CA; neutralized to pH 7.0 with 1 M sodium hydroxide), 20% (v/v) minimum essential medium (Sigma, St Louis, MO, USA) and 10% (v/v) FBS (Cambrex, East Rutherford, NJ, USA).51
Seeding scaffolds
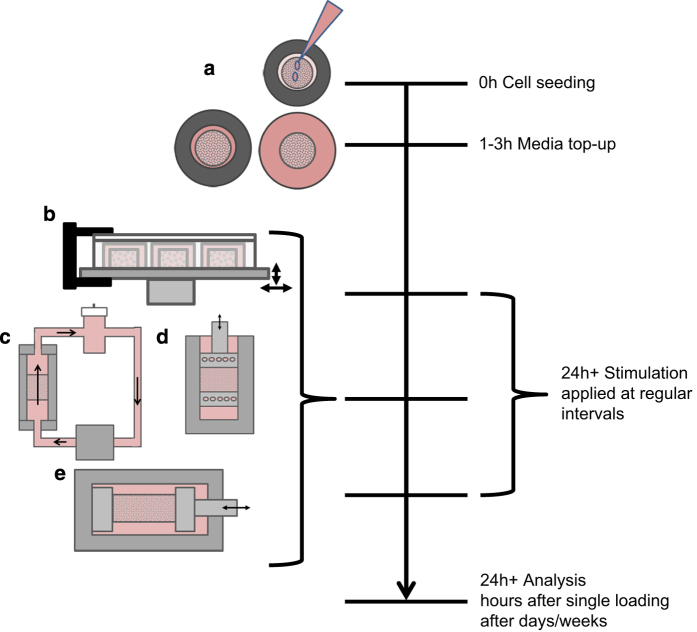
Cell suspensions are generally introduced into scaffolds in small volumes of media (100–300 μl) added dropwise and left for a few hours to allow cells to attach50,54 before more media are then added to cover the scaffolds and to ensure cell survival. Dental wire may be used to prevent scaffolds from floating when additional media are added,50 after which scaffolds are often left overnight49,54 or for several days50 before stimulation is applied (Figure 5a).
Figure 5.
Setting up and loading 3D cell cultures. (a) Cell suspension is added dropwise on to scaffolds and incubated for 1–3 h for cell attachment, medium topped up and scaffolds incubated for cell growth. Scaffolds can be loaded from as early as 24 h after seeding. Loading may be applied by (b) vibration,55,56 (c) fluid flow,57,58,59,60,61,62 (d) compression64,65 or (e) strain.67,68
3D vibration
As in monolayer, 3D cultures in collagen- or human bone-derived scaffolds housed in culture plates are placed on a vibrating platform for short periods of stimulation at 0.3 g and 30–40 Hz55,56 (Figure 5b). In 3D culture, this has been shown to cause increases in osteogenic gene expression, including alkaline phosphatase, collagen I and osteocalcin over culture of several weeks55 or several days for vascular endothelial growth factor, osteoprotegerin and fibronectin.56
3D fluid flow
As in monolayer, fluid flow affects the activity of osteoblasts in 3D culture systems. Cell–scaffold constructs are usually confined within a chamber such that fluid is forced through the scaffold and past the cells (Figure 5c). Unidirectional,57,58 pulsatile59 and oscillatory fluid flow54,59,60 have all been tested and are often compared in studies. Physiological FSS on osteocyte processes is estimated to be in the range of 8–30 dyn/cm2.17,18 The wall shear stress inside a scaffold has been estimated mathematically61 (Equation (4)), where τ is the shear stress, μ is the dynamic viscosity of the culture medium and dpore is the pore diameter. The average fluid velocity inside the scaffold, μscaf, is related to the inlet flow rate (Q), radius of the scaffold chamber (rchamber) and scaffold porosity (ϕ) as follows: 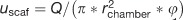 . Flow rates used in bioreactors are generally low (<1 ml min1) but some studies have used much higher rates (up to 120 ml min−1), particularly when applying dynamic flow25
. Flow rates used in bioreactors are generally low (<1 ml min1) but some studies have used much higher rates (up to 120 ml min−1), particularly when applying dynamic flow25
 |
Another method of applying fluid flow is the use of a rotating bioreactor. Scaffolds are placed within a larger vessel and are free to move around as the vessel rotates,62,63 creating unconfined flow within the constructs; however, flow is not well regulated in this system and as such there is no tight regulation of the forces involved.
3D compression
Compressive mechanical loading applies a load through one surface of a tissue construct to stimulate cells. The 3D construct can be placed between two platens (Figure 5d) and subjected to static or cyclic mechanical compression at a specified strain. The use of a load cell allows the monitoring and adjustment of the applied forces, even if the mechanical properties change during the culture period because of extracellular matrix deposition.48,50 In direct compression, the material may also be confined or unconfined,50 the latter allowing the flow of fluid in and out of the construct during loading. This flow of fluid may act like the flow of interstitial fluid within canaliculi during loading of bones in vivo.
Many different regimens have been applied, often applying the compressive load through a sinusoidal waveform at a frequency of 1 Hz.3,50 The magnitude of compressive loading for the bone is presented as the applied strain and many studies have found favorable results at 5%50,64 or 10%65 strain. Compression is usually applied for several hours and often repeated several times during the culture period.50,66
3D tensile strain
For the application of tensile forces in 3D, cell seeded constructs (usually made of collagen or silicone) can be fixed by a set of grips on either side of the scaffold and stretched by pulling (uniaxial) the construct67 (Figure 5e). As with other methods, tension is applied cyclically (typically 1 Hz) to induce changes within the cells,51 using strain magnitude (5–10%) similar to compressive loading.67,68
3D Models for loading osteocyte-osteoblast cocultures
Most 3D models of loading use only one cell type, either osteoblasts or osteocytes. However, as is widely recognized, osteocytes are capable of detecting mechanical stimuli and using this information to regulate osteoblast activity, thus it is important that researchers can investigate these interactions.
In previous studies, cells have not been embedded within a matrix, but instead attached to a scaffold surface and therefore do not accurately capture the environment of an osteocyte within bone. However, these systems have proven the feasibility of reproducing the synthesis of an organized matrix69 and cell-mediated matrix degradation.70,71,72 Several studies have embedded bone cells within type I collagen gels in an attempt to mimic the bone matrix;73,74,75 however, few studies set up such cultures and applied loading. Until recently, none of the available 3D in vitro bone models investigated osteocyte–osteoblast interactions, which lead to mechanically induced bone formation.
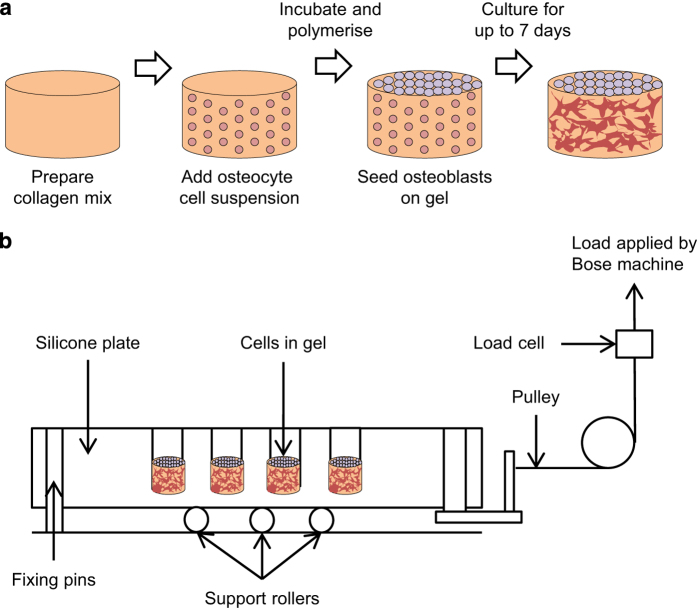
This has recently been overcome via a new 3D in vitro osteocyte–osteoblast coculture mechanical loading model, cultured within a custom-built multiwell silicone loading plate, using MLO-Y4 osteocyte-like cells and MC3T3-E1(14) or MG63 osteoblast like cells.26 In this model, MLO-Y4 cells were incorporated within rat tail tendon type I collagen gels (2–2.6 mg ml−1) and either MC3T3-E1(14) or MG63 cells layered on the top. MLO-Y4 cells (1.5 × 106 cells per ml gel) diluted in the culture medium (<10% of total gel volume) were embedded in the collagen and distributed into multiwell plates for polymerization (37 °C, 1 h). MC3T3-E1(14) or MG63 cells (1.5 × 105 cells per well) in the culture medium were then applied onto the surface of the gel incubated at 37 °C (Figure 6a).26 The ratio of osteocytes and osteoblasts was 10:1 to mimic in vivo bone where there are 10 times more osteocytes than osteoblasts.76 The cells within the model were viable, maintained typical osteocyte morphology and phenotype and appeared to connect to neighboring cells. RNA was extracted separately from surface and embedded cells of cocultures by dispensing 1 ml TRIzol (Invitrogen) onto the surface for 10 s to extract osteoblast RNA, and subsequently dissolving the underlying gel within a separate 1 ml TRIzol aliquot to extract RNA from gel-embedded cells.
Figure 6.
Coculture preparation and compressive loading. (a) Collagen mix is prepared and added into wells. MLO-Y4 cell suspension is mixed with collagen that is allowed to polymerize with incubation. MC3T3-E1 cell suspension is added to the surface of the gel followed by incubation for up to 7 days. (b) Cocultures are then loaded via a 16-well silicone loading plate of the same dimensions (10 mm diameter) as a standard 48-well tissue culture plate but with a 150-μm-thick base and with holes for its attachment to a BOSE loading instrument. The collagen gels are contained in the wells of the silicone plate, and the entire plate is stretched to apply a strain to the gels.26,70,76,77
To expose 3D cultures to cyclic compression (5 min, 10 Hz, 2.5 N), a 16-well loading plate was developed from solid silicone and with the dimensions of a 48-well tissue culture plate but with a thinner base and a series of holes on each side for attachment to a BOSE loading instrument via a custom-made device (Figure 6b), allowing stretching of one side of the plate. The system was validated using Digital Image Correlation, which revealed strains of 4000–4500 μɛ when a force of 2.5 N was applied to the silicone plate.26 Currently, there are two published devices that are similar to the one developed by Vazquez et al.77,78 These are a six-well silicone plate used to mechanically load vascular smooth muscle cells in monolayers,78 and a single-well silicone plate designed to load 3D collagen cultures of intervertebral disc cells.77 Both devices applied cyclic mechanical stimuli in a similar manner to the device described by Vazquez et al., but instead used higher strains and lower frequencies (24 h, 0.1 Hz, 10 000 μɛ;77 6–72 h, 1 Hz, 10–20% strain).78 Using the device by Vazquez et al., it was shown that embedded osteocytes respond to loading by increasing PGE2 release 0.5 h post-load, and that loading of 3D cocultures increased type I pro-collagen synthesis, a marker of bone formation.26
This coculture model allows the formation of a 3D osteocytic network in vitro, which can be exposed to loading stimuli, facilitating the elucidation of osteocyte-controlled osteoblast bone formation as a result of mechanical loading.
Specific materials: Custom-built multiwell silicone loading plate,26 2–2.6 mg ml−1 type I collagen gels (rat tail tendon type I collagen in acetic acid (Sigma) mixed 4:1 with 5 × MEM (Invitrogen) containing 11 g l−1 sodium bicarbonate and neutralized (1 M tris(hydroxymethyl)aminomethane (Tris) base, pH 11.5)).
A summary of the mechanical stimuli described is provided in Table 1.
Table 1. A summary of the different loading systems, mechanical stimulus and specific parameters with references.
| System | Mechanical stimulus | Parameters | References |
|---|---|---|---|
| Vibration | Low-magnitude, high-frequency vibration | 30–100 Hz, 0.3 g | 16,17 |
| Fluid flow: see-saw rocking platform | Oscillatory fluid flow-induced shear | 0.001–0.25 Pa | 28,29 |
| Fluid flow: parallel-plate flow chamber | Steady, pulsing, oscillatory fluid flow-induced shear | 0.1–3 Pa | 30,31 |
| Substrate strain: FlexCell | Non-uniform substrate strain deformation | 0.5–1 Hz, 3–12% uniaxial strain (up to 120 000 μɛ) | 37,38,39,40,41,42 |
| Substrate strain: four-point bending | Uniform substrate strain deformation | 1 Hz square wave, 3400 μɛ | 6,12,43,86,87 |
| 3D vibration | Low-magnitude, high-frequency vibration | 30–40 Hz, 0.3 g | 55,56 |
| 3D fluid flow: perfusion | Steady, pulsing, oscillatory fluid flow-induced shear | 1–120 ml min−1 | 57,58,59,60,61,62 |
| 3D compression | Cyclic compression | 1 Hz, 5–10% compression | 64,65 |
| 3D tensile strain | Tensile strain/cyclic tensile strain | 1 Hz, 5–10% strain magnitude | 67,68 |
| 3D cocultures | Cyclic compression | 10 Hz, 2.5 N | 26,70,76,77 |
Abbreviation: 3D, three dimensional.
Discussion
In this paper, we have described the methods for the in vitro replication of the main forms of mechanical stimuli found within the bone microenvironment in vivo. Each in vitro model brings its own benefits: vibration and fluid flow in monolayer are tightly controlled systems, each focused on a single form of mechanical stimulus; substrate strain more closely represents the situation in bone in vivo where strain and strain-induced flow coexist; 3D models more closely resemble the bone microenvironment compared with cell monolayers, and cocultures allow the interactions that exist between cell types to be studied.
The effects of multiple forms of mechanical stimuli upon early signals from SaOS-2 osteoblastic cells have been compared within a single study.3 ATP release was rapidly increased in response to fluid shear (to 23 dyn cm−2) with elevated responses in 3D. In monolayer, strain engendered by four point bending led to gradual increases in ATP release (at 3400 μɛ), however, in 3D, there were few effects of compressive loading (and associated strain), suggesting that the PU scaffold used was not transducing the compressive force in such a way that SaOS-2 cells could detect.3 This comparison demonstrates that one cell type may exhibit specific responses to different mechanical stimuli. The responses to compressive loading were in contrast to other studies using the same scaffold material but different osteoblast-like cells where osteogenic responses were engendered.50,79 This demonstrates that even similar cell types can exhibit different responses to the same mechanical stimulus. When designing an in vitro loading experiment, it is important to take into account the specific cell line being used, the mechanical stimulus and the substrate.
Despite osteocytes being regarded as the primary mechanosensors of bone, most previous studies have used osteoblasts.8 One reason for this is because primary osteocytes are so difficult to isolate and culture owing to their position within bone. To date, only chick,80,81 mouse82,83,84 and rat85 primary osteocytes have successfully been isolated using sequential bone digestion. The yield of live osteocytes after digestion is low and they do not proliferate in culture, limiting their use for experimental procedures. As this paper shows, even in the absence of osteocytes, osteoblasts still respond to an array of mechanical stimuli in vitro. Models such as the 3D osteocyte-osteoblast coculture26 will improve our understanding of the combined response of these cells to loading.
Although the response of osteoblasts and osteocytes in response to loading appears to be reasonably well understood, or at least is gaining traction, there is a distinct lack of data on the effect of loading upon osteoclasts. RAW cells and primary osteoclasts have been cultured on plastic substrates and subjected to substrate strain which led to decreased proliferation.13,14 However, such substrates do not allow osteoclasts to resorb and as such it is difficult to assert that osteoclasts would respond to strain in the same way within the bone microenvironment. Nevertheless, these results do demonstrate that osteoclasts can respond to mechanical stimuli. It is therefore vital that further research focuses on the osteoclastic response to mechanical stimuli.
These findings show that while in vitro loading models have contributed greatly to our understanding of the cellular responses to mechanical stimuli, there is still a wide scope for further investigation. As such, in vitro loading provides a vital array of tools for the bone biologist.
Recommended further reading
Delaine-Smith RM, Reilly GC. The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Vitamins Horm 2011; 87: 417–480.
McCoy RJ, O'Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng B 2010; 16 587–601.
Skerry TM. One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J Musculoskelet Neuronal Interact 2006; 6(2): 122–127.
Vazquez M, Evans BaJ, Riccardi D, Evans SL, Ralphs JR, Dillingham CM, Mason DJ. A new method to investigate how mechanical loading of osteocytes controls osteoblasts. Front Endocrinol 2014; 5, 208.
Acknowledgments
We gratefully acknowledge Dr Aymen Idris for inviting this review. We thank Dr Gwendolen Reilly, Dr Bronwen Evans and Dr Gabriel Galea for their suggestions. Dr Behzad Javaheri wishes to acknowledge his funding from the BBSRC (BB/I014608/1).
Footnotes
The authors declare no conflict of interest.
References
- Klein-Nulend J, Bacabac RG, Mullender MG. Mechanobiology of bone tissue. Pathol Biol 2005; 53: 576–580. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 1995; 57: 344–358. [DOI] [PubMed] [Google Scholar]
- Rumney RMH, Sunters A, Reilly GC, Gartland A. Application of multiple forms of mechanical loading to human osteoblasts reveals increased ATP release in response to fluid flow in 3D cultures and differential regulation of immediate early genes. J Biomech 2012; 45: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Sunters A, Meakin LB, Zaman G, Sugiyama T, Lanyon LE et al. Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett 2011; 585: 2450–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol 1999; 276: E171–E178. [DOI] [PubMed] [Google Scholar]
- Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 1999; 14: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Kawata A, Mikuni-Takagaki Y. Mechanotransduction in stretched osteocytes—temporal expression of immediate early and other genes. Biochem Biophys Res Commun 1998; 246: 404–408. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone 2013; 54: 182–190. [DOI] [PubMed] [Google Scholar]
- Delaine-Smith RM, MacNeil S, Reilly GC. Matrix production and collagen structure are enhanced in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. Eur Cells Mater 2012; 24: 162–174. [DOI] [PubMed] [Google Scholar]
- Lane WO, Jantzen AE, Carlon TA, Jamiolkowski RM, Grenet JE, Ley MM et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J Vis Exp 2012; 59: 3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE et al. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol 1997; 273: C810–C815. [DOI] [PubMed] [Google Scholar]
- Javaheri B, Sunters A, Zaman G, Suswillo RFL, Saxon LK, Lanyon LE et al. Lrp5 is not required for the proliferative response of osteoblasts to strain but regulates proliferation and apoptosis in a cell autonomous manner. PLoS One 2012; 7: e35726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Guo C, Yan YX, Guo Y, Li RX, Song M et al. Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Mol Med Rep 2012; 6: 409–415. [DOI] [PubMed] [Google Scholar]
- Rubin J, Fan X, Biskobing DM, Taylor WR, Rubin TC. Osteoclastogenesis is repressed by mechanical strain in an In vitro model. J Orthop Res 1999; 17: 639–645. [DOI] [PubMed] [Google Scholar]
- Uzer G, Manske SL, Chan ME, Chiang FP, Rubin CT, Frame MD et al. Separating fluid shear stress from acceleration during vibrations in vitro: identification of mechanical signals modulating the cellular response. Cell Mol Bioeng 2012; 5: 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 2010; 46: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech 2009; 41: 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 1994; 27: 339–360. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Smith RN. Measurements of bone strain in the walking animal. Res Vet Sci 1969; 10: 93–94. [PubMed] [Google Scholar]
- Baggott DG, Lanyon LE. An independent ‘post-mortem' calibration of electrical resistance strain gauges bonded to bone surfaces ‘in vivo'. J Biomech 1977; 10: 615–622. [DOI] [PubMed] [Google Scholar]
- Wright TM, Hayes WC. Strain gage application on compact bone. J Biomech 1979; 12: 471–475. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones' adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res 2012; 27: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman R, Spatz J, Cloutier A, Palme R, Christiansen BA, Bouxsein ML. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J Bone Miner Res 2013; 28: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J 2005; 19: 482–484. [DOI] [PubMed] [Google Scholar]
- McCoy RJ, O'Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B 2010; 16: 587–601. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Evans B a J, Riccardi D, Evans SL, Ralphs JR, Dillingham CM et al. A new method to investigate how mechanical loading of osteocytes controls osteoblasts. Front Endocrinol (Lausanne) 2014; 5: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan UA, Akkus O. The mechanical environment of bone marrow: A review. Ann Biomed Eng 2008; 36: 1978–1991. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu D, You L, Wang L. Quantifying fluid shear stress in a rocking culture dish. J Biomech 2010; 43: 1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Henningsson P, Franklin SL, Chen D, Ventikos Y, Bomphrey RJ et al. See-saw rocking: an in vitro model for mechanotransduction research. J R Soc Interface 2014; 11: 20140330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology 2003; 40: 591–603. [PubMed] [Google Scholar]
- Morris HL, Reed CI, Haycock JW, Reilly GC. Mechanisms of fluid-flow-induced matrix production in bone tissue engineering. Proc Inst Mech Eng H 2010; 224: 1509–1521. [DOI] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem 2001; 276: 13365–13371. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech 1998; 31: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res 2012; 27: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing D, Lu XL, Luo E, Sajda P, Leong PL, Guo XE. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone 2013; 53: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue TLH, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech 2003; 36: 1363–1371. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci 1985; 75: 35–42. [DOI] [PubMed] [Google Scholar]
- Bhatt KA, Chang EI, Warren SM, Lin Se, Bastidas N, Ghali S et al. Uniaxial mechanical strain: an in vitro correlate to distraction osteogenesis. J Surg Res 2007; 143: 329–336. [DOI] [PubMed] [Google Scholar]
- Granet C, Vico AGL, Alexandre C, Lafage-Proust MH. MAP and src kinases control the induction of AP-1 members in response to changes in mechanical environment in osteoblastic cells. Cell Signal 2002; 14: 679–688. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA. Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of alphavbeta3-integrin expression. J Bone Miner Res 2000; 15: 1731–1745. [DOI] [PubMed] [Google Scholar]
- Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology 1995; 136: 528–535. [DOI] [PubMed] [Google Scholar]
- Vande Geest JP, Di Martino ES, Vorp DA. An analysis of the complete strain field within FlexercellTM membranes. J Biomech 2004; 37: 1923–1928. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Suswillo RF, Rawlinson SC, Zaman G, Lee K, Das-Gupta V et al. Osteoblast-like cells from estrogen receptor alpha knockout mice have deficient responses to mechanical strain. J Bone Miner Res 2004; 19: 938–946. [DOI] [PubMed] [Google Scholar]
- Earthman JC, Li Y, VanSchoiack LR, Sheets CG, Wu JC. Reconstructive materials and bone tissue engineering in implant dentistry. Dent Clin N Am 2006; 50: 229–244. [DOI] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010; 31: 461–466. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater 2003; 5: 29–39 discussion 39–40. [DOI] [PubMed] [Google Scholar]
- Wiesmann HP, Joos U, Meyer U. Biological and biophysical in extracorporal bone tissue engineering Part II. Int J Oral Maxillofac Surg 2004; 33: 523–530. [DOI] [PubMed] [Google Scholar]
- Dumas V, Perrier A, Malaval L, Laroche N, Guignandon A, Vico L et al. The effect of dual frequency cyclic compression on matrix deposition by osteoblast-like cells grown in 3D scaffolds and on modulation of VEGF variant expression. Biomaterials 2009; 30: 3279–3288. [DOI] [PubMed] [Google Scholar]
- Vance J, Galley S, Liu D, Donahue SW. Mechanical stimulation of MC3T3 osteoblastic cells in a bone tissue-engineering bioreactor enhances prostaglandin E2 release. Tissue Eng 2005; 11: 19–22. [DOI] [PubMed] [Google Scholar]
- Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone 2009; 44: 822–829. [DOI] [PubMed] [Google Scholar]
- Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell-seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A 2009; 88: 778–786. [DOI] [PubMed] [Google Scholar]
- Bye FJ, Wang L, Bullock AJ, Blackwood KA, Ryan AJ, MacNeil S. Postproduction processing of electrospun fibres for tissue engineering. J Vis Exp 2012; 66: 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003; 24: 2077–2082. [DOI] [PubMed] [Google Scholar]
- Barron MJ, Tsai C-J, Donahue SW. Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J Biomech Eng 2010; 132: 041005. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Guan X, Zhu Z, Gao S, Zhang C, Li C et al. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. Eur Cell Mater 2011; 22: 12–25. [DOI] [PubMed] [Google Scholar]
- Kim IS, Song YM, Lee B, Hwang SJ. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J Dent Res 2012; 91: 1135–1140. [DOI] [PubMed] [Google Scholar]
- Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci USA 2002; 99: 12600–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PHM, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A 2003; 64: 235–241. [DOI] [PubMed] [Google Scholar]
- Jaasma MJ, O'Brien FJ. Mechanical stimulation of osteoblasts using steady and dynamic fluid flow. Tissue Eng Part A 2008; 14: 1213–1223. [DOI] [PubMed] [Google Scholar]
- Du D, Furukawa KS, Ushida T. 3D culture of osteoblast-like cells by unidirectional or oscillatory flow for bone tissue engineering. Biotechnol Bioeng 2009; 102: 1670–1678. [DOI] [PubMed] [Google Scholar]
- Jungreuthmayer C, Donahue SW, Jaasma MJ, Al-Munajjed AA, Zanghellini J, Kelly DJ et al. A comparative study of shear stresses in collagen-glycosaminoglycan and calcium phosphate scaffolds in bone tissue-engineering bioreactors. Tissue Eng Part A 2009; 15: 1141–1149. [DOI] [PubMed] [Google Scholar]
- Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc Natl Acad Sci USA 2004; 101: 11203–11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-Y, Teoh SH, Teo EY, Khoon Chong MS, Shin CW, Tien FT et al. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 2010; 31: 8684–8695. [DOI] [PubMed] [Google Scholar]
- Waldman S, Spiteri C, Grynpas M, Pilliar R, Kandel R. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue 2004; 10: 1323–1331. [DOI] [PubMed] [Google Scholar]
- Thorpe SD, Buckley CT, Vinardell T, O'Brien FJ, Campbell VA, Kelly DJ. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Ann Biomed Eng 2010; 38: 2896–2909. [DOI] [PubMed] [Google Scholar]
- Mann V, Huber C, Kogianni G, Jones D, Noble B. The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J Musculoskelet Neuronal Interact 2006; 6: 408–417. [PMC free article] [PubMed] [Google Scholar]
- Byrne EM, Farrell E, McMahon LA, Haugh MG, O'Brien FJ, Campbell VA et al. Gene expression by marrow stromal cells in a porous collagen-glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J Mater Sci Mater Med 2008; 19: 3455–3463. [DOI] [PubMed] [Google Scholar]
- Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng 2010; 38: 1767–1779. [DOI] [PubMed] [Google Scholar]
- Tortelli F, Pujic N, Liu Y, Laroche N, Vico L, Cancedda R. Osteoblast and osteoclast differentiation in an in vitro three-dimensional model of bone. Tissue Eng Part A 2009; 15: 2373–2383. [DOI] [PubMed] [Google Scholar]
- Tortelli F, Cancedda R. Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro. Eur Cells Mater 2009; 17: 1–14. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Abukawa H, Shin MY, Terai H, Troulis MJ, Vacanti JP. Osteoclastogenesis on tissue-engineered bone. Tissue Eng 2004; 10: 93–100. [DOI] [PubMed] [Google Scholar]
- Domaschke H, Gelinsky M, Burmeister B, Fleig R, Hanke T, Reinstorf A et al. In vitro ossification and remodeling of mineralized collagen I scaffolds. Tissue Eng 2006; 12: 949–958. [DOI] [PubMed] [Google Scholar]
- Murshid SA, Kamioka H, Ishihara Y, Ando R, Sugawara Y, Takano-Yamamoto T. Actin and microtubule cytoskeletons of the processes of 3D-cultured MC3T3-E1 cells and osteocytes. J Bone Miner Metab 2007; 25: 151–158. [DOI] [PubMed] [Google Scholar]
- Qi J, Chi L, Faber J, Koller B, Banes AJ. ATP reduces gel compaction in osteoblast-populated collagen gels. J Appl Physiol 2007; 102: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials 2009; 30: 3672–3681. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption—bone flow theory. Clin Orthop Relat Res 1977; 127: 236–247. [PubMed] [Google Scholar]
- Neidlinger-Wilke C, Würtz K, Liedert A, Schmidt C, Börm W, Ignatius A et al. A three-dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J Neurosurg Spine 2005; 2: 457–465. [DOI] [PubMed] [Google Scholar]
- Tata U, Xu H, Rao SMN, Chuong C, Nguyen KT. A novel multiwell device to study vascular smooth muscle cell responses under cyclic strain. 2011; 2: 1–6.
- Sittichokechaiwut A, Edwards JH, Scutt AM, Reilly GC. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur Cell Mater 2010; 20: 45–57. [DOI] [PubMed] [Google Scholar]
- Nijweide PJ, van der Plas A, Alblas MJ, Klein-Nulend J. Osteocyte isolation and culture. Methods Mol Med 2003; 80: 41–50. [DOI] [PubMed] [Google Scholar]
- Van der Plas A, Nijweide PJ. Isolation and Purification of Osteocytes. J Bone Miner Res 2005; 20: 706–714. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011; 17: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Halleux C, Kramer I, Allard C, Kneissel M. Isolation of mouse osteocytes using cell fractionation for gene expression analysis. Methods Mol Biol 2012; 816: 55–66. [DOI] [PubMed] [Google Scholar]
- Stern AR, Stern MM, van Dyke ME, Jähn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 2012; 52: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Nars M, Hentunen TA, Metsikkö K, Väänänen HK. Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res 2006; 323: 263–271. [DOI] [PubMed] [Google Scholar]
- Cheng Mz, Zaman G, Rawlinson SC, Mohan S, Baylink DJ, Lanyon LE. Mechanical strain stimulates ROS cell proliferation through IGF-II and estrogen through IGF-I. J Bone Miner Res 1999; 14: 1742–1750. [DOI] [PubMed] [Google Scholar]
- Zaman G, Sunters A, Galea GL, Javaheri B, Saxon LK, Moustafa A et al. Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-i axis-dependent. J Biol Chem 2012; 287: 3946–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]