Abstract
Background:
Helicobacter pylori colonizes not only on the surface of mucous membrane, but also beneath the surface mucous gel layer (SMGL). As diclofenac Na decreases the secretion of SMGL, in this study we examined this drug as an adjuvant therapy to a quadruple therapy for H. Pylori eradication.
Materials and Methods:
One hundred and seventy-two patients were randomly assigned to three groups. Fifty four patients received quadruple therapy, that is, azithromycine 250 mg, amoxicillin 500 mg, bismuth subcitrate 240 mg, and omeprazole 20 mg bid for 1 week (group A) and 65 patients received the same dosage of those agents plus diclofenac Na tab, 100 mg daily for 1 week (group B). Sixty two patients received the quadruple therapy for 2 weeks (group C). Eradication of the infection was assessed 4-6 weeks after completion of treatment by stool antigen assay for H. pylori.
Results:
While the rate of H. pylori eradication in the groups A and B was 66.7% and 82.1%, respectively (P = 0.062), the rate of H. pylori eradication in groups B and C were 82.1% and 82.3% respectively (P = 0.987).
Conclusions:
It seems that diclofenac Na can shorten anti-H. pylori regimens for 1 week. More investigations are needed for more clarification of the efficacy of NSAIDs for successful eradication of H. pylori. (IRCT code: IRCT201204059256N2)
Keywords: Diclofenac Na, eradication, Helicobacter pylori
INTRODUCTION
The most common bacterial infection in human is caused by Helicobacter pylori. H. pylori has been demonstrated all around the world and this germ could affect human in all ages and it is estimated that up to 50% of the world's population is infected by H. pylori.[1]
In developing countries (such as Iran) H. pylori is more frequent in younger adults compared to industrialized nations. Infection in H. pylori is persistent and it may or may not cause gastroduodenal disease.[2]
H. pylori colonized not only in the surface of the surface mucous cells, but also the surface mucous gel layer (SMGL). The urease, motility of the germ and its adhesive ability allows H. pylori to survive and proliferate in the gastric milieu.[3]
The aim of H. pylori eradication is to achieve a high eradication rate at the first try because the risk of antibiotic resistance is very high after anti H. pylori treatment. Many regimens have been recommended for H. pylori eradication,[4,5,6] however, considering the costs, complications, and ease of administration; the optimal therapeutic regimen has not been defined yet.
Successful H. pylori eradication could prevent spreading of resistant strains in the society and there is still no general agreement in treatment duration to receive best result.[7]
Several therapeutic approaches have been used for H. pylori eradication (triple therapy, sequential therapy, quadruple therapy, and dual therapy). However, failure to treat is still reports from many cases in all regimes.[8,9,10,11]
For example, Albrecht et al., had reported that about 20% of their samples were failure to treat during H. pylori eradication due to antibiotic resistance, side effects, or differences in physiological conditions.[12]
Nonsteroidal anti-inflammatory drugs (NSAIDs) decreases the secretion of surface mucous gel layer (SMGL),[13] in this study the efficacy of diclofenac-Na as a NSAIDs in adjuvant therapy with a traditional quadruple therapy in shortening the H. pylori eradication period or increasing the rate of H. pylori eradication was evaluated.
MATERIALS AND METHODS
This was an open label study that was conducted in Isfahan from April 2010 to August 2011. At the baseline, patients were evaluated for inclusion or exclusion criteria. This study was approved by medical ethics committee of Isfahan university of medical sciences and Iranian Registry of Clinical Trials (IRCT) and the code is: IRCT201204059256N2.
We had included adults with H. pylori infectious. Diagnosis of H. pylori infection was based on positivity of a rapid urease test (RUT) or based on histology. In all patients, five biopsies (two antrum, two bodies, and one angulus) specimens were taken for histological assessment and two specimens (one from antrum and one from body) were taken for RUT and histology evaluations. In the cases that RUT was negative, biopsy samples were sent to the pathology laboratory for specific histological test of H. pylori. Our exclusion criteria were: Previous treatment for H. pylori infection, use of PPI (proton pomp inhibitors) during 2 weeks and/or antibiotics during 4 weeks before the study, peptic ulcer, GERD, gastrointestinal malignancy, previous gastro-oesophageal surgery, severe concomitant cardiovascular, hypertension, respiratory or endocrine diseases, clinically significant renal or hepatic disease, hematological disorders, any other clinically significant medical conditions that could increase risk, history of allergy to any of the drug used in the study, pregnancy or lactation, alcohol abuse, drug addiction, severe neurological or psychiatric disorders, contraindication of consumption of NSAID, and long-term use of corticosteroids or anti-inflammatory drugs.
Patients were then randomly assigned to three treatment groups (Simple Randomization Method was used) and follow-up evaluation was done to assess the eradication rate of the infection. A total number of 172 patients were included in final design of the study, who were randomly divided in three groups: (1) 54 patients received the following regime: Traditional quadruple therapy including azithromycin 250 mg, amoxicillin 500 mg, bismuth subcitrate 240 mg, and omeprazole 20 mg, twice a day for 1 week (group A); (2) 56 patients received a regime same as group A regime plus diclofenac-Na, 100 mg daily for 1 week (group B); 62 patients received a regime same as group A, but for 2 weeks (group C) [Figure 1].
Figure 1.
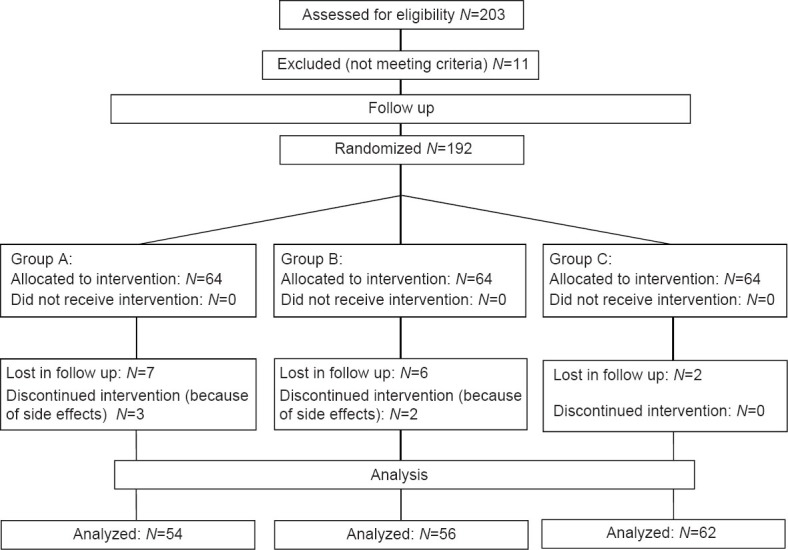
Participants’ retention versus attrition
Patients were enrolled by gastrointestinal specialist at the hospitals after assessment of appropriate indication and ruling out any contraindication to the treatments. Patients were randomly allocated to receive one of the three schedules of treatment to receive the relevant drugs.
Patients were interviewed at completion of therapy to assess adherence to the therapeutic regimen and adverse events by medical personnel blinded to the eradication regimen of each patient. In particular, first, open-ended questions on side effects and then specific questions on anticipated adverse events were asked. Four weeks after completion of therapy, H. pylori status was re-evaluated by stool antigen assay, by personnel unaware of the eradication regimen of each patient to assess whether the infection had been successfully eradicated. Infection was considered eradicated in the patent, if the test was negative.
Statistical procedure was done by Statistical Program for Social Sciences software (SPSS) version 18. Differences between ratio of eradication in each regime were evaluated by χ2 test. The significance level was set at P < 0.05. Power of this study was 0.8.
This study had been registered in Iranian Registry of Clinical Trials (IRCT) and the code is: IRCT201204059256N2.
RESULTS
In this study, a total number of 172 patients were assigned to three A, B, and C groups. Twenty nine in group A, 33 in group B, and 29 in last group were male. There was no significant difference between groups in sex (P = 0.414).
Mean age in group A was 40.54 (SD = 12.77), in group B was 40.02 (SD = 13.49) and in group C was 41.14 (SD = 13.87) (P = 0.919).
Tables 1 show the sex and age frequency of the tested patients, respectively. After statistical analysis, there were not any significant difference among the three groups regarding sex and age (P > 0.05).
Table 1.
Comparison of recovery rate of H. pylori based on the stool antigen assay in the three groups

The rate of H. pylori eradication in groups A and B were 66.7% and 82.1%, Fisher exact test showed a significant difference between groups (P = 0.048). The rate of H. pylori eradication in groups A and C were 66.7% and 82.3%, respectively (P = 0.044). The rate of H. pylori eradication in groups B and C were 82.1% and 82.3%, respectively (P > 0.0.987).
Patients reports complications during their treatment, Abdominal pain was the most common side effect in patients which was seen in 26 patients but there was no significant difference between groups (P = 0.263), nausea was seen in 24 patients (P = 0.232) and diarrhea was reported by 18 patients (P = 0.548).
DISCUSSION
One hundred and seventy two participants were enrolled to this study, all patients had a positive rapid urease test. Participants were divided in to three groups; group A received quadruple regime for 1 week, group B received quadruple regime and diclofenac-Na for 1 week, and third group regime was the same as second group but their treatment duration was 2 weeks. The eradication rate in group A was 66.7%, 82.1% in group B and 82.3% in group C. Our results show a significant difference between groups (P < 0.05).
The cause of better effect of treatment in group B and C proposed that diclofenac decreases of thickness of the mucosal gel layer and so the drugs effect better on H. pylori bacteria that get under mucosal layer.
Some others papers had evaluated the effect of different NSAIDs agents on H. pylori treatment. Gokturk et al. had evaluated the effect of Asprin in H. pylori eradication. In their study, 77 participants were Asprin users with dyspepsia symptoms and 79 years of age and sex-matched dyspeptic patients. Their eradication regime was Lansoprazole (30 mg twice a day), Clarithromycin (500 mg twice a day), and Amoxicillin (1 g twice a day) for 14 days. They had reported that in dyspeptic patients who used Asprin eradication ratio was 64/77 (83%) and in other group was 42/79 (53%). There was a significant difference between their findings (P < 0.05).[14] Our findings in groups which used NSAIDs in addition to our therapeutic regimen was almost the same with Gokturk's report (82.1% in group B and 82.3 in group C vs. 83% in Gokturk's study). Our findings are agreed with Gokturk's results.
Park et al. had reported that adding aspirin to Omeprazole, Amoxicillin, and Clarithromycin regime had increased H. pylori eradication rate (86.7% in patients who used Asprin added to their regime vs. 80.3% in other group). There was no statistical differences between their groups (P > 0.05).[15] Our eradication rate was lower than their results were significant.
Zhang et al. in 2009 had evaluated the effect of Celecoxib as a selective cyclooxygenase 2 (COX-2) inhibitor in H. pylori eradication. They had reported that H. pylori eradication followed by Celecoxib had improved gastric paraneoplastic lesions.[16]
According to our findings and other reports using NSAIDs could increase the H. pylori eradication ratio. Mechanisms by that NSAIDs increase eradication in not well recognized but some of them may be: NSAIDs could increase antibiotics endocellular concentrations and inhibiting the virulence factors of H. pylori. Another factor that could affect the eradication rate is the bacterial load. Moshkowitz et al. used triple therapy for H. pylori eradication and they had proved that low bacterial load is associated with a higher eradication rate.[17] Patients H. pylori infection.[18] It was proved that NSAIDs such as: Sodium salicylate, aspirin, indomethacin and celecoxib have a dose-dependent effect on H. pylori eradication and it may be because of the effect of NSAIDs on H. pylori virolence factors.[19]
Finally we can conclude that NSAIDs such as Diclofenac-Na, Asprin, celecoxib could increase the H. pylori eradication rate. In this study, if we had a larger sample size our findings might be significant. We suggest that further studies should be established to evaluate the effect of NSAIDs on H. pylori eradication but sample size and treatment duration should to be considered and we also recommend to use RUT for follow ups in H. pylori eradication.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Steininger S, Pelz C, Vogelmann R. Purpose of recently detected inhibitory domain of the Helicobacter pylori protein CagA. Gut Microbes. 2011;2:167–72. doi: 10.4161/gmic.2.3.15872. [DOI] [PubMed] [Google Scholar]
- 2.Taghvaei T, Talebi Bezmin Abadi A, Ghasemzadeh A, Naderi BK, Mohabbati Mobarez A. Prevalence of horB gene among the Helicobacter pylori strains isolated from dyspeptic patients: First report from Iran. Intern Emerg Med. 2012;7:505–8. doi: 10.1007/s11739-011-0614-7. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T, Akamatsu T, Sugiyama A, Ota H, Katsuyama T. Helicobacter pylori and the surface mucous gel layer of the human stomach. Helicobacter. 1996;1:207–18. doi: 10.1111/j.1523-5378.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 4.Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971–5. doi: 10.3748/wjg.v17.i35.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggiero P. Helicobacter pylori infection: What's new. Curr Opin Infect Dis. 2012;25:337–44. doi: 10.1097/QCO.0b013e3283531f7c. [DOI] [PubMed] [Google Scholar]
- 6.Selgrad M, Malfertheiner P. Treatment of Helicobacter pylori. Curr Opin Gastroenterol. 2011;27:565–70. doi: 10.1097/MOG.0b013e32834bb818. [DOI] [PubMed] [Google Scholar]
- 7.Hu CT, Wu CC, Lin CY, Cheng CC, Su SC, Tseng YH, et al. Resistance rate to antibiotics of Helicobacter pylori isolates in eastern Taiwan. J Gastroenterol Hepatol. 2007;22:720–3. doi: 10.1111/j.1440-1746.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: Duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–62. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fischbach L, Evans EL. Meta-analysis: The effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, et al. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter. 2010;15:233–8. doi: 10.1111/j.1523-5378.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905–13. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht P, Kotowska M, Szajewska H. Sequential therapy compared with standard triple therapy for Helicobacter pylori eradication in children: A double-blind, randomized, controlled trial. J Pediatr. 2011;159:45–9. doi: 10.1016/j.jpeds.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Garner A, Allen A, Rowe PH. Gastroduodenal mucosal defence mechanisms and the action of non-steroidal anti-inflammatory agents. Scand J Gastroenterol Suppl. 1987;127:29–34. doi: 10.3109/00365528709090947. [DOI] [PubMed] [Google Scholar]
- 14.Gokturk HS, Demir M, Unler GK, Erbayrak M, Sakalli M, Yilmaz U. Does long-term aspirin use have any effect on Helicobacter pylori eradication? Am J Med Sci. 2011;342:15–9. doi: 10.1097/MAJ.0b013e3182174cf1. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Park DI, Kim SH, Kim HJ, Cho YK, Sung IK, et al. Effect of high-dose aspirin on Helicobacter pylori eradication. Dig Dis Sci. 2005;50:626–9. doi: 10.1007/s10620-005-2547-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang LJ, Wang SY, Huo XH, Zhu ZL, Chu JK, Ma JC, et al. Anti-Helicobacter pylori therapy followed by celecoxib on progression of gastric precancerous lesions. World J Gastroenterol. 2009;15:2731–8. doi: 10.3748/wjg.15.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshkowitz M, Konikoff FM, Peled Y, Santo M, Hallak A, Bujanover Y, et al. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut. 1995;36:845–7. doi: 10.1136/gut.36.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caselli M, Pazzi P, LaCorte R, Aleotti A, Trevisani L, Stabellini G. Campylobacter-like organisms, nonsteroidal anti-inflammatory drugs and gastric lesions in patients with rheumatoid arthritis. Digestion. 1989;44:101–4. doi: 10.1159/000199898. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XP, Wang WH, Tian Y, Gao W, Li J. Aspirin increases susceptibility of Helicobacter pylori to metronidazole by augmenting endocellular concentrations of antimicrobials. World J Gastroenterol. 2009;15:919–26. doi: 10.3748/wjg.15.919. [DOI] [PMC free article] [PubMed] [Google Scholar]


