Abstract
Background and Aims Evaluation of population projection matrices (PPMs) that are focused on asymptotically based properties of populations is a commonly used approach to evaluate projected dynamics of managed populations. Recently, a set of tools for evaluating the properties of transient dynamics has been expanded to evaluate PPMs and to consider the dynamics of populations prior to attaining the stable-stage distribution, a state that may never be achieved in disturbed or otherwise ephemeral habitats or persistently small populations. This study re-evaluates data for a tropical orchid and examines the value of including such analyses in an integrative approach.
Methods Six small populations of Lepanthes rubripetala were used as a model system and the R software package popdemo was used to produce estimates of the indices for the asymptotic growth rate (lambda), sensitivities, reactivity, first-time step attenuation, maximum amplification, maximum attenuation, maximal inertia and maximal attenuation. The response in lambda to perturbations of demographic parameters using transfer functions and multiple perturbations on growth, stasis and fecundity were also determined. The results were compared with previously published asymptotic indices.
Key Results It was found that combining asymptotic and transient dynamics expands the understanding of possible population changes. Comparison of the predicted density from reactivity and first-time step attenuation with the observed change in population size in two orchid populations showed that the observed density was within the predicted range. However, transfer function analysis suggests that the traditional approach of measuring perturbation of growth rates and persistence (inertia) may be misleading and is likely to result in erroneous management decisions.
Conclusions Based on the results, an integrative approach is recommended using traditional PPMs (asymptotic processes) with an evaluation of the diversity of dynamics that may arise when populations are not at a stable-stage distribution (transient processes). This method is preferable for designing rapid and efficient interventions after disturbances, and for developing strategies to establish new populations.
Keywords: Transient population dynamics, stable-stage equilibrium, lambda, reactivity, first-time step attenuation, maximum amplification, maximum attenuation, maximum inertia, transfer function, population projection matrices, PPM, orchids, Lepanthes rubripetala, Orchidaceae, Puerto Rico
INTRODUCTION
Biologists, conservation managers and decision makers with interest in the trajectories of population dynamics need tools that are easy to use and interpret, apply across a wide range of taxa and give results that predict future population sizes with the highest possible confidence. The preferred approach has been to use asymptotically based population projection matrices (PPMs) as a set of models that have tractable outcomes, including calculations of asymptotic growth rates, population stable distributions, elasticities, sensitivities, damping ratios etc. (Caswell, 2001). Stochastic PPM models are a modification of these approaches and explore the likely changes in population dynamics over time as a consequence of demographic, spatial and environmental variation (Tuljapurkar, 1997; Fieberg and Ellner, 2001; Lande et al., 2003).
Although these models are commonly employed in the ecological and conservation literature, little attention has been given to the accuracy and effectiveness of PPM models (Kephart and Paladino, 1997; Bierzychudek, 1999; Coulson et al., 2001; Lindborg and Erhlén, 2002; Van Mantgem and Stephenson, 2005; Schödelbauerová et al., 2010; Jäkäläniemi et al., 2013). So what is the predictive power of population projection analyses? In a recent review of the population dynamics of 82 populations of 20 species, Crone et al. (2013) found that in more than half of the studies using PPMs the actual population sizes over a period of time were either above or below the projected confidence intervals, suggesting weaknesses in the methods, the data or both.
An alternative, novel and infrequently considered approach for characterizing population dynamics over time is to use transient dynamics, which are fluctuations describing how much populations vary as a consequence of stochastic events prior to reaching asymptotic growth and achieving a stable-stage distribution. Originally part of the robust control theory of engineering, several attempts have been made to include transfer functions in the population ecology and conservation management toolbox (Rebarber and Townley, 1995; Hodgson and Townley, 2004). Caswell (2007) developed an approach to analyse transient sensitivities and elasticities using matrix calculus, but more recently Stott et al. (2010a, 2011, 2012a, b) offered a series of set of functions, which are available in R (popdemo package; R Project for Statistical Computing, 2013), to investigate short-term time series as a consequence of ecologically, environmentally and anthropologically induced perturbations. The traditional way to assess the effects of perturbations on demographic parameters using sensitivity analysis relies on eigenvectors and gives a linear approximation that could be misleading for non-linear responses. This was addressed by Stott et al. (2012b), who calculated sensitivities without using eigenvectors through a derivative process based on transfer functions (McCarthy et al., 2008). One of the advantages of the transfer function is that it only needs to specify the magnitude of the perturbation of a demographic parameter to describe the non-linear changes in that parameter. Furthermore, transient dynamics may be more appropriate for conservation because it addresses outcomes that are more likely to occur within the time frame of a management project (Ezard et al., 2010).
To complement asymptotic and transient analysis, we use transfer functions to perform an analysis of the population dynamics of a Neotropical orchid by assessing the non-linear changes in asymptotic growth produced by changes in transition parameters. We re-analyse the data of Schödelbauerová et al. (2010) on Lepanthes rubripetala using transient dynamics and transfer function tools and compare the observed dynamics and likely persistence of the population with the stochastic simulations from Schödelbauerová et al. (2010). We hypothesize that for many small populations, using only the traditional PPM models, based on stable-stage distribution and asymptotic growth rate, is likely to be either misleading or give a different perspective on the likely population dynamics under study.
METHODS
Data collection
Our model species was Lepanthes rubripetala, Orchidaceae, which is endemic to the Caribbean island of Puerto Rico and is a member of a large genus (>1100 species; Govaerts et al., 2014) renowned for its narrow endemism (Crain and Tremblay, 2014). All but one of 120 species in the Caribbean are single-island endemics (Ackerman, 2012; Luer, 2014). Lepanthes rubripetala is a small epiphytic or epipetric species restricted to shady, montane wet habitats. The caespitose plants grow sympodially, with each slender stem bearing a single leaf. The terminal, fasciculate racemes are adpressed to the underside of the leaf and produce flowers in succession, usually one at a time, throughout the year. The flowers are 3–4 mm long. Fruit and seed production is pollinator-dependent and flowers are protandrous (Tremblay et al., 2006), and infrequently pollinated; pollination is probably the result of fungus gnat pseudocopulation (Blanco and Barbosa, 2005; Tremblay and Ackerman, 2001).
The small scattered populations of this species make a good model system because plant size and lifespan are manageable for demographic work. Furthermore, relatively few studies have been conducted on the demographic dynamics of tropical plants, especially herbaceous ones.
Our data are the same as those of Schödelbauerová et al. (2010), who selected six populations distributed along three streams: Río Grande and Quebrada Grande along the western slopes of the Luquillo Mountains in the El Yunque National Forest; and Río Patillas at Charco Azul in the Carite State Forest. Three hundred and eighty-one individuals of L. rubripetala were marked and observed monthly from June 1994 to January 1996. Population sizes at the first survey were 84, 17, 49, 86, 101 and 44. At each observation period, the numbers of leaves, flowers and fruits were counted and individuals from all populations were classified as seedlings, juveniles, non-reproductive adults and reproductive adults. These categories are defined as follows (Tremblay and Hutchings, 2003; Rivera-Gómez et al., 2006): seedlings are small plants without lepanthiform sheaths on any shoot; juveniles are individuals with at least one lepanthiform sheath on the stem and lack evidence of past or current inflorescences; non-reproductive adults have dried inflorescences from a previous flowering event, but they are not currently flowering; reproductive adults have active inflorescences that have buds, flowers and/or fruits (the theoretical life cycle of L. rubripetala is illustrated in Fig. 1).
Fig. 1.

The theoretical life cycle of Lepanthes rubripetala. For successful germination, seeds must be colonized by mycorrhizal fungi to form protocorms, which subsequently grow into juveniles, non-reproductive adults or reproductive adults. Once adulthood is achieved, the plant remains an adult; however, adults move readily among reproductive and non-reproductive phases within a year.
Population dynamics analysis and comparisons
The traditional approach of asymptotic analysis of PPMs focuses on long-term population dynamics to predict population trajectories. Stable-stage distributions (the predicted population structure when asymptotic growth rate is attained) and sensitivities (a measure evaluating the effect of absolute change of a parameter on growth rate) are calculated, which are density-independent and time-invariant indices (Caswell, 2001). Transient-based evaluation of PPMs is also time-invariant, but by varying the starting demographic distribution as a consequence of demographic stochasticity, whether of biotic, abiotic or anthropogenic origin, an initial stage distribution is obtained that diverges from the stable-stage distribution. This difference leads initially to either a short-term increase in population size/density (amplifications) or a short-term decrease (attenuation). If no other perturbations or disturbances are present (e.g. hurricanes, flash floods, landslides, biotic invasions), then the transient dynamics models are expected to settle to the stable-stage distribution. The time it takes to reach the stable stage is the transient period (Stott et al., 2011). Transient population dynamics are usually not evaluated in most published PPM articles; however, indices such as the damping ratio (Caswell, 2001), Keyfitz’s delta (Keyfitz, 1968) and Cohen’s cumulative distance metric (Cohen, 1979) are sometimes considered surrogates for considering the transient dynamics of populations, but they have limitations (Stott et al., 2011).
We calculated, using the package popdemo in R (R Project for Statistical Computing, 2013), the following indices of transient dynamics as described by Stott et al. (2011), and includes the expected lower and upper limits of change in the first-time step: reactivity (maximum population growth in a single time step relative to stable-stage growth), first-time attenuation (minimum population growth in a single time step relative to stable-stage growth), maximal amplification (the largest possible future population size relative to a stable growth rate and same initial population size) and maximal attenuation (the smallest possible future population size relative to a stable growth rate and the same initial population size). We also calculated two other indices: amplified inertia (the largest long-term population size relative to a population with stable growth rate and the same initial density) and attenuated inertia (the smallest long-term population size relative to a population with stable growth rate and the same initial density). In other words, populations that have not achieved a stable-stage distribution are likely to achieve long-term population density at a fixed ratio below or above the expected stable-stage distribution (Stott et al., 2012b).
Natural perturbations affect the demographic parameters of a population to different degrees (Hodgson and Townley, 2004; Hodgson et al., 2006). Consequently, we present the response of asymptotic growth rate (lambda) to perturbations of demographic parameters using a transfer function and multiple perturbations of growth, stasis and fecundity.
Moreover, we investigate the expected effect of changes in parameter estimates on lambda using a non-linear function (Stott et al., 2012b). Transfer functions are commonly used in the analysis of linear, time-invariant systems such as single-input, single-output filters. They allow one to calculate the relationship between lambda (λ) and the intensity of perturbation (δ). We only need to define the position of the vital rate perturbed through two vectors, e and d, the intensity of the perturbation, with one scalar, δ, to obtain the new asymptotic growth rate as a consequence of the perturbed vital rate. The exact relationship between perturbation and growth rate was given by Hodgson and Townley (2004) as: δ–1 = eT(λI – A)–1d. A is a generic way to indicate the matrix population of the species we are working with, in our case all of six populations of L. rubripetala have (4 × 4) dimension. I is the identity matrix with the same dimension of A. Inertia in the context of transient dynamics measures how much larger or smaller a population changes compared with an equivalent population at stable-stage distribution (Stott et al., 2012b). Non-stable populations show different growth patterns compared with populations at stable distribution, ultimately resulting in long-term population densities above or below those predicted if the population was at a stable distribution (Stott et al., 2012b). This long-term persistence is termed inertia. Inertia is calculated as the ratio between the long-term population densities at non-equilibrium above an equivalent stable population (Koons et al., 2007; Stott et al., 2012b). Consequently, populations with inertia values >1 become and remain larger whereas those with inertia <1 are smaller and remain smaller. Transfer function plots of inertia for the whole life cycle for the six populations of L. rubripetala were evaluated for the upper and lower bounds and the case-specific inertia for the current population structure.
Non-linear sensitivities were calculated (tfamatrix function in the popdemo package) and compared with the original linear approach. We tested the assumptions of reducibility and ergodicity for matrices using the tests as described in Stott et al. (2010b), and all were met.
To evaluate how transient dynamics mirror real population growth rates, we compared the change in population size across 17 time periods with the extremes of population growth and reduction (reactivity and first-time attenuation), which evaluated the likely range of change in the first time step with a time lag of 1 with n equal to the number of observed individuals in the time period in each of the observed stages.
The PPMs are available in Supplementary Data S1.
RESULTS
Transient dynamics
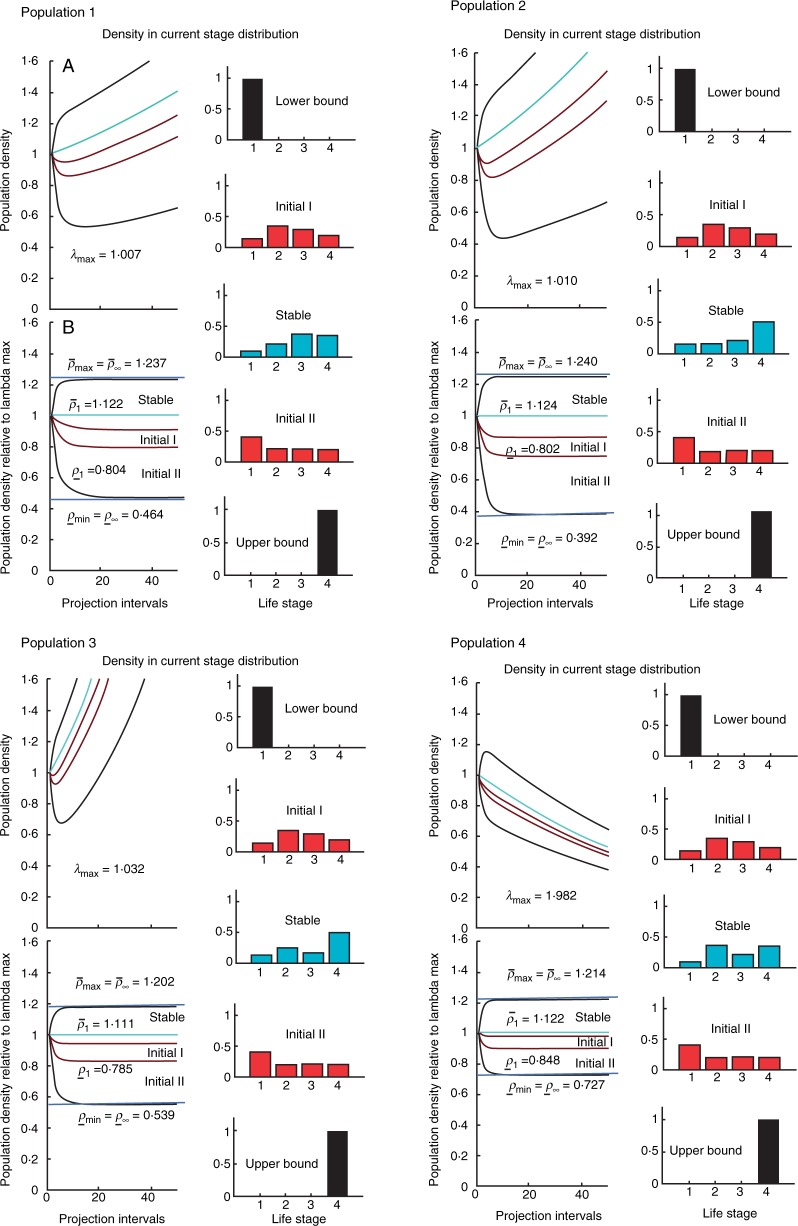
The transient indices suggest that the range of amplitude of predicted population densities over the first time interval is substantial. Populations in which the structure is dominated by seedlings are expected to have large reductions in population size (density), resulting in a first-time attenuation ranging from 0·84 to 0·57, suggesting that in one time period populations could be reduced to 57 % of the expected stable-stage distribution. When populations are dominated by reproductive adults, the expected population densities increase due to reactivity in the first time step, suggesting that populations could increase by 11–18 % (Fig. 2, Table 1).
Fig. 2.
(A) Absolute population dynamics, including transient and asymptotic influences, for the six populations of Lepanthes rubripetala. (B) Standardized transient dynamics, excluding the influence of asymptotic growth for L. rubripetala. All demographic distributions are scaled to an initial population density sum 1. The transient bounds for L. rubripetala are superimposed on the graph. We simulated five initial conditions. The two black bar plots at top and bottom correspond to having all individuals in the smallest and largest class, respectively. The two red bar plots correspond to distributions between the above distributions, and the blue bar plot corresponds to a stable demographic distribution. Bias distribution of individuals among stages: bias1 = [1 0 0 0]; bias2 = [0·15 0·35 0·30 0·20]; bias3 = stable-stage distribution (varies among matrices); bias4 = [0·4 0·2 0·2 0·2]; bias5 = [0 0 0 1].
Table 1.
Predicted stochastic asymptotic growth (lambda) of six Lepanthes rubripetala populations deterministic and stochastic lambda (the 95 % confidence interval of the s.e of the stochastic lambda) and transient dynamics: reactivity, first-time step attenuation, maximum amplification, maximum attenuation and upper and lower bound inertia (see Methods section for explanation of indices, stochastic lambdas from Schödelbauerová et al., 2010)
| Population | Deterministic lambda/stochastic lambda (s.e.) | Reactivity | First-time step attenuation | Maximum amplification | Maximum attenuation | Amplitude inertia (upper bound) | Attenuation inertia (lower bound) |
|---|---|---|---|---|---|---|---|
| 1 | 1·007/1·0072 (1·0070–1·0074) | 1·122 | 0·805 | 1·237 | 0·464 | 1·237 | 0·464 |
| 2 | 1·010/1·0161 (1·0159–1·0163) | 1·125 | 0·802 | 1·240 | 0·393 | 1·240 | 0·393 |
| 3 | 1·028/1·011 (1·0108–1·0113) | 1·112 | 0·786 | 1·202 | 0·539 | 1·202 | 0·539 |
| 4 | 0·982/0·9824 (0·9782–0·9801) | 1·123 | 0·848 | 1·214 | 0·728 | 1·214 | 0·728 |
| 5 | 1·024/1·0233 (1·0230–1·0236) | 1·118 | 0·837 | 1·191 | 0·627 | 1·191 | 0·627 |
| 6 | 0·987/1·0075 (1·0072–1·0079) | 1·180 | 0·567 | 1·301 | 0·133 | 1·301 | 0·133 |
The same two initial conditions, only seedlings or only reproductive adults, could have long-term effects on population density. The long-term expected reduction, maximal attenuation, could be dramatic, with density reductions ranging from 13 to 73 % in the transient period (Fig. 2, Table 1). On the other hand, the expected pattern of maximal amplification is no more than 19–30 %, a much smaller proportional change compared with the maximal attenuation. The amplified inertia also ranges from 19 to 30 %, whereas the expected range of attenuated inertia is 13–73 % (Fig. 2, Table 1).
Transfer functions
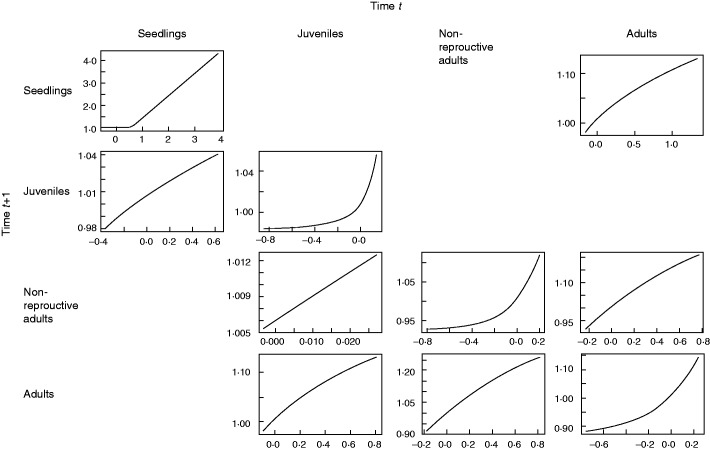
The general pattern for the non-linear sensitivities, i.e. transfer function analysis on lambda (Fig. 3, population 1; and Supplementary Data S2, populations 2–6), shows that parameters below the diagonal, which indicate the probability of growth from one growth class to another in one time interval, are more or less linear, whereas the diagonal (the probability of individuals staying in the same stage) are clearly non-linear. Thus, change in stasis could be misleading solely by using the traditional method of calculating sensitivities as a likely predictor of the impact of absolute changes of the parameter on lambda (Supplementary Data S4).
Fig. 3.
Transfer functions between transition parameters and the resulting changes in asymptotic growth rate, lambda, for population 1 of L. rubripetala (see Supplementary Data S2 for other populations). Each graph represents the effect of change in lambda as a function of change in the parameter while holding the other parameters constant. A graph is produced only if a parameter is present in the matrix for the specific transition. In general, we see that most changes in the transition of growth (below the diagonal) have close to a linear effect on lambda (but none is linear), but in stasis (the diagonal) the effect of changes in parameters is clearly non-linear.
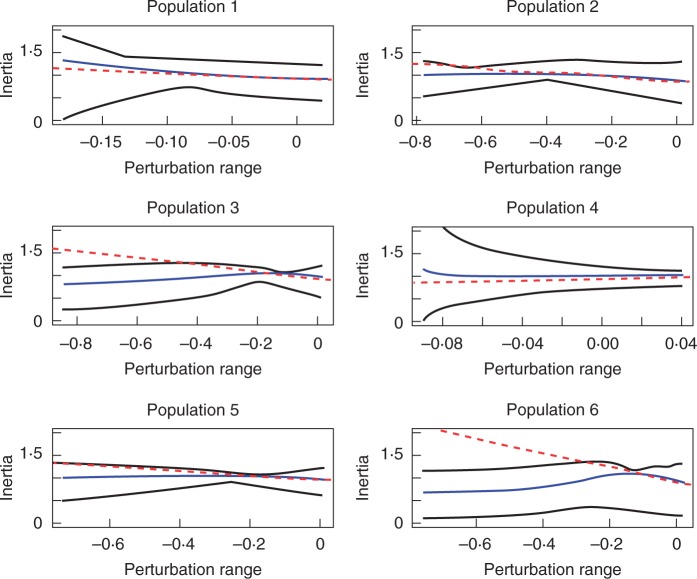
The non-linear response of inertia to perturbation shows a more complex pattern of relationships compared with the effect on lambda (Fig. 4; Supplementary Data S3). The effects of perturbation on inertia were evaluated for the upper and lower bounds and case-specific results for each population are shown in Appendix 3. As an example of the complex dynamics, the non-reproductive adults remaining in this stage show a double-hump inertia response. The density of the population would be expected to increase by 24 % as result of a small reduction in this stasis parameter in population 1, whereas no such density change is expected in population 2. However, in both populations a density increase is expected. Another equally variable response in inertia is observed in the transition from the non-reproductive to the reproductive stage for the case-specific population structure, where the response is a decreasing function (populations 1, 2 and 5), an increasing function (population 3) or a complex response (populations 4 and 6). These complex variable patterns of inertia are observed in many of the transitions.
Fig. 4.
Transfer function plots of population inertia for the elements that have the maximum sensitivity value of the whole life cycle for each of the six populations of Lepanthes rubripetala. On each plot, the two black lines represent the upper and lower bounds of population inertia, respectively; the blue line represents the transfer function of the current population structure for each population; and the dashed red line represents sensitivity at zero perturbation.
Observed versus expected population density changes
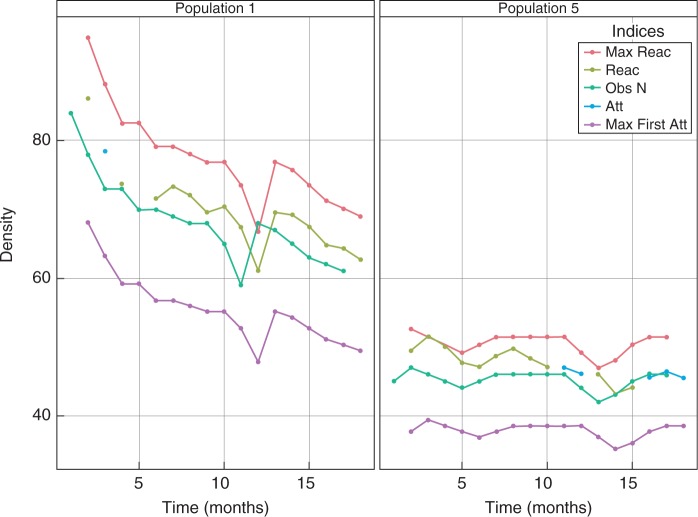
Observed population densities of L. rubripetala were similar to the predicted first-time attenuation and reactivity for the complete 17 time periods in the two populations evaluated (populations 1 and 5) (Fig. 5). The number of individuals was too low in the other populations for a reasonable comparison.
Fig. 5.
Observed population density, first-time attenuation and reactivity for the 17 time periods for populations 1 and 5. First-time attenuation and reactivity are the expected changes in density for a given time period as determined by the change in population demographic structure. Maximal reactivity (Max Reac) and first-time step (Max Att) attenuation are the results of biasing the population towards reproductive adults or seedlings, respectively, attenuation (Att) and reactivity (Reac) are the expected decrease or increase in density as a function of the observed sample size at time t – 1, and the observed line (Obs N) is the density of the surveyed population at time t.
DISCUSSION
Schödelbauerová et al. (2010) reported results of PPM analysis using an asymptotic approach and noted that if population growth rates are consistent and the expected stable population distributions are reached, four populations of L. rubripetala (populations 1, 2, 3 and 5) are expected to grow, whereas two are expected to decline (populations 4 and 6). The asymptotic growth rate (non-stochastic) ranged from 0·987 to 1·029 (Fig. 2, Table 1). With the same data set but integrating transient dynamics and transfer function tools, we found that the road to stable-stage distribution is non-linear and the starting population structure at the beginning influences population densities.
The expected transient dynamics of the surveyed populations varied among populations of L. rubripetala and population density could be expected to change readily. The transients among populations varied in their predicted projection in the different indices. One of the conspicuous patterns is that populations are much less likely to have large increases in density (reactivity and maximum amplification) compared with reduction (first-step attenuation and maximum attenuation). For species that are rarely if ever found in large numbers (Tremblay, 1997), this suggests that populations could be reduced or even disappear much more readily than they can increase in size, thus not only influencing population persistence (Morris and Doak, 2002) but also leading to the possibility of reduction in the effective population size (Tremblay and Ackerman, 2001).
The asymptotic behaviour of all six populations of L. rubripetala showed growth rates near to 1, five of them slightly above 1, and one slightly below 1, which means that a population of L. rubripetala as a whole tends to show long-term stationary behaviour. However, due to uncertainty associated with the process of demographic stochasticity and the fact that parameters are estimated from small sample sizes, it is risky to make a statement regarding the ‘true’ state of the transition parameters. Therefore, parameter estimates should always include confidence intervals and some measure of uncertainty in the PPMs. Furthermore, parameters for each population could be better estimated using a Bayesian approach by including information from all populations (Tremblay and McCarthy, 2014). Demographic stochasticity (important for small population sizes) and environmental stochasticity (flash floods, hurricanes, tree falls) can result in a loss of many individuals and result in variation in parameter estimates and ultimately variation in the population growth rate (Morris and Doak, 2002). The likely effect of demographic stochasticity was explicitly modelled by Schödelbauerová et al. (2010), who showed that in all populations of this orchid the density of future populations could be drastically reduced, and most simulation outcomes included the probability of populations going extinct.
A comparison of transient dynamics and population growth outcomes after 13 years (from Schödelbauerová et al., 2010) shows that population 4, which went extinct, had the lowest transient dynamic range among all surveyed populations. This population went extinct after a flash flood (December 1995) that resulted in the removal of all but one large individual from the population due to the force of the water. The remaining plants perished in the following months. In populations that are continuously perturbed by the environment, as are these orchids, evaluation of the growth pattern by asymptotic processes may not reflect the possible change in population size even if the species is long-lived, because attaining a stable-stage distribution may take much longer than the frequency of disturbance. Disturbance is probably a common process in epiphytic and lithophytic species, so realization of a stable-stage distribution may be an uncommon phenomenon. Thus, limiting the application of PPMs to the evaluation of asymptotic population dynamics may not appropriately reflect extinction risks.
The observed number of individuals was consistently similar to the predicted number of individuals (Fig. 3). In the absence of immigration and migrations or disturbance, population dynamics should be similar. Noting densities outside the predicted bounds should be considered a rare event. For example, strongly biased dynamics would be expected for founder populations comprising only seedlings or relocated populations of a few reproductive adults.
The absolute density of the population may not follow the attenuation predicted by the transient dynamics, as in Fig. 3, if lambda >1; thus, population density could be relatively lower at t + 1 compared with an equivalent population at stable-stage distribution, but absolutely higher than at t. Biologically, the PPM may not necessarily represent adequately the recruitment process in Lepanthes. Fruits can have many seeds (∼6000), and the recruitment process is variable and unpredictable and unlikely to follow a simple pattern, whereas stochastic demographic recruitment will influence density by overestimating attenuation. In this PPM model recruitment distribution may be unrealistic, because the recruitment is likely to have a Poisson distribution with low mean probability but an extremely flat and wide probability distribution. However, we are still ignorant of recruitment processes across time and space in natural populations of orchids and how best to model these in a realistic fashion (Ackerman et al., 1996; Murren and Ellison, 1998; Raventos et al., 20 11; Jacquemyn et al., 2012).
Transfer function analyses were consistent among all six populations. The slope represents the likely rates of change of lambda as a function of change in the parameter estimates. Consequently, if small changes (increase or decrease) in survivorship/stasis are applied to juveniles, non-reproductive adults and reproductive adults staying in the same stage would have the largest effect on growth rates compared with equivalent changes in parameters with smaller slopes. Environmental fluctuations will result in changes in the parameters of stasis and transitions, so that population growth rates will probably change as well. Although a shift in environmental conditions will cause a proportional change in the parameters, it does not necessarily result in proportional change in growth rate, nor is the proportional response likely to be linear. Thus, predicting the influence of growth rate as a function of linear responses (sensitivities) is likely to be misleading. Consequently, we suggest that transfer functions be used to evaluate non-linear responses to changes in parameters instead of the traditional measures of sensitivities. In addition, Hodgson and Townley (2004) showed a similar pattern in that the curvature of response seems to be more pronounced in the stasis parameters than in the growth parameters in the desert tortoise.
Inertia, the persistence of the increase or decrease in population density as a consequence of perturbations and not being at a stable-stage equilibrium is extremely variable and varies among populations. This extreme variation in the pattern of inertia among populations would make it difficult to predict the response to an equal perturbation in an unevaluated sister population. Nevertheless, some patterns are evident: populations dominated by the later life stages result in positive inertia, whereas populations dominated by early life stages are likely to have persistent small population densities. Consequently, founder events are likely to be either disastrous or result in a long period at low densities.
For populations that are frequently perturbed because of flash floods as a consequence of tropical storms and hurricanes, the concept of stable-stage/age distribution and the parameters that are dependent on this index may be unrealistic. Transient indices may be more amenable to the needs of wildlife managers and conservation biologists because they describe the range of possible outcomes from diverse starting points in population size and structure. Pielke et al. (2003) pointed out that many locations in the tropics had at least a 10 % chance of experiencing a hurricane annually. In these areas the occurrence of hurricanes shows high inter-annual variability and large multidecade changes.
An additional difficulty is in the use of elasticities and sensitivities, which occurs when categorizing continuous variables to make the model fit a discrete model of stage structure, as in the PPM approach. Biases may emerge if different categorical cutoff points that are selected influence the relative importance of growth stage and survival rates (Enright et al., 1995). When the matrices are constructed from continuous variables, integral projection models (IPMs) should be the preferred way to evaluate PPMs (Easterling et al., 2000; Ellner and Rees, 2006; Metcalf et al., 2013), but these are limited as they do not yet incorporate transient analysis.
As a general pattern, transient dynamics are strongly dependent on initial conditions (i.e. a population composed only of seedlings will have trajectories different from those of a population composed only of adults; Stott et al., 2010a). Fortunately, the first years of a reintroduction programme or new founding populations can be evaluated to determine the range of likely density changes in subsequent years. Such analyses could provide better choices for determining the initial population structure that could maximize long-term persistence.
In situ species conservation requires knowledge not only of ecological variables that influence life history stages, but also of how they enhance the likelihood of species/population persistence. A drawback of asymptotic growth rates and related indices is that they do not readily include estimates of the transient dynamics and variation in growth rates and stage distributions prior to attaining the asymptotic growth rate and the expected stable-stage distribution. Thus, an integrative approach could elucidate alternative scenarios of population dynamics for the conservation management of species. We recommend unifying the study of PPMs using the traditional asymptotic analysis with an evaluation of the diversity of dynamics that may arise when populations are not at a stable-stage distribution.
SUPPLEMENTARY DATA
Supplementary Data are available online at www.aob.oxfordjournals.org and consist of the following. Data S1: population matrices of the six populations of L. rubripetala and calculation of transition parameters. Data S2: sensitivities of non-linear relationship between changes (permutations) in transition parameters and the resulting change in the asymptotic growth rate, lambda, for the six populations of L. rubripetala. Data S3: transfer functions plots of inertia for the whole life cycle for the six populations of L. rubripetala. Data S4: sensitivity matrices as 3-D surface plots for the six populations of L. rubripetala.
ACKNOWLEDGEMENTS
Partial funding for this work was provided by the Center for Applied Tropical Ecology and Conservation and a grant from the US National Science Foundation to the University of Puerto Rico (E. Cuevas, project director; HRD 0206200).
LITERATURE CITED
- Ackerman JD. 2012. Orchidaceae. In: Acevedo-Rodríguez P, Strong MT. eds. Catalogue of seed plants of the West Indies. Smithsonian Contributions to Botany 98: 622–667. [Google Scholar]
- Ackerman JD, Sabat A, Zimmerman JK. 1996. Seedling establishment in an epiphytic orchid: an experimental study of seed limitation. Oecologia 106: 192–198. [DOI] [PubMed] [Google Scholar]
- Blanco M, Barbosa G. 2005. Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Annals of Botany 95: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierzychudek P. 1999. Looking backwards: assessing the projections of a transition matrix model. Journal of Applied Ecology 9: 1278–1287. [Google Scholar]
- Caswell H. 2001. Matrix population models: construction, analysis and interpretation, 2nd edn Sunderland: Sinauer. [Google Scholar]
- Caswell H. 2007. Sensitivity analysis of transient population dynamics. Ecology Letters. 10: 1–15. [DOI] [PubMed] [Google Scholar]
- Cohen JE. 1979. The cumulative distance from an observed to a stable age structure. SIAM Journal of Applied Mathematics 36: 169–175. [DOI] [PubMed] [Google Scholar]
- Coulson T, Mace GM, Hudson E, Possingham H. 2001. The use and abuse of population viability analysis. Trends in Ecology and Evolution 16: 219–221. [DOI] [PubMed] [Google Scholar]
- Crain B, Tremblay RL. 2014. Do richness and rarity hotspots really matter for orchid conservation in light of anticipated habitat lost? Diversity and Distributions 20: 652–662. [Google Scholar]
- Crone EE, Ellis MM, Morris WF, et al. 2013. Ability of matrix models to explain the past and predict the future of plant populations. Conservation Biology 27: 968–978. [DOI] [PubMed] [Google Scholar]
- Easterling MR, Ellner SP, Dixon PM. 2000. Size-specific sensitivity: applying a new structured population model. Ecology 81: 694–708. [Google Scholar]
- Ellner SP, Rees M. 2006. Integral projection models for species with complex demography. American Naturalist 167: 410–418. [DOI] [PubMed] [Google Scholar]
- Enright NJ, Franco M, Silvertown J. 1995. Comparing plant life histories using elasticity analysis: the importance of life span and the number of life-cycle stages. Oecologia 104: 79–84. [DOI] [PubMed] [Google Scholar]
- Ezard THG, Bullock JM, Dalgleish HJ, et al. 2010. Matrix models for a changeable world: the importance of transient dynamics in population management. Journal of Applied Ecology 47: 515–523. [Google Scholar]
- Fieberg J, Ellner SP. 2001. Stochastic matrix models for conservation and management: a comparative review of methods. Ecology Letters 4: 244–266. [Google Scholar]
- Govaerts R, Kratochvil K, Gerlach G, et al. 2014. World checklist of Lepanthes . Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp. [Google Scholar]
- Hodgson DJ, Townley S. 2004. Linking management changes to population dynamic responses: the transfer function of a projection matrix perturbation. Journal of Applied Ecology 41: 1155–1161. [Google Scholar]
- Hodgson DJ, Townley S, McCarthy D. 2006. Robustness: predicting the effects of life history perturbations on stage-structured population dynamics. Theoretical Population Biology 70: 214–224. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Lievens B, Wiegand T. 2012. Spatial variation in below-ground seed germination and divergent mycorrhizal associations correlate with spatial segregation of three co-occurring orchids species. Journal of Ecology 100: 1328–1337. [Google Scholar]
- Jäkäläniemi A, Postila H, Tuomi J. 2013. Accuracy of short-term demographic data in projecting long-term fate of populations. Conservation Biology 27: 552–559. [DOI] [PubMed] [Google Scholar]
- Kephart SR, Paladino C. 1997. Demographic change and microhabitat variability in a grassland endemic, Silene douglasii var. oraria (Carophyllaceae). American Journal of Botany 84: 179–189. [PubMed] [Google Scholar]
- Keyfitz N. 1968. Introduction to mathematics of populations . Reading: Addison-Wesley. [Google Scholar]
- Koons DN, Holmes RR, Grand JB. 2007. Population inertia and its sensitivity to changes in vital rates and population structure. Ecology 88: 2857–2867. [DOI] [PubMed] [Google Scholar]
- Lande R, Engen S, Saether BE. 2003. Stochastic population dynamics in ecology and conservation . Oxford: Oxford University Press. [Google Scholar]
- Lindborg R, Ehrlén J. 2002. Evaluating the extinction risk of a perennial herb: demographic data versus historical records. Conservation Biology 16: 683–690. [Google Scholar]
- Luer CA. 2014. Lepanthes. In: Ackerman JD, collaborators. Orchid flora of the Greater Antilles. Memoirs of the New York Botanical Garden, Vol. 109. New York: NYBG Press: 232–300. [Google Scholar]
- Van Mantgem PJ, Stephenson NL. 2005. The accuracy of matrix population model projections for coniferous trees in the Sierra Nevada, California. Journal of Ecology 93: 737–747. [Google Scholar]
- McCarthy D, Townley S, Hodgson DJ. 2008. On second order sensitivity for stage-based population projection matrix models. Theoretical Population Biology 74: 68–73. [DOI] [PubMed] [Google Scholar]
- Metcalf CJE, McMahon SN, Gómez RS, Jongejans E. 2013. Application IPMpack: an R package for integral projection models. Methods in Ecology and Evolution 4: 195–200. [Google Scholar]
- Morris WF, Doak DF. 2002. Quantitative conservation biology. The theories and practice of population viability analysis. Sunderland: Sinauer. [Google Scholar]
- Murren CJ, Ellison AM. 1998. Seed dispersal characteristics of Brassavola nodosa (Orchidaceae). American Journal of Botany 85: 675–680. [PubMed] [Google Scholar]
- Pielke RA, Rubiera J, Landsea C, Fernández ML, Klein R. 2003. Hurricane vulnerability in Latin America and the Caribbean: normalized damage and loss potentials. Natural Hazards Review 4: 101–114. [Google Scholar]
- R Project for Statistical Computing. 2013. The R project for statistical computing. www.R-project.org. [PubMed] [Google Scholar]
- Raventos J, Mújica E, Wiegand T, Bronet A. 2011. Analyzing the spatial structure of Broughtonia cubensis (Orchidaceae) populations in the dry forests of Guanahacabibes, Cuba. Biotropica 43: 173–182. [Google Scholar]
- Rebarber R, Townley S. 1995. Robustness and continuity of the spectrum of the spectrum for uncertain distributed-parameter systems. Automatica 31: 1533–1546. [Google Scholar]
- Rivera-Gómez N, Tremblay RL, Meléndez-Ackerman E. 2006. Density dependent effects in a lithophytic and epiphytic orchid. Folia GeoBotanica 41: 107–120. [Google Scholar]
- Schödelbauerová I, Tremblay RL, Kindlmann P. 2010. Prediction vs. reality: can a PVA model predict population persistence 13 years later? Biodiversity and Conservation 19: 637–650. [Google Scholar]
- Stott I, Franco M, Carslake D, Townley S, Hodgson DJ. 2010a. Boom or bust? A comparative analysis of transient population dynamics in plants. Journal of Ecology 98: 302–311. [Google Scholar]
- Stott I, Townley S, Carslake D, Hodgson DJ. 2010b. On reducibility and ergodicity of population projection matrix models. Methods in Ecology and Evolution 1: 242–252. [Google Scholar]
- Stott I, Townley S, Hodgson DJ. 2011. A framework for studying transient dynamics of population projection matrix models. Ecological Letters 14: 959–970. [DOI] [PubMed] [Google Scholar]
- Stott I, Hodgson DJ, Townley S. 2012a. Popdemo: an R package for population demography using projection matrix analysis. Methods in Ecology and Evolution 3: 797–802. [Google Scholar]
- Stott I, Hodgson DJ, Townley S. 2012b. Beyond sensitivity: nonlinear perturbation analysis of transient dynamics. Methods in Ecology and Evolution 3: 673–684. [Google Scholar]
- Tremblay RL. 1997. Distribution and dispersion patterns of individuals in nine species of Lepanthes (Orchidaceae). Biotropica 29: 38–45. [Google Scholar]
- Tremblay RL, Ackerman JD. 2001. Gene flow and effective population size in Lepanthes (Orchidaceae): a case for genetic drift. Biological Journal of the Linnean Society 72: 47–62. [Google Scholar]
- Tremblay RL, Hutchings MJ. 2003. Population dynamics in orchid conservation: a review of analytical methods based on the rare species Lepanthes eltoroensis. In: Dixon K, Kell S, Barrett R, Cribb P, eds. Orchid conservation. Kota Kinabalu: Natural History Publications (Borneo), 183–204. [Google Scholar]
- Tremblay RL, McCarthy MA. 2014. Bayesian estimates of transition probabilities in seven small lithophytic orchid populations: maximizing data availability from many small samples. PLoS One doi: 10.1371/journal.pone.0102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RL, Pomales-Hernández G, Méndez-Cintrón ML. 2006. Flower phenology and sexual maturation: partial protandrous behavior in three species of orchids. Caribbean Journal of Science 42: 75–80. [Google Scholar]
- Tuljapurkar S. 1997. Stochastic matrix models . In: Tuljapurkar S, Caswell H, eds. Structured-population models in marine, terrestrial and freshwater systems. New York: Chapman & Hall, 59–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.