Abstract
Background and Aims The evolution of interspecific reproductive barriers is crucial to understanding species evolution. This study examines the contribution of transitions between self-compatibility (SC) and self-incompatibility (SI) and genetic divergence in the evolution of reproductive barriers in Dendrobium, one of the largest orchid genera. Specifically, it investigates the evolution of pre- and postzygotic isolation and the effects of transitions between compatibility states on interspecific reproductive isolation within the genus.
Methods The role of SC and SI changes in reproductive compatibility among species was examined using fruit set and seed viability data available in the literature from 86 species and ∼2500 hand pollinations. The evolution of SC and SI in Dendrobium species was investigated within a phylogenetic framework using internal transcribed spacer sequences available in GenBank.
Key Results Based on data from crossing experiments, estimations of genetic distance and the results of a literature survey, it was found that changes in SC and SI significantly influenced the compatibility between species in interspecific crosses. The number of fruits produced was significantly higher in crosses in which self-incompatible species acted as pollen donor for self-compatible species, following the SI × SC rule. Maximum likelihood and Bayesian tests did not reject transitions from SI to SC and from SC to SI across the Dendrobium phylogeny. In addition, postzygotic isolation (embryo mortality) was found to evolve gradually with genetic divergence, in agreement with previous results observed for other plant species, including orchids.
Conclusions Transitions between SC and SI and the gradual accumulation of genetic incompatibilities affecting postzygotic isolation are important mechanisms preventing gene flow among Dendrobium species, and may constitute important evolutionary processes contributing to the high levels of species diversity in this tropical orchid group.
Keywords: Character reconstruction, Dendrobium, evolution, Orchidaceae, post-mating barriers, reproductive isolation, self-compatibility, self-incompatibility, speciation
INTRODUCTION
Speciation can be viewed as a dynamic process in which previously interbreeding groups of individuals acquire reproductive isolation, thus impeding or limiting the homogenizing effect of gene flow among diverging lineages (Mayr, 1942; Dobzhansky, 1970; Grant, 1981; Morjan and Rieseberg, 2004; Lexer and Widmer, 2008). The understanding of the events that cause speciation is a primary goal of evolutionary biology and would in principle require disentangling which reproductive barrier acted in the early phase of species divergence, i.e. which was directly responsible for the reduction of gene flow among formerly interbreeding populations (Coyne and Orr, 1989). This goal could be directly achieved only through the investigation of isolating mechanisms among divergent intraspecific populations (Etterson et al., 2007; Scopece et al., 2010; Pinheiro et al., 2013) because well-established species may have gathered changes that can potentially mask the relative contributions of various isolating mechanisms during incipient species formation (Kay, 2006). However, divergent populations will rarely become new species and, as a consequence, studies on speciation are complicated by a surplus of speculations either on the future (in the case of diverging populations) or on the past (in the case of well-established species) (Butlin et al., 2008).
To overcome this problem, Coyne and Orr (1989) proposed an approach based on the measurement of the strength of reproductive isolating mechanisms across large groups of taxa that vary in divergence time and on its comparison with genetic distances considered as a proxy of time divergence. Such an approach, however, requires a huge experimental effort and, consequently, so far only few studies have employed it in plants (Moyle et al., 2004; Archibald et al., 2005; Scopece et al., 2007, 2008; Jewell et al., 2012). Such studies often rely on literature data, thus suffering from an intrinsic lack of specific experimental design or of appropriate sample sizes. Furthermore, these studies often encompass few isolating mechanisms, whilst reproductive isolation can be achieved through a combination of many different pre- or postzygotic barriers (Ramsey et al., 2003; Lowry et al., 2008). Despite these shortcomings, such studies have contributed significantly to more general hypotheses on speciation in the plant kingdom. Indeed, only by employing a similar approach was it possible to shed light on the evolutionary rates of different types of isolating mechanisms and to show that prezygotic mechanisms generally evolve rapidly, whereas the evolution of postzygotic mechanisms appears to be more gradual (Coyne and Orr, 1989; Moyle et al., 2004). Particularly in Mediterranean orchids, it has been shown that, excluding the groups in which mechanisms such as allopolyploidy have a dominant role (e.g. Hedrén, 1996, 2001; Trávníček et al., 2010), postzygotic isolation evolves gradually (Scopece et al., 2007) and that late-acting mechanisms (such as hybrid sterility and inviability) evolve faster than early-acting ones (fruit formation, embryo mortality) (Scopece et al., 2008). These evolutionary patterns suggest an overall difference in the genetic background of different reproductive barriers, with prezygotic ones likely due to few genes and postzygotic ones with a multigenic basis (e.g. Coyne and Orr, 1998; Edmands, 2002).
Reproductive isolation can also evolve as a by-product of evolutionary processes related to different ecological requirements (Bomblies et al., 2007). For instance, shifts between different compatibility states can have a direct impact on reproductive isolation among species and populations (reviewed by Brandvain and Haig, 2005). In this context, studies performed with self-incompatible (SI) and self-compatible (SC) species of plants have shown a strong asymmetrical pattern of reproductive incompatibility, in which SI species pollen grows in SC species styles, but SC species pollen is inhibited in SI species styles, the so called SI × SC rule (Lewis and Crowe, 1958; de Nettancourt, 1977; Murfett et al., 1996; Hiscock et al., 1998; Brandvain and Haig, 2005). Barriers in SI species styles may have a multigenic origin associated with the S locus (Murfett et al., 1996; Hiscock et al., 1998), which plays a role in many SI systems (Charlesworth and Charlesworth, 1979; Hiscock and Dickinson, 1993; Igic et al., 2006). In SI species, the potential for sexual conflicts is higher because pollen tubes are normally unrelated to the sporophytic tissues through which they grow. In contrast, SC species often lack such incompatibilities since pollen tubes approach genetic identity with the sporophytic tissues through which they grow by constant cycles of selfing (Brandvain and Haig, 2005). Since changes in compatibility states intensely affect the levels of reproductive isolation between populations, transitions between SC and SI may accelerate the accumulation of reproductive barriers among distinct lineages, contributing to speciation (Hiscock et al. 1998; Brandvain and Haig, 2005).
The evolution of reproductive isolation has been extensively studied in Mediterranean deceptive orchids (Cozzolino and Scopece, 2008; Xu et al., 2011; Zitari et al., 2012; Scopece et al., 2013). Abundant sympatric populations composed of species showing different ecological attributes (food versus sexual deceptive systems) have provided an interesting biological model to investigate the evolutionary mechanisms underlying speciation in groups with different levels of pollinator specialization (Cozzolino and Scopece, 2008). Unfortunately, no such model is available for tropical regions, where most of the orchid species occur. For this reason, using literature data, we investigated the evolution of pre- and postzygotic reproductive isolation and the potential effect of the SI × SC rule on speciation patterns in Dendrobium, one of the largest orchid genera in the tropical region. Crossing compatibility among Dendrobium species was studied by Wilfret (1968) and Johansen (1990), who performed a large amount of crossing experiments in order to understand taxonomic affinities and self-incompatibility systems within the genus. By combining large and diverse datasets (crossing experiments, DNA sequences and breeding system information), this study specifically asks about patterns of evolution of pre- and postzygotic isolation and explores the effect of changes between compatibility states on the evolution of reproductive isolation within the genus Dendrobium.
MATERIALS AND METHODS
Plant group and dataset origin
Dendrobium is one of the largest orchid genera in the tropical region, with ∼1200 species (Adams, 2011). Most species are distributed in tropical Asia, Australasia and Australia, and many endemic species are reported along its distribution range (Cribb and Govaerts, 2005; Wood, 2006; Zhu et al., 2009). The genus shows extensive morphological variation, which challenges classification systems and taxonomic decisions regarding species and infrageneric boundaries (Adams, 2011). Several authors have studied the genus from a phylogenetic perspective (Clements, 2003; Burke et al., 2008, 2013; Yuan et al., 2009; Li et al., 2012; Xiang et al., 2013), providing a sequence dataset for the nuclear internal transcribed spacer (ITS) region and a phylogenetic background for comparative studies. The ornamental value and medicinal properties of many species place the genus as a target for a large array of studies regarding the development of improved cultivars (reviewed by Kamemoto et al., 1999), gene expression (Xu et al., 2006), phytochemistry and physiology (reviewed by Ng et al., 2012). Furthermore, the genus Dendrobium encompasses SC and SI species (Kerr, 1909; Johansen, 1990; Kamemoto et al., 1999).
In order to examine interspecific compatibility and the breeding system of Dendrobium species, extensive crossing experiments were performed by Wilfret (1968) and Johansen (1990), who used a total of 86 species (Table 1) and carried out ∼2500 hand pollinations. Self, intraspecific and interspecific pollinations were performed by both authors, and the number of flowers used in pollination experiments, the number of fruits produced (fruit set) and the proportion of viable seeds (seed viability) were measured. Interspecific crosses from both datasets are summarized in Supplementary Data Table S1.
Table 1.
Total number of Dendrobium species analysed in this study. Crossing experiments results were available for species indicated by W (dataset from Wilfret, 1968) and J (dataset from Johansen, 1990)
| Species | Compatibility system | Dataset of origin1 | Types of cross performed2 | GenBank number3 |
|---|---|---|---|---|
| D. aciculare† | SI | J | SE, intra, inter | |
| D. acinaciforme† § | SI | J | SE, inter | HQ114253# |
| D. aggregatum* † | SI | W | SE, inter | |
| D. albosanguineum | SI | J | SE | EU477498# |
| D. aloifolium† § | SI | J | SE, inter | AY239951# |
| D. alterum† | SI | J | SE, inter | |
| D. aphyllum† § | SI | J | SE, intra, inter | KF143430# |
| D. arachnites† | SI | W | SE, inter | |
| D. bellatulum§ | – | W | inter | KF143431# |
| D. bicameratum | SI | – | – | HM054581# |
| D. bigibbum† | SC | W | inter | |
| D. bilobulatum† | SC/SI | J | SE, intra, inter | |
| D. blumei | SI | J | SE | |
| D. brevimentum† | SI | J | SE, inter | |
| D. brymerianum | SC | J | SE | KF143432# |
| D. bullenianum† | SI | W | SE, inter | |
| D. capillipes | SI | – | – | KF143433# |
| D. cariniferum† § | SI | W, J | SE, inter | JN388583# |
| D. chrysotoxum† § | SI | W, J | SE, intra, inter | KF143444# |
| D. compactum | SI | J | SE | KF143445# |
| D. concinnum | – | J | inter | |
| D. crepidatum | SC | KF143446# | ||
| D. crumenatum† § | SI | W, J | SE, inter | HM590370# |
| D. crystallinum† § | SC/SI | J | SE, inter | HQ114243# |
| D. dalbertsii† | SC | W | SE, inter | |
| D. delacourii† | SC | W | SE, inter | |
| D. densiflorum | SC | – | – | KF143451# |
| D. denudans | SI | J | SE | KF143452# |
| D. devonianum† § | SI | J | SE, intra, inter | KF143453# |
| D. disticum† | SI | J | SE, inter | |
| D. dixanthum† § | SC | W | SE, inter | KF143454# |
| D. draconis† § | SC/SI | W, J | SE, inter | HM054628# |
| D. ellipsophyllum† § | SI | J | SE, inter | KF143455# |
| D. erostelle | SI | J | SE | |
| D. exile | SC | J | SE | KF143457# |
| D. falconeri | SI | J | intra, inter | KF143458# |
| D. farmeri† § | SI | W, J | SE, intra, inter | HM054631# |
| D. fimbriatum† § | SI | W | SE, inter | JN388588# |
| D. formosum† § | SI | W, J | SE, inter | AY239967# |
| D. friedericksianum§ | – | W | inter | EU477505# |
| D. gibsonii† § | SI | J | SE, inter | HQ114256# |
| D. gouldii† | SC | W | SE, inter | |
| D. grantii | – | W | inter | |
| D. gratiosissimum† § | SI | J | SE, inter | KF143464# |
| D. griffithianum† | SI | J | SE, inter | |
| D. hancockii | SC | – | – | KF143467# |
| D. hendersonii† | SI | J | SE, intra | |
| D. hercoglossum | SC | – | – | KF143472# |
| D. heterocarpum† § | SC | W, J | SE, inter | KF143473# |
| D. hildebrandii† | SC | W | SE, inter | |
| D. indivisum† § | SI | J | SE, inter | AY239972# |
| D. infundibulum | SC/SI | J | SE | KF143477# |
| D. jenkinsii | SC | – | – | KF143479# |
| D. keithii† | SI | J | SE, intra, inter | |
| D. kingianum | SI | – | – | EU430386# |
| D. lamellatum | SI | J | SE | |
| D. leonis† § | SI | W, J | SE, intra, inter | AY239978# |
| D. leptocladum | SI | – | – | HM590373# |
| D. linawianum | SI | – | – | HM590371# |
| D. lindleyi† § | SI | J | SE, intra, inter | JN388568# |
| D. linguella† | SI | W, J | SE, intra, inter | |
| D. lituiflorum† § | SI | W | SE, inter | AB593602# |
| D. loddigesii | SC | – | – | HM590374# |
| D. maccarthiae | – | W | inter | |
| D. macrophyllum† § | SC | W | SE, inter | AY239979# |
| D. macrostachyum† § | SC | W | SE, inter | HM054696# |
| D. mannii | SI | J | SE | |
| D. monile** † § | SI | W | SE, inter | KF143489# |
| D. moschatum† § | SI | W, J | SE, intra, inter | KF143492# |
| D. mucronatum | SI | J | SE | |
| D. nathanielis† | SI | J | SE, inter | |
| D. nobile† § | SC | J | inter | HQ114219# |
| D. officinale | SI | – | – | HQ114245# |
| D. pachyglossum† | SI | J | SE, inter | |
| D. pachyphyllum† | SI | J | SE, inter | |
| D. panduriferum† | SI | J | SE, inter | |
| D. parcum | SI | J | SE | |
| D. parishii† § | SI | W, J | SE, intra, inter | HM590378# |
| D. pendulum | SC | J | SE | KF143498# |
| D. phalaenopsis† | SC/SI | W, J | SE, inter | |
| D. planibulbe† | SI | J | SE, inter | |
| D. podagraria | SI | J | SE | |
| D. primulinum† § | SI | W, J | SE, inter | HQ114242# |
| D. pulchellum† § | SI | J | SE, intra, inter | KF143503# |
| D. salaccense† § | SC | J | SE, inter | KF143506# |
| D. secundum† § | SI | J | SE, intra, inter | AY239993# |
| D. senile† § | SI | W, J | SE, inter | EU477509# |
| D. setifolium† | SI | J | SE, inter | |
| D. sinense | SI | – | – | KF143511# |
| D. speciosum | SI | – | – | AY239998# |
| D. spectabile† | SC | W | SE, inter | |
| D. stratiotes† | SC | W | SE, inter | |
| D. strebloceras† | SC | W | SE, inter | |
| D. stuposum | SC | – | – | KF143516# |
| D. subulatum | SI | J | SE | |
| D. sulcatum | SC | – | – | KF143517# |
| D. tetrodon | SC | J | SE | |
| D. thyrsiflorum† § | SI | J | SE, intra, inter | KF143519# |
| D. tortile† | SC | W, J | SE, inter | EU477511# |
| D. undulatum† | SC | W | SE, inter | |
| D. unicum§ | – | J | intra, inter | KF143523 |
| D. virgineum† | SI | J | SE, inter | |
| D. wardianum | SC | – | – | JN388600# |
1Dendrobium species not used in crossing experiments (–) by Wilfret (1968) or Johansen (1990) were not included in the correlation between reproductive isolation and genetic distances.
2Reciprocal interspecific crosses are indicated in bold.
3ITS sequences used to calculate genetic distances among species and to build the phylogenetic tree where transitions between SC and SI were mapped (Fig. 1).
†Species used to investigate the transitions between SC and SI and the evolution of reproductive isolation.
#Species used to infer the phylogenetic hypothesis.
§Species used to test the association between reproductive isolation and genetic distances.
*Synonym of D. lindleyi.
**Synonym of D. moniliforme.
SC, self-compatible species; SI, self-incompatible species; SC/SI species, both; –, unknown; SE, self-pollination; intra, intraspecific pollination; inter, interspecific pollination.
Asymmetry in reproductive compatibility and transitions between SC and SI
To examine the role of SC and SI changes in reproductive compatibility among species, differences in fruit set and seed viability were tested using results of interspecific crosses from the Wilfret (1968) and Johansen (1990) datasets. Specifically, interspecific crosses were tested for differences in fruit set and seed viability when SC species and SI species acted as pollen donors and pollen receptors, and between interspecific crosses involving SC species × SC species and SI species × SI species. Information regarding the SC and SI status of most species was collected from the studies of Wilfret (1968) and Johansen (1990). Further information was retrieved from other reproductive biology studies conducted on Dendrobium species (Kerr, 1909; Slater and Calder, 1988; Bartareau, 1995; Wood, 2006; Li et al., 2009a, b; Vasudevan and Staden, 2010; Huda and Wilcock, 2012; Pang et al., 2012). Quantitative differences in fruit set and seed viability were tested using the Mann–Whitney U-test with the statistical package SPSS 13.0 (SPSS Inc., Chicago, IL).
Transitions between SC and SI in Dendrobium species were examined in a phylogenetic framework. The phylogenetic inference was based on ITS sequences found in GenBank for the 61 taxa for which the compatibility state was known (Table 1). All sequences used in this study were previously analysed and published in peer-reviewed journals, thus increasing our confidence in the sequence-specific names. Of the 61 taxa for which sequence data were obtained, there were representatives from all the main phylogenetic clades found by Xiang et al. (2013). Each ITS sequence accession was aligned using the ClustalW option in BioEdit v.7.1.9 (Hall, 1999). The resulting automated alignment was manually edited in BioEdit v.7.1.9 and then exported as a Phylip 4 file for maximum parsimony (MP) and maximum likelihood (ML) analyses, following Pessoa et al. (2012).
Homogeneity of the dataset was tested with the incongruence-length difference test (Farris et al., 1995) as implemented in PAUP 4.0 (Swofford, 2002). The MP analysis used the criterion of Fitch (1971), excluding uninformative characters, and with ACCTRAN optimization. Robustness of MP tree topologies was tested by bootstrap analysis (Felsenstein, 1985). Ten thousand addition sequence replicates were performed by stepwise addition and holding ten trees per replicate, and tree bisection and reconnection branch swapping on the best trees. The MP analysis was performed with PAUP. The ML analysis was conducted using RAxML v. 7.0.4 (Stamatakis, 2006) and RAxML-GUI v. 1.1 (Silvestro and Michalak, 2011). The GTR + Γ substitution model, which allows rate variation among sites, was determined using jModeltest v. 0.1.1 (Posada, 2008) under the Akaike information criterion (AIC). To find the optimal likelihood tree, we ran 100 independent tree searches on the ITS matrix. Support for individual branches was evaluated using non-parametric bootstrapping (Felsenstein, 1985) with 1000 thorough bootstrap replicates. Trees were rooted with D. macrophyllum and D. salaccense, which are members of the most basal clade in Dendrobium according to the phylogeny published by Xiang et al. (2013).
Ancestral character state reconstruction analysis was used to map the transitions between SC and SI in Dendrobium species. The SC? and SI? states were coded as binary data (0, 1) and optimized onto the best scoring ML tree under an MP criterion using the package Mesquite v. 2.75 (Maddison and Maddison, 2011). A polymorphic state (0and1) was used for species where the character is variable (i.e. SC? and SI? in the same species). The number and directionality of transitions between SC? and SI? states were quantified using the Summarize State Changes Over Trees function in Mesquite v. 2.75.
Following Escobar et al. (2010), ML and Markov chain Monte Carlo (MCMC) procedures were applied to test the significance of transitions between SC and SI in Dendrobium species, using the 1000 bootstrap trees from which we obtained support values of nodes. Analyses of bootstrap trees allowed assessment of the uncertainty of transitions in nodes not fully supported. The ML and MCMC analyses on bootstrap trees were performed with the BayesMultistates program (Pagel et al., 2004) implemented in BayesTraits 1.0 v. 2.0 (http://www.evolution.rdg.ac.uk/BayesTraits.html). The MCMC analyses were run for 5 050 000 generations, a uniform prior distribution and a burn-in of 50 000 generations.
Three different models were compared using likelihood ratio tests, following Escobar et al. (2010): (1) the unrestricted model, in which the probability of the two types of transitions, from SI? to SC? (qIC) and the converse (qCI), were calculated; (2) a restricted model in which only SI? to SC? transitions were permitted (i.e. qCI = 0); and (3) an alternative, restricted model in which only SC? to SI? transitions were permitted (i.e. qIC = 0). The likelihood ratio test was used to compare the two likelihoods derived from unrestricted and each of the restricted models (unrestricted versus restricted qCI = 0, and unrestricted versus restricted qIC = 0). In the restricted models, ancestral states were fixed to SI or SC.
Reproductive isolation indices
Results from interspecific crosses were used to calculate two postmating reproductive isolation indices: one prezygotic (pollen–stigma incompatibility, RIprezygotic) and one postzygotic (embryo mortality, RIpostzygotic). From Wilfret (1968) and Johansen (1990) we collected data on the proportion of fruits produced, which was used as the measure of pollen–stigma incompatibility, and on the proportion of viable seeds, used as the measure of embryo mortality. There was considerable overlap between the approaches employed in performing hand pollinations and estimating fruit and seed production. From the Wilfret (1968) and Johansen (1990) datasets we only selected bidirectional interspecific crosses and reproductive isolation was calculated as the average of crossing results where each species was used as both pollen donor and receiver. The traditional method of calculating reproductive isolation (Coyne and Orr, 2004) involves a comparison between interspecific crosses and intraspecific performances used as reference. However, the studies from which data were collected were focused on the investigation of SIy mechanisms and therefore mainly included intraspecific self-pollinations rather than intraspecific cross-pollinations, which would be the ideal reference for the calculation of reproductive isolation. To circumvent this issue, we assumed as reference the maximum hypothetic value of performance (i.e. 100 %). The pollen–stigma incompatibility isolation index was thus defined as RIprezygotic = 1 – (mean percentage of fruits in bidirectional interspecific crosses/total potential compatibility, i.e.100 %), where RI is the reproductive isolation index. The embryo mortality isolation index was defined as RIpostzygotic = 1 – (mean percentage seed viability in bidirectional interspecific crosses/total potential compatibility, i.e. 100 %). A few species pairs, present in both original datasets (Wilfret, 1968; Johansen, 1990), were averaged as a single data point to avoid duplication. All measures of isolation varied between 0 (no isolation) and 1 (complete isolation).
Correlations between reproductive isolation indices and genetic distances
Genetic distances were calculated from ribosomal ITS sequences available in GenBank, using 37 species (Table 1). Sequences were hand-aligned in BioEdit v. 7.1.9 and genetic distances were then calculated in PAUP 4.0 under the best-fit model of molecular evolution chosen with jModeltest v. 0.1.1. Because species of Dendrobium may be circumscribed very differently and receive different names in different studies, and to avoid the potential risk of estimating reproductive isolation and genetic distances not between but within the same Dendrobium species, potential synonyms were checked in the Plant List website (http://www.theplantlist.org/). Names were corrected for D. aggregatum (synonym of D. lindleyi) and D. monile (synonym of D. moniliforme), as both species were used to estimate correlations between reproductive isolation stages and genetic distances.
In order to test for significant associations between genetic distances and reproductive isolation, the non-parametric Kendall’s τ rank correlation was used for full and strictly independent datasets. Since species were often involved in multiple crosses, most points in our dataset were not statistically independent. To circumvent this problem, we selected strictly phylogenetically independent species pairs to maximize the number of pairs that could be obtained from the available dataset (Felsenstein, 1985). All analyses were conducted using SPSS 13.0 (SPSS, Chicago).
Results
Breeding system transitions
The transition between SC and SI and the evolution of reproductive isolation were analysed for 63 species, for which 759 interspecific crosses were performed (Table 1). Self-incompatibility was the predominant breeding system in 43 out of 63 species used in the crossing experiments (Table 1). Self-compatibility was observed in 16 species, and both states were present in only four species, specifically in individuals from different localities (Johansen, 1990; Table 1, Appendix 1).
The types of compatibility system significantly influenced the results of interspecific crosses (Table 2). The number of fruits produced was significantly higher in crosses where SI species acted as pollen donor for SC species, in comparison with the converse situation (Mann–Whitney U = 9129·0; P < 0·001). In this case, no significant differences were observed for seed viability (Mann–Whitney U = 131·5; P =0·184). Furthermore, significant higher fruit set (Mann–Whitney U = 17 739·0; P < 0·001) and seed viability values (Mann-Whitney U = 820·5; P < 0·01) were observed in crosses between SC species, in comparison with crosses between SI species (Table 2).
Table 2.
Summary of interspecific crosses results obtained by Wilfret (1968) and Johansen (1990), used to test for differences in interspecific compatibilities when SC and SI species acted as pollen donors and pollen receptors, and vice versa (groups 1 and 2), and between interspecific crosses involving SI × SI species (group 3) and SC × SC species (group 4). The total number of crosses performed (number of crosses that produced fruits), number of pollinated flowers, mean number of fruits and mean seed viability are indicated
| Crossing group1 | Pollen receptor | Pollen donor | Number of species | Crosses | Pollinated flowers | Mean fruits produced (s.d.) | Mean seed viability2 (%) |
|---|---|---|---|---|---|---|---|
| 1 | SI species | SC species | 39 | 137 (9) | 171 | 0·07 (0·28) | 10·68 |
| 2 | SC species | SI species | 38 | 164 (41) | 219 | 0·34 (0·67) | 10·72 |
| 3 | SI species | SI species | 16 | 304 (41) | 919 | 0·41 (1·31) | 22·78 |
| 4 | SC species | SC species | 46 | 154 (63) | 218 | 0·48 (0·63) | 42·86 |
1Significant differences were detected between groups 1 and 2 in fruit production (P = 0·000) and between groups 3 and 4 in fruit production (P = 0·000) and seed viability (P < 0·01).
2Only crosses that produced fruits.
The ITS matrix comprised 61 species for which the compatibility system information was available (Table 1). The aligned matrix was 669 bp long after exclusion of regions of ambiguous alignment. A total of 386 (58 %) substitutions were parsimony-informative (225 were constant and 58 were parsimony-uninformative) with 198 of these (52 %) informative within Dendrobium. The topologies of MP and ML trees recovered in this study were very similar and, for this reason, only the result of the ML analysis is shown (Fig. 1). Ancestral state reconstruction suggested high levels of homoplasy, with frequent shifts between SC and SI across the tree (Fig. 1). Indeed, the number of character steps (23) and the consistency and retention indices (0·17 and 0·05, respectively) confirmed the homoplasious nature of the transitions between SC and SI in Dendrobium species. Ancestral states were difficult to interpret at several nodes (grey branches in Fig. 1), particularly when a node was poorly supported by bootstrap values (Fig. 1).
Fig. 1.
Evolutionary transitions between SC and SI in Dendrobium, using the best maximum likelihood tree based on ITS sequences. White and black branches indicate self-compatible and self-incompatible lineages, respectively. Grey branches indicate undetermined compatibility systems. *Species in which both SC and SI are present. White, grey and black diamonds indicate bootstrap support values above 50 % obtained by maximum parsimony, maximum likelihood and both methods, respectively.
The number of transitions from SI to SC (minimum 12, maximum 21, average 15·48) was higher than the converse situation (minimum 2, maximum 9, average 6·1). However, ML and MCMC analyses suggested that the log-likelihood of the unrestricted model, allowing transitions from SI to SC (qIC) and from SC to SI (qCI), was significantly better than both restricted models tested (only transitions from SI to SC, qCI = 0; only transitions from SC to SI, qIC = 0). Thus, the best model describing changes in compatibility systems across the Dendrobium phylogeny was that in which both transitions between SI and SC were allowed (Table 3).
Table 3.
Models testing transitions between SI and SC across the Dendrobium phylogeny, using ML and Bayesian MCMC methods, including the mean log-likelihoods for ML analyses and harmonic mean of log-likelihoods for MCMC analyses (lnL), the deviance of harmonic means between unrestricted and restricted models (Dev) with the respective probability (P), the probability of SC-to-SI transitions (q01), the probability of SI-to-SC transitions (q10), the probability of self-compatibility at the root of the tree [Root P(0)] and the probability of self-incompatibility at the root of the tree [Root P(1)]
| Model | lnL | Dev (P) | q01 | q10 | Root P(0) | Root P(1) |
|---|---|---|---|---|---|---|
| ML unrestricted | –42·09 | 1000·00 | 596·33 | 0·50 | 0·50 | |
| ML 01 = 0 | –63·96 | 43·74 (0·00) | 0·00 | 6·65 | 0·00 | 1·00 |
| ML 10 = 0 | –54·13 | 24·08 (0·00) | 12·18 | 0·00 | 1·00 | 0·00 |
| MCMC unrestricted | –38·27 | 88·55 | 53·32 | 0·49 | 0·50 | |
| MCMC 01 = 0 | –60·85 | 45·16 (0·00) | 0·00 | 7·73 | 0·00 | 1·00 |
| MCMC 10 = 0 | –47·29 | 18·04 (0·00) | 14·92 | 0·00 | 1·00 | 0·00 |
Impact of genetic distances on reproductive isolation patterns
Reproductive isolation indices were calculated for 310 interspecific bidirectional crosses involving 66 species (Appendix 1). The maximum reproductive isolation index (RI = 1) was observed for most crosses considering either fruit set (no fruit formation in 272 out of 310 crosses) or seed viability (0 % of seed viability in 22 out of 39 crosses that produced fruits).
ITS sequences were available for 36 species involved in the interspecific crosses. Consequently, the association between genetic distances and reproductive isolation was estimated for 105 species pairs. Using the full dataset, RIprezygotic was not correlated with genetic distance (Kendall’s τ = 0·085; P = 0·272) but RIpostzygotic was positively correlated with genetic distance (Kendall’s τ = 0·326; P = 0·026) (Fig. 2). Using strictly independent species pairs, RIprezygotic was not correlated with genetic distance (Kendall’s τ = 0·645; P = 0·079), but also RIpostzygotic was not correlated with genetic distance (Kendall’s τ = 0·086; P = 0·822).
Fig. 2.
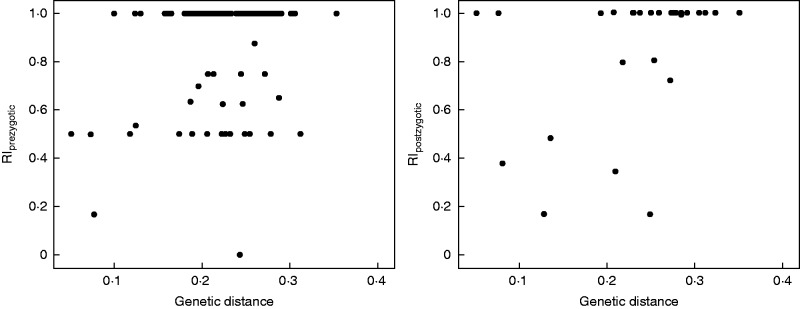
Strength of pre- and postzygotic reproductive isolation versus genetic distance in Dendrobium.
DISCUSSION
The investigation of interspecific reproductive barriers is crucial to the understanding of species origin and evolution (Coyne and Orr, 2004). Because reproductive barriers can act at different stages of the reproductive cycle, from mate recognition to offspring fertility, integrated approaches are critical to the understanding of the contribution of different barriers to the formation and maintenance of species boundaries. In this study, datasets from crossing experiments and DNA sequences were used to investigate the strength of two postmating (pre- and postzygotic) reproductive isolation mechanisms, the relative rates of reproductive barrier evolution and the role of transitions between SC and SI in speciation. The genetic distance among species was significantly correlated with interspecific seed production, suggesting a gradual accumulation of reproductive barriers, probably with a multigenic origin, as already observed in other food-deceptive orchids (Scopece et al., 2007). Transitions from SI to SC were the most common, suggesting the existence of an evolutionary advantage in self-pollination. This is in agreement with the traditional dead-end hypothesis (Stebbins, 1974; Busch and Delph, 2012), by which SCble lineages often evolve from SIble ancestors in flowering plants, and SCble lineages with a prevalence of self-pollination are thought to show limited potential for adaptation due to the accumulation of deleterious mutations. However, reverse changes from SC to SI could not be rejected by our analysis. In fact, the dead-end hypothesis only applies when SCble lineages display high levels of self-pollination, which is not a common mating system observed in orchids (Squirrell et al., 2001; Tremblay et al., 2005). Strong and significant asymmetrical patterns of reproductive isolation between SI and SC species follow the SI × SC rule, suggesting that changes in the compatibility system may be linked with the evolution of reproductive isolation during speciation events. Our data not only inform us about the strength and direction of reproductive barriers among Dendrobium species, but also contain important information about the potential ecological scenarios and genetic mechanisms underlining the diversification of this species-rich plant genus.
Relationship between reproductive isolation and time divergence
The few studies investigating relationships between reproductive isolation and genetic divergence in plants showed contrasting patterns ranging from consistently positive associations to weak or no correlation, depending on the plant lineage investigated (Moyle et al., 2004; Scopece et al., 2007; Jewell et al., 2012). Our data show a significant correlation between postzygotic but not prezygotic isolation and genetic distance, confirming previous results in the same plant family but in a phylogenetically distant lineage (Scopece et al., 2007). This finding suggests that, also in the genus Dendrobium, postzygotic isolation between species increases with increasing time since species divergence, in agreement with traditional theories of speciation in which reproductive isolation is considered a by-product of gradual genetic divergence (Presgraves, 2002). Furthermore, a gradual evolution of postzygotic reproductive isolation suggests that it can be shaped by the amount of genome divergence between parental species (Coyne and Orr, 1989; Moyle et al., 2004; Scopece et al., 2007), achieved through the accumulation of differences in many genes of small individual effects (Coyne and Orr, 1998; Edmands, 2002). Differently, prezygotic isolation has been generally found to evolve more rapidly and erratically than postzygotic isolation (Coyne and Orr, 1998; Scopece et al., 2007). In agreement, we found no correlation between prezygotic isolation and genetic distance between Dendrobium species. The stage of prezygotic isolation investigated here is likely linked to biochemical processes involving the recognition between pollen and stigma and to the consequent triggering of female gametophyte development (Zhang and O’Neill, 1993). This process, typical of the orchid family, is likely to be affected by ecological factors or by the action of natural selection in sympatry, which may explain why closely related species pairs often display strong prezygotic isolation in spite of presumed recent divergence (e.g. D. cariniferum × D. unicum and D. gibsonii × D. moschatum; Fig. 1).
To avoid phylogenetic constraints, we also selected a set of phylogenetically independent species pairs. The correlations, however, showed a lack of significance in both pre- and postzygotic isolation. This pattern is likely due to the reduced sample size (only six independent species pairs) and to the small interval of genetic distance in which the independent pairs fall. In addition, it should also be noted that several closely related species (e.g. D. acinaciforme × D. leonis) show elevated postzygotic isolation, which suggests the existence of mechanisms other than the mere accumulation of incompatibilities in shaping postzygotic barriers.
Directionality of SC and SI transitions and its effect on reproductive isolation in Dendrobium
The inferred phylogeny based on available ITS sequences indicates that SC may be the ancestral breeding system in Dendrobium. Accordingly, most species of section Grastidium, which is the ancestral group within Dendrobium (Xiang et al., 2013) are reported to be SC species (Catling, 1990; Wood, 2006), such as D. macrophyllum, which was used to root our tree (Fig. 1). Likelihood and Bayesian tests could not reject transitions from SC to SI (Table 3), and the breakdown of SI occurred independently in different clades (Fig. 1). In addition, most of the transitions occurred from SI to SC, in agreement with the intuition that SI is lost more frequently than gained (Takebayashi and Morrell, 2001; Igic et al., 2006; Escobar et al., 2010). According to Davis et al. (2013), inferences of character evolution using trees with fewer than 300 terminal taxa have low power and should be interpreted with caution. Thus, we will not discuss the results of SC and SI transitions in relation to the directionality of changes, but only the transitions between SC and SI observed in different clades over the tree, a result that probably would not change if additional species were analysed in future studies. For instance, SC and SI species occur within the same clades in the phylogeny published by Xiang et al. (2013), which includes 192 accessions of 109 Dendrobium species.
A breakdown in SI has been shown to evolve in populations in which sexual reproduction is limited by mate availability, such as small or colonizing populations (Levin, 2012; Barrett, 2013), as found in epiphyte species (Gentry and Dodson, 1987; Vasquez et al., 2003; Tremblay et al., 2005, 2006). Fragmented distribution and pollen limitation are common ecological attributes of orchid populations (Gentry and Dodson, 1987; Larson and Barrett, 2000; Tremblay and Ackerman, 2001; Tremblay et al., 2005; Phillips et al., 2011). Population studies have shown that the colonization of new paths by epiphytic orchids is often due to a small number of individuals (Tremblay et al., 2006), with subsequent population expansion resulting from in situ reproduction (Trapnell and Hamrick 2005; Trapnell et al., 2013).
The founding of new populations by long-distance seed dispersal combined with further divergent selection in these novel selective environments has been proposed as an important mechanism of orchid speciation (reviewed by Phillips et al., 2012). The observation that most Dendrobium species are epiphytes suggests that founding events from long-distance seed dispersal followed by the breakdown of SI may be an important mechanism of speciation in the genus. In this scenario, genetic incompatibilities and reproductive isolation barriers would accumulate faster by exposing the newly founded populations to different selective environments (Phillips et al., 2012) and changes to SC (Squirrell et al., 2002). Furthermore, outbreeding depression was observed in some crosses between individuals from distant populations (e.g. D. aciculare, D. devonianum, D. lindleyi; Johansen, 1990), suggesting that genetic incompatibilities that accumulate among divergent populations may reduce compatibility, triggering early stages of speciation, as observed in other plant groups (Scopece et al., 2010), including orchids (Pinheiro et al., 2013). Further population-level studies are thus necessary to shed light on these speciation stages, using sister species with different compatibility systems or, even better, different populations in species with SC? and SI? systems, e.g. in D. aphyllum, D. crystallinum and D. draconis.
Our results show that transitions between SC and SI in Dendrobium are followed by asymmetrical patterns of reproductive isolation (Table 2). This finding strongly agrees with the unilateral incompatibility explained by the SI × SC rule, which states that pollen from SI individuals fertilizes ovules of SC plants but the reciprocal cross fails (Hiscock et al., 1998; Brandvain and Haig, 2005). Additional support for this hypothesis came from crosses between SC species, which showed significantly higher fruit and seed set compared with crosses between SI species. According to the SI × SC rule, pollen tubes from SI species are normally unrelated to the sporophytic tissues through which they grow, a situation not observed for SC species (Lewis and Crowe, 1958; Hiscock and Dickinson 1993; Murfett et al., 1996). Thus, SI styles contain barriers to fertilization that are not retained in SC styles (Hiscock and Dickinson, 1993), decreasing overall fruit and seed set, as observed in crosses between SI species, in agreement with the results observed here (Table 2). According to the observed patterns, a higher frequency of interspecific hybridization would be expected between SC species, and genomic studies using new sequencing technologies (Twyford and Ennos, 2012) may shed light on this question.
Unilateral incompatibility was also observed in crosses between SI species (e.g. D. bilobulatum × D. leonis, D. cariniferum × D. virgineum, D. devonianum × D. crystallinum) and between SC species (e.g. D. phalaenopsis × D. delacourii, D. undulatum × D. macrophyllum, D. strebloceras × D. stratiotes) (Appendix 1), suggesting that variable SI systems and different degrees of outcrossing are present in SI and SC species. Usually orchid species show very low levels of selfing (Tremblay et al., 2005), even considering only SC species (Squirrell et al., 2001; Soliva and Widmer, 2003; Jacquemyn et al., 2006; Pinheiro et al., 2011). By showing high levels of outcrossing, asymmetrical incompatibilities between SC species are also expected to occur because the occurrence of pollen–pistil conflicts is proportional to the number of partners involved in pollination (Brandvain and Haig, 2005). Thus, reproductive isolation is expected between outcrossers and inbreeders, or in species in which SC was recently acquired (Lewis and Crowe, 1958; Brandvain and Haig, 2005). Future studies using non-model organisms should examine divergence time estimates between species pairs in which transitions in compatibility systems are present and absent. If divergence time estimates are shorter for lineages experiencing transitions between SC and SI, the reproductive isolation expected by the SI × SC rule may play a role in speciation events (Brandvain and Haig, 2005).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxford journals.org and consist of Table S1: full details of the interspecific reciprocal crosses performed by Wilfret (1968) and Johansen (1990).
ACKNOWLEDGEMENTS
We thank Ming-Xun Ren for help during Dendrobium bibliographic searching, and Clarisse Palma da Silva and Alexandre Antonelli for comments on this manuscript. This work was supported by grants to F.P. from FAPESP (2009/15052-0, BEPE 2013/02453-2), a PNPD/CAPES fellowship and a University of Naples Short mobility grant to F.P. This work was also supported by a CNPq/CNR International Cooperation grant (CNPq 4905102013-2).
LITERATURE CITED
- Ackerman JD. 2012. Orchids gone wild: discovering naturalized orchids in Hawaii. Orchids 81: 88–93. [Google Scholar]
- Adams PB. 2011. Systematics of Dendrobiinae (Orchidaceae), with special reference to Australian taxa. Botanical Journal of the Linnean Society 166: 105–126. [Google Scholar]
- Archibald JK, Mort ME, Crawford DJ, Kelly JK. 2005. Life history affects the evolution of reproductive isolation among species of Coreopsis (Asteraceae). Evolution 59: 2362–2369. [PubMed] [Google Scholar]
- Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible? Proceedings of the Royal Society B: Biological Sciences 280: 20130913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartareau T. 1995. Pollination limitation, costs of capsule production and the capsule-to-flower ratio in Dendrobium monophyllum F. Muell. (Orchidaceae). Austral Ecology 20: 257–265. [Google Scholar]
- Bomblies K, Lempe J, Epple P, et al. 2007. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biology 5: 1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandvain Y, Haig D. 2005. Divergent mating systems and parental conflict as a barrier to hybridization in flowering plants. American Naturalist 166: 330–338. [DOI] [PubMed] [Google Scholar]
- Burke JM, Bayly MJ, Adams PB, Ladiges PY. 2008. Molecular phylogenetic analysis of Dendrobium (Orchidaceae), with emphasis on the Australian section Dendrocoryne, and implications for generic classification. Australian Systematic Botany 21: 1–14. [Google Scholar]
- Burke JM, Ladiges PY, Batty EL, Adams PB, Bayly MJ. 2013. Divergent lineages in two species of Dendrobium orchids (D. speciosum and D. tetragonum) correspond to major geographical breaks in eastern Australia. Journal of Biogeography 40: 2071–2081. [Google Scholar]
- Busch JW, Delph LF. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin RK, Galindo J, Grahame JW. 2008. Sympatric, parapatric or allopatric: the most important way to classify speciation? Philosophical Transactions of the Royal Society B: Biological Sciences 363: 2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling RM. 1990. Auto-pollination in Orchidaceae. In: Arditti J. ed. Orchid biology, reviews and perspectives , 5 Portland: Timber Press, 121–158. [Google Scholar]
- Clements MA. 2003. Molecular phylogenetic systematics in the Dendrobiinae (Orchidaceae), with emphasis on Dendrobium section Pedilonum. Telopea 10: 247–298. [Google Scholar]
- Charlesworth D, Charlesworth B. 1979. The evolution and breakdown of s-allele systems. Evolution 43: 41–55. [Google Scholar]
- Coyne JA, Orr HA. 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. 1998. The evolutionary genetics of speciation. Philosophical Transactions of the Royal Society of London B: Biological Sciences 353: 287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. 2004. Speciation. Sunderland: Sinauer Associates. [Google Scholar]
- Cozzolino S, Scopece G. 2008. Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 3037–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb P, Govaerts R. 2005. Just how many orchids are there? In: Raynal-Roques A, Roguenant A, Prat D. eds. Proceedings of the 18th World Orchid Conference. Dijon: Naturalia Publications, 161–172. [Google Scholar]
- Davis MP, Midford PE, Maddison W. 2013. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evolutionary Biology 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. 1970. Genetics of the evolutionary process. New York: Columbia University Press. [Google Scholar]
- Edmands S. 2002. Does parental divergence predict reproductive compatibility? Trends in Ecology & Evolution 17: 520–527. [Google Scholar]
- Escobar JS, Cenci A, Bolognini J, et al. 2010. An integrative test of the dead-end hypothesis of selfing evolution in Triticeae (Poaceae). Evolution 64: 2855–2872. [DOI] [PubMed] [Google Scholar]
- Etterson JR, Keller SR, Galloway LF. 2007. Epistatic and cytonuclear interactions govern outbreeding depression in the autotetraploid Campanulastrum americanum. Evolution 61: 2671–2683. [DOI] [PubMed] [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. 1995. Testing significance of incongruence. Cladistics 10: 315–319. [Google Scholar]
- Felsenstein J. 1985. Confidence-limits on phylogenies with a molecular clock. Systematic Zoology 34: 152–161. [Google Scholar]
- Fitch WM. 1971. Toward defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology 20: 406–416. [Google Scholar]
- Gentry AH, Dodson CH. 1987. Diversity and biogeography of neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74: 205–233. [Google Scholar]
- Grant V. 1981. Plant speciation, 2nd edn New York: Columbia University Press. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hedrén M. 1996. Genetic differentiation, polyploidization and hybridization in northern European Dactylorhiza (Orchidaceae): evidence from allozyme markers. Plant Systematics and Evolution 201: 31–55. [Google Scholar]
- Hedrén M. 2001. Systematics of the Dactylorhiza euxina/incarnata/maculata polyploid complex (Orchidaceae) in Turkey: evidence from allozyme data. Plant Systematics and Evolution 229: 23–44. [Google Scholar]
- Hiscock SJ, Dickinson HG. 1993. Unilateral incompatibility within the Brassicaceae – further evidence for an involvement of the self-incompatibility (S)-locus. Theoretical and Applied Genetics 86: 744–753. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Doughty J, Dickinson HG. 1998. Unilateral incompatibility and the S (self-incompatibility) locus. In: Owens SJ, Rudall PJ. eds. Reproductive biology in systematics, conservation and economic botany. Kew, UK: Royal Botanic Gardens, 31–46. [Google Scholar]
- Huda MK, Wilcock CC. 2012. Rapid flower senescence following male function and breeding systems of some tropical orchids. Plant Biology 14: 278–284. [DOI] [PubMed] [Google Scholar]
- Igic B, Bohs L, Kohn JR. 2006. Ancient polymorphism reveals unidirectional breeding system shifts. Proceedings of the National Academy of Sciences of the USA 103: 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Vandepitte K, Honnay O, Roldán-Ruiz I. 2006. Fine-scale genetic structure of life history stages in the food-deceptive orchid Orchis purpurea. Molecular Ecology 15: 2801–2808. [DOI] [PubMed] [Google Scholar]
- Jewell C, Papineau AD, Freyre R, Moyle LC. 2012. Patterns of reproductive isolation in Nolana (Chilean bellflower). Evolution 66: 2628–2636. [DOI] [PubMed] [Google Scholar]
- Johansen B. 1990. Incompatibility in Dendrobium (Orchidaceae). Botanical Journal of the Linnean Society 103: 165–196. [Google Scholar]
- Kamemoto H, D’Amore T, Kuehnle A. 1999. Breeding Dendrobium orchids in Hawaii. Honolulu: University of Hawaii Press. [Google Scholar]
- Kay KM. 2006. Reproductive isolation between two closely related hummingbird-pollinated neotropical gingers. Evolution 60: 538–552. [PubMed] [Google Scholar]
- Kerr AFG. 1909. Notes on the pollination of certain species of Dendrobium . Scientific Proceedings of the Royal Dublin Society 12: 47–53. [Google Scholar]
- Larson BMH, Barrett SCH. 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69: 503–520. [Google Scholar]
- Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D, Crowe LK. 1958. Unilateral incompatibility in flowering plants. Heredity 12: 233–256. [Google Scholar]
- Lexer C, Widmer A. 2008. The genic view of plant speciation: recent progress and emerging questions. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 3023–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li ZJ, Mao P, Yan XF, Chun Z, Ma XR. 2012. Phylogenetic analysis and identification of Dendrobium species based on ribosomal DNA internal transcribed spacer (ITS) sequence. Acta Horticulturae Sinica 39: 1539–1550. [Google Scholar]
- Li ZJ, Wang Y, Yu Y, Zhang Y, Miao K. 2009a. Studies on floral and pollination biology in endangered Dendrobium orchid. Guangdong Agricultural Sciences 6: 43–45. [Google Scholar]
- Li ZJ, Kang XP, Wang Y, Yu Y, Miao K. 2009b. Studies on the pollination biology and microscopic structure of Dendrobium hercoglossum Rchb. f. (Orchidaceae). Acta Botanica Boreali-Occidentalia Sinica 29: 1804–1810. [Google Scholar]
- Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH. 2008. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 3009–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2011. Mesquite 2.75. A modular system for evolutionary analysis. http://mesquiteproject.org. [Google Scholar]
- Mayr E. 1942. Systematics and the origin of species. New York: Columbia University Press. [Google Scholar]
- Morjan CL, Rieseberg LH. 2004. How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Molecular Ecology 13: 1341–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle LC, Olson MS, Tiffin P. 2004. Patterns of reproductive isolation in three angiosperm genera. Evolution 58: 1195–1208. [DOI] [PubMed] [Google Scholar]
- Murfett J, Strabala TJ, Zurek DM, Mou BQ, Beecher B, McClure BA. 1996. S RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8: 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nettancourt D. 1977. Incompatibility in angiosperms. Heidelberg: Springer. [Google Scholar]
- Ng TB, Liu J, Wong JH, et al. 2012. Review of research on Dendrobium, a prized folk medicine. Applied Microbiology and Biotechnology 93: 1–9. [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A, Barker D. 2004. Bayesian estimation of ancestral character states on phylogenies. Systematic Biology 53: 673–684. [DOI] [PubMed] [Google Scholar]
- Pang S, Pan K, Wang Y, Li W, Zhang L, Chen Q. 2012. Floral morphology and reproductive biology of Dendrobium jiajiangense (Orchidaceae) in Mt. Fotang, southwestern China. Flora 207: 469–474. [Google Scholar]
- Pessoa EM, Alves M, Alves-Araújo A, Palma-Silva C, Pinheiro F. 2012. Integrating different tools to disentangle species complexes: a case study in Epidendrum (Orchidaceae). Taxon 61: 721–734. [Google Scholar]
- Phillips RD, Brown AP, Dixon KW, Hopper SD. 2011. Orchid biogeography and factors associated with rarity in a biodiversity hotspot, the Southwest Australian Floristic Region. Journal of Biogeography 38: 487–501. [Google Scholar]
- Phillips RD, Dixon KW, Peakall R. 2012. Low population genetic differentiation in the Orchidaceae: implications for the diversification of the family. Molecular Ecology 21: 5208–5220. [DOI] [PubMed] [Google Scholar]
- Pinheiro F, Barros F, Palma-Silva C, Fay MF, Lexer C, Cozzolino S. 2011. Phylogeography and genetic differentiation along the distributional range of the orchid Epidendrum fulgens: a Neotropical coastal species not restricted to glacial refugia. Journal of Biogeography 38: 1923–1935. [Google Scholar]
- Pinheiro F, Cozzolino S, Barros F, et al. 2013. Phylogeographic structure and outbreeding depression reveal early stages of reproductive isolation in the Neotropical orchid Epidendrum denticulatum. Evolution 67: 2024-–039. [DOI] [PubMed] [Google Scholar]
- Posada D. 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56: 1168–1183. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Bradshaw HD, Schemske DW. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) . Evolution 57: 1520–1534. [DOI] [PubMed] [Google Scholar]
- Scopece G, Musacchio A, Widmer A, Cozzolino S. 2007. Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution 61: 2623–2642. [DOI] [PubMed] [Google Scholar]
- Scopece G, Widmer A, Cozzolino S. 2008. Evolution of postzygotic reproductive isolation in a guild of deceptive orchids. American Naturalist 171: 315–326. [DOI] [PubMed] [Google Scholar]
- Scopece G, Lexer C, Widmer A, Cozzolino S. 2010. Polymorphism of postmating reproductive isolation within plant species. Taxon 59: 1367–1374. [Google Scholar]
- Scopece G, Croce A, Lexer C, Cozzolino S. 2013. Components of reproductive isolation between Orchis mascula and Orchis pauciflora. Evolution 67: 2083–2093. [DOI] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. 2011. RaxmlGUI: a graphical front-end for RAxML. Organism Diversity and Evolution 12: 335–337. [Google Scholar]
- Slater AT, Calder DM. 1988. The pollination biology of Dendrobium speciosum Smith: a case of false advertising? Australian Journal of Botany 36: 145–158. [Google Scholar]
- Soliva M, Widmer A. 2003. Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution 57: 2252–2261. [DOI] [PubMed] [Google Scholar]
- Squirrell J, Hollingsworth PM, Bateman RM, et al. 2001. Partitioning and diversity of nuclear and organelle markers in native and introduced populations of Epipactis helleborine (Orchidaceae). American Journal of Botany 88: 1409–1418. [PubMed] [Google Scholar]
- Squirrell J, Hollingsworth PM, Bateman RM, Tebbitt MC, Hollingsworth ML. 2002. Taxonomic complexity and breeding system transitions: conservation genetics of the Epipactis leptochila complex (Orchidaceae). Molecular Ecology 11: 1957–1964. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stebbins G. 1974. Flowering plants: evolution above the species level. Cambridge: Belknap Press. [Google Scholar]
- Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland: Sinauer. [Google Scholar]
- Takebayashi N, Morrell PL. 2001. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany 88: 1143–1150. [PubMed] [Google Scholar]
- Trapnell DW, Hamrick JL. 2005. Mating patterns and gene flow in the Neotropical epiphytic orchid, Laelia rubescens. Molecular Ecology 14: 75–84. [DOI] [PubMed] [Google Scholar]
- Trapnell DW, Hamrick JL, Ishibashi C, Kartzinel TR. 2013. Genetic inference of epiphytic orchid colonization; it may only take one. Molecular Ecology 22: 3680–3692. [DOI] [PubMed] [Google Scholar]
- Trávníček P, Kubátová B, Čurn V, et al. 2010. Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): evidence from flow cytometry. Annals of Botany 110: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD. 2001. Gene flow and effective population size in Lepanthes (Orchidaceae): a case for genetic drift. Biological Journal of the Linnean Society 72: 47–62. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2005. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society 84: 1–54. [Google Scholar]
- Tremblay RL, Meléndez-Ackerman E, Kapan D. 2006. Do epiphytic orchids behave as metapopulations? Evidence from colonization, extinction rates and asynchronous population dynamics. Biological Conservation 129: 70–81. [Google Scholar]
- Twyford AD, Ennos RA. 2012. Next-generation hybridization and introgression. Heredity 108: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez R, Ibisch PL, Gerkmann B. 2003. Diversity of Bolivian Orchidaceae – a challenge for taxonomic, floristic and conservation research. Organism Diversity and Evolution 3: 93–102. [Google Scholar]
- Vasudevan R, Staden JV. 2010. Fruit harvesting time and corresponding morphological changes of seed integuments influence in vitro seed germination of Dendrobium nobile Lindl. Plant Growth Regulation 60: 237–246. [Google Scholar]
- Wilfret GJ. 1968. Genome and karyotype relationships in the genus Dendrobium (Orchidaceae) . PhD Thesis, University of Hawaii. [Google Scholar]
- Wood HP. 2006. The Dendrobiums. Liechtenstein: A.R.G. Gantner. [Google Scholar]
- Xiang XG, Schuiteman A, Li DZ, et al. 2013. Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Molecular Phylogenetics and Evolution 69: 950–960. [DOI] [PubMed] [Google Scholar]
- Xu SQ, Schlüter PM, Scopece G, et al. 2011. Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution 65: 2606–2620. [DOI] [PubMed] [Google Scholar]
- Xu Y, Teo LL, Zhou J, Kumar PP, Yu H. 2006. Floral organ identity genes in the orchid Dendrobium crumenatum . Plant Journal 46: 54–68. [DOI] [PubMed] [Google Scholar]
- Yuan ZQ, Zhang JY, Liu T. 2009. Phylogenetic relationship of China Dendrobium species based on the sequence of the internal transcribed spacer of ribosomal DNA. Biologia Plantarum 53: 155–158. [Google Scholar]
- Zhang XS, O’Neill SD. 1993. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell 5: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GH, Ji ZH, Wood JJ, Wood HP. 2009. Dendrobium. In: Wu CY, Raven PH, Hong DY. eds. Flora of China. Beijing: Scientific Press, 367–397. [Google Scholar]
- Zitari A, Scopece G, Helal AN, Widmer A, Cozzolino S. 2012. Is floral divergence sufficient to maintain species boundaries upon secondary contact in Mediterranean food-deceptive orchids? Heredity 108: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



