Abstract
Background Manganese (Mn) is an essential micronutrient that is phytotoxic under certain edaphic and climatic conditions. Multiple edaphic factors regulate Mn redox status and therefore its phytoavailability, and multiple environmental factors including light intensity and temperature interact with Mn phytotoxicity. The complexity of these interactions coupled with substantial genetic variation in Mn tolerance have hampered the recognition of Mn toxcity as an important stress in many natural and agricultural systems.
Scope Conflicting theories have been advanced regarding the mechanism of Mn phytotoxicity and tolerance. One line of evidence suggests that Mn toxicity ocurs in the leaf apoplast, while another suggests that toxicity occurs by disruption of photosynthetic electron flow in chloroplasts. These conflicting results may at least in part be attributed to the light regimes employed, with studies conducted under light intensities approximating natural sunlight showing evidence of photo-oxidative stress as a mechanism of toxicity. Excessive Mn competes with the transport and metabolism of other cationic metals, causing a range of induced nutrient deficiencies. Compartmentation, exclusion and detoxification mechanisms may all be involved in tolerance to excess Mn. The strong effects of light, temperature, precipitation and other climate variables on Mn phytoavailability and phytotoxicity suggest that global climate change is likely to exacerbate Mn toxicity in the future, which has largely escaped scientific attention.
Conclusions Given that Mn is terrestrially ubiquitous, it is imperative that the heightened risk of Mn toxicity to both managed and natural plant ecosystems be factored into evaluation of the potential impacts of global climate change on vegetation. Large inter- and intraspecific genetic variation in tolerance to Mn toxicity suggests that increased Mn toxicity in natural ecosystems may drive changes in community composition, but that in agroecosystems crops may be developed with greater Mn tolerance. These topics deserve greater research attention.
Keywords: Manganese phytotoxicity, photo-oxidative stress, climate change, Mn phytoavailability
INTRODUCTION
Current dogma that manganese (Mn) phytotoxicity is primarily associated with acid soils warrants re-evaluation on the basis of unresolved theories in the literature. Given that Mn is ubiquitous in soil and is also a common source of plant abiotic stress, contrasting hypotheses concerning Mn phytotoxicity demand careful re-evaluation, particularly in the context of global climate change. Their interpretation is pivotal to decisions affecting the welfare of natural and managed ecosystems, and also toward genetic manipulation of crop Mn tolerance. While the problem of plant overexposure to Mn has in the past drawn considerable attention, it is now a neglected issue, with research at an all-time low. The warning signs for increased prevalence and severity of Mn phytotoxicity in future climates need to be seriously considered. The Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, 2014) states that atmospheric temperatures across the globe are rising, as are frequencies of flooding events and duration of droughts. Variability in plant physiological processes associated with elevated Mn phytoavailability remain poorly understood in regard to external drivers such as climate.
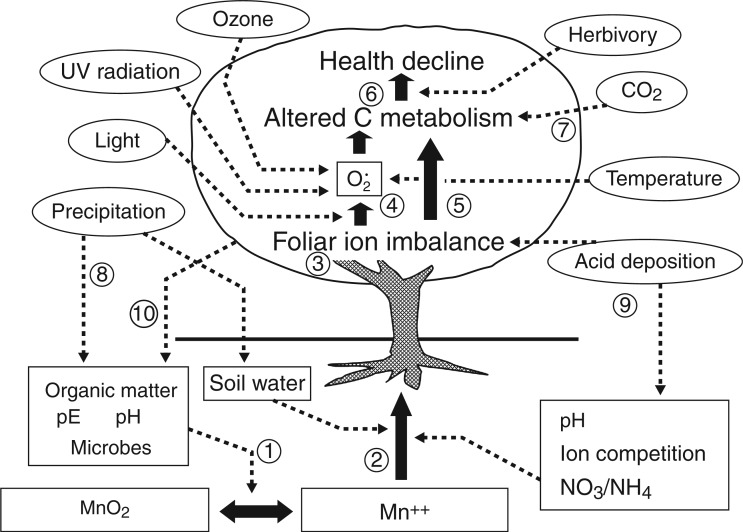
The overexposure of plants to Mn is intrinsic to certain geographic regions of the world. Shifting climate patterns (IPCC, 2014) are set to widen constraints imposed by enhanced Mn phytoavailabilty, thereby endangering productivity of important ecosystems. Although the toxic extent of this is difficult to predict, cases can be cited of previously healthy forest ecosystems now exhibiting Mn and other mineral stresses from changed environmental conditions (Bernier and Brazeau, 1988; Horsley et al., 2000; Drohan et al., 2002; Tomlinson, 2003; StClair and Lynch, 2005a, b; StClair et al., 2005). Altered precipitation, rainwater acidity, flooding events, increased length and intensity of sunlight hours, increased atmospheric ozone levels and elevated ambient temperatures are anthropogenic environmental effects capable of altering plant–Mn interactions, even within areas previously unknown for Mn phytotoxicity. The dynamic nature of soil–Mn bioavailability is one of several contributors to these complex plant–soil systems (Fig. 1) susceptible to climatic factors (Siman et al., 1974; Rufty et al., 1979; Sparrow and Uren, 1987; González et al., 1998; Driscoll et al., 2001; StClair and Lynch, 2004; Fernando et al., 2009a).
Fig. 1.
Conceptual model of Mn biogeochemistry and phytotoxicity. Mn reduction (1) is regulated by soil redox potential, pH, microbial activity, and soil organic matter content and composition. The uptake of reduced Mn by plant roots (2) is influenced by interactions with several plant nutrients, rhizosphere pH (hence the NO3:NH4 ratio), and soil water status, which regulates mass transport of soluble ions to the root. Mn phytotoxicity occurs in leaves through interference with the metabolism of essential metals (3) and via the production of reactive oxygen species (4), and by interfering with nutrient acquisition by roots; atmospheric factors affecting stomatal transpiration (light, temperature, ozone, humidity and CO2) will also affect leaf nutrient balance, as the uptake of some nutrients is driven by mass flow of water in soil (3). Free radicals generated by foliar nutrient imbalances and increased by high light intensity are exacerbated in the presence of other pro-oxidants (high ozone, temperature extremes and UV radiation), resulting in oxidative damage of the photosynthetic apparatus, which reduces carbon fixation (4). Foliar nutrient deficiencies will also directly affect carbon metabolism as nutrients are a principal component of the cellular machinery (e.g. enzymes, metabolites) that drives carbon metabolism (5). Carbon resource depletion over time limits maintenance, growth and defence, which, in combination with pathogen attack and herbivory, leads to health decline (6). Increases in atmospheric CO2 can alter carbon gain with various effects on plant processes including tissue dilution of nutrients (7). Precipitation influences soil pE, soil microbial processes and soil water status, all of which influence Mn availability (8). Acid deposition lowers soil pH and inhibits mycorrhizal associations, which has strong effects on ion acquisition by roots; acid precipitation has also been shown to deplete base cations from foliage by leaching (9). There are important biogeochemical feedbacks of plant function and productivity on soil nutrient availability: root exudation and leaf litter chemistry and quantity affect soil organic matter, litter-derived nutrients, soil redox potential, pH, and microbial composition and function (10). Human effects on many of the atmospheric and soil factors shown here could increase the severity of Mn phytoxicity in the future. From Lynch and St.Clair (2004).
New perspectives on the Mn stress effects of multiple drivers, both within and across edaphic and aerial environments, contribute to a revised understanding of Mn phytotoxicity by revealing the importance of certain mechanisms while also implicating the potential exacerbating role of global climate change. These also provide a platform for improved management strategies via crop breeding and soil modification. This review presents a synthesis of current ideas on Mn phytotoxicity, and is not intended as a comprehensive literature review.
Mn PHYTOAVAILABILITY IN SOIL
Several interactive factors that elicit highly heterogeneous net effects drive Mn phytoavailability (Graham et al., 1988; El-Jaoual and Cox, 1998; Sparrow and Uren, 2014). Soil Mn exists in three oxidation states, i.e. the phytoavailable form Mn2+, as well as the insoluble yet easily reducible forms Mn3+ and Mn4+. The redox status of Mn and therefore its bioavailability is largely influenced by soil Mn content, proton (pH) and electron (Eh/pE) activities. Soil acidity impacts substantially on plants below around pH 5·3, a problem common in high-rainfall regions supporting much of the world’s vegetation. Manganese toxicity is one of the most important threats to plant growth on acid soils (Foy, 1984). Redox potential or electron activity reflects the reduction stability of a soil system, and is at least equally as important as pH, particularly when considering easily reducible Mn-oxides. However, it is often overlooked in discussion of Mn bioavailability. Hypoxic soils, for example, are highly reducing. Both acidity and hypoxia solubilize higher Mn-oxides to Mn2+, the latter capable of inducing Mn phytotoxicity even at a pH as high as 7. In contrast, upper threshold soil pH values leading to Al phytotoxicity are comparatively far lower at pH <5 (Graham et al., 1988).
The bioavailability of other soil solutes affects that of Mn via ion antagonism and/or their capacity to alter Mn availability in the rhizosphere. For example, base cations such as Mg2+ and Ca2+ moderate soil acidity and compete with Mn2+ for plant uptake. Similarly, diminished Fe2+ uptake due to Mn2+ oversupply can lead to Fe2+ deficiency (Marschner, 2002). The alleviation of Mn phytotoxicity by Si supply as observed in some crops is not clearly explicable, and will be addressed in the following section. Nitrogen, depending on its form, i.e. as NO3– or NH4+, can affect Mn soil solubility and shoot Mn uptake by altering rhizosphere pH (Elamin and Wilcox, 1986). The solubilization of higher Mn-oxides in soil is also facilitated by its organic matter content, and extreme heating and drying (Moraghan, 1985). While Mn is terrestrially common, i.e. the tenth most abundant element on land, certain geographic regions, including eastern Australia, Hawaii, Puerto Rico, Brazil and parts of tropical Africa, are known for their inherently Mn-toxic soils (Graham et al., 1988). Such habitats are vulnerable to Mn phytotoxicity arising from ‘natural’ processes as well as induced climate effects. On the Allegheny Plateau of North America, unbuffered soils contain relatively low total soil Mn concentrations; however, repeated clear-cutting and soil acidification by acid rain has increased Mn phytoavailability, albeit to levels far lower than the aforementioned naturally enriched substrates. Yet here Mn oversupply is recognized as a key contributor to the broad-scale disruption of forest composition and productivity in that region.
PHYTOTOXICITY MECHANISMS
The trace mineral nutrient Mn is integral to several key plant physiological functions including photosynthesis, mitigation of damage from reactive oxygen species (ROS), and redox processes (Graham et al., 1988). It serves as an enzyme co-factor in the water-splitting reaction of photosystem II (PSII) as well as aiding the sructural integrity and light harvesting by chloroplast lamellae. The regulation of transition metal nutrients is vulnerable to excessive cellular concentrations of these metals, including Mn, and can lead to harmful mechanisms including ROS production via the Fenton reaction (Ducic and Polle, 2005). Elevated tissue Mn2+ concentrations also drive toxicity via antagonism with similar ions, which when outcompeted by excess Mn2+ cannot perform their normal functional roles, e.g. as enzyme co-factors in ROS damage mitigation (González et al., 1998; StClair and Lynch, 2004; StClair et al., 2005).
Plant overexposure to Mn manifests most obviously as leaf chlorosis, dark inclusions and/or crinkling symptoms (Fig. 2), generally interpreted as stress. Genetics, certain environmental conditions including light exposure and temperature, soil chemistry and Mn supply affect the type and extent of symptoms. Not only do these parameters vary greatly, they often interact with each other to added effect. The resultant heterogeneity in plant response to Mn overexposure coupled with consideration of less conspicuous drivers of long-term Mn stress has contributed to ongoing confusion around the issue of Mn phytotoxicity.
Fig. 2.

Typical symptoms of Mn stress in common bean (Phaseolus vulgaris) as evident in fully expanded leaves of 3-week-old plants on day 6 of Mn treatment (100 µm) in solution culture: (A) interveinal chlorosis and early leaf crinkle in young leaves; (B) dark leaf speckles in old leaves; and (C) advanced leaf crinkle, interveinal chlorosis and dark spots on mature leaves.
To date, two major divergent theories have been proposed to explain Mn phytoxicity caused by excessively accumulated foliar Mn. Plant experimental growth conditions, interpretation of results and ensuing research priorities have steered differing arguments via independent lines of investigation. The first of these began with experiments on bean (Phaseolus vulgaris var. Red Kidney) and cowpea [Vigna unguiculata (L.) Walp.] exposed to Mn stress in solution culture, and illuminated at levels 4–17 times below that of sunlight (Horst and Marschner, 1978; Wissemeier and Horst, 1992). The resultant hypothesis was that Mn phytotoxicity is primarily mediated in the apoplast, causing callose and dark leaf-spot formation from apoplastically deposited Mn-oxides and oxidized phenols. These findings fuelled the still widely held notion that leaf black spot is the most important and reliable indicator of Mn phytotoxicity, with added inference that the converse applies. These authors susbequently tested an observation (Horiguchi, 1987, 1988) that light exacerbated Mn toxicity, by examining light effects on leaf black spot formation in cowpea (Wissemeier and Horst, 1992). They concluded that light did not exacerbate Mn toxicity, as interpreted by observing a lack of increase in leaf black spot under light. It is noteworthy that Horiguchi’s plants were illuminated by full sunlight, whereas the so-called ‘high light treatments’ imposed by Wissemier and Horst were around 250 μmol photons m−2 s−1, which was less than 15 % that of full sunlight. It is unsurprising, therefore, that no ‘light effects’ were observed in the latter case. Conclusions that Mn toxicity is most importantly mediated via apoplastic processes (Horst and Marschner, 1978; Wissemeier and Horst, 1992) have been followed up more recently in detailed studies on cowpea, also conducted under relatively low light exposures; and interpreted as showing phenotypic Mn-sensitivity-driven activities of oxidases and free-radical-mitigating agents in the apoplast (Fecht-Christoffers et al., 2006). It is unclear whether symplastic components were analysed separately so as to justify the underlying assumption that major Mn stress responses were indeed restricted to the apoplast.
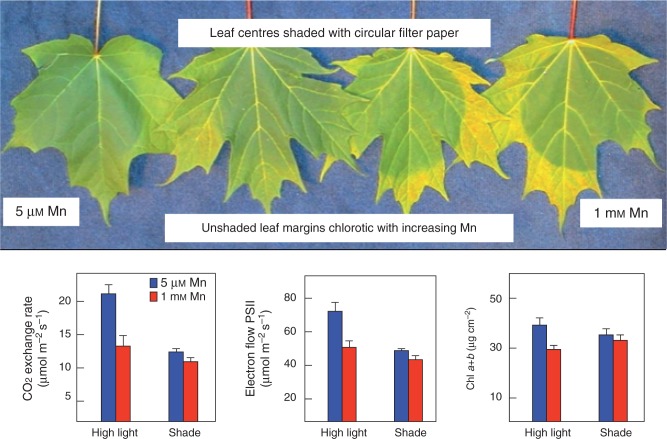
A somewhat different approach to that of Horst et al. (1978; Wissemeier and Horst, 1992) was employed in a series of investigations designed at the outset to interrogate plant Mn overexposure under ‘real’ climate conditions (González et al., 1998; González and Lynch, 1999b; StClair and Lynch, 2004, 2005a; StClair et al., 2005). These studies were instrumental in clarifying the physiological basis of Mn phytotoxicity exacerbated by sunlight. Common bean and North American maple species exposed to excess Mn and illuminated by full sunlight were affected by photobleaching and photo-oxidative stress, depending on phenotype and species. These studies have collectively demonstrated that notwithstanding numerous variables ultimately contributing to tissue Mn overaccumulation and phytoxicity, solar radiation serves as a particularly potent trigger for Mn toxicity mainly because light exposure at this level generates several ROS in planta. They demonstrated that Mn phytotoxicity is mediated via the inhibition of free-radical-mitigating antioxidative enzymes such as ascorbate peroxidase and glutathione reductase (González et al., 1998; StClair and Lynch, 2004; StClair et al., 2005) and demonstrated that excess Mn causes oxidative stress via metal-antagonism-driven deficiencies in co-factors integral to anti-oxidative enzyme activity. Chlorosis, originally recognized by Horiguchi et al. (1988) as a persistent and notable symptom of Mn toxicity, has since been shown by Gonzalez et al. (1998) to arise from chlorophyll destruction or photobleaching. Gonzalez et al. (1998) have also demonstrated that in common bean at least, the presence of dark leaf speckles associated with excessively accumulated Mn does not correlate with physiological stress. These latter experiments on bean and maple have provided unequivocal evidence of the distinct bleaching effect of solar radiation on leaves with elevated Mn concentrations (Fig. 3). This insidious or latent form of Mn phytotoxcity would vary with geography and changing weather patterns, manifesting only under certain conditions. Furthermore, it is realistic to assume that such an impact would interact with other stressors to compromise the overall health of a mature tree, for example, ultimately only noticeable in a broad sense, i.e. by forest decline and diminished pest resistance across several seasons (Tomlinson, 2003). As well as exacerbating Mn toxicity by photobleaching and photo-oxidative stress, sunlight is also known to enhance foliar Mn accumulation (Fernando et al., 2009a). An oversupply of Mn can also impose stress by competing with other ions for plant uptake, thereby disrupting Mg, Ca, K and Fe nutrition (StClair and Lynch, 2005b; StClair et al., 2005).
Fig. 3.
Upper panel: photobleaching effects on sugar maple leaves exposed to elevated Mn. Lower panel: the influence of Mn availability and light intensity (1500 and 500 μmol m−2 s−1) on the light and dark reactions of photosynthesis (CER, ETR) and chlorophyll a + b in sugar maple leaves. Error bars represent ±1 s.e. From StClair and Lynch (2004).
Natural plant communities contrast markedly in their abilities to tolerate excess Mn, depending on a range of factors including their genetics, whether Mn overexposure is ecologically intrinsic and/or whether it arises from induced conditions such as climate change (Foyer et al., 1994). Native floras evolved on the eastern Australian seaboard where Mn-rich acidic host substrates contain phytoavailable Mn concentrations as high as 5000 μg g–1 dry weight (d. wt) do not exhibit any obvious symptoms of physiological stress or forest decline (Fernando et al., 2009b). For example, Gossia (Myrtaceae) and Macadamia (Proteaceae) are mid-storey rainforest tree genera native to these soils and taxonomically delineated by an inherent capacity to overaccumulate foliar Mn and/or Al. This trait manifests in foliar concentrations of these metals that well exceed those in other plants on the same soils (Fernando et al., 2009b). Field studies have demonstrated that several Gossia species accumulate extraordinary foliar Mn concentrations of 10 000–35 000 μg g–1 d. wt, while controlled experiments on Macadamia point to the possible role of excess foliar Mn in chemical defence against insect herbivory (Fernando et al., 2009a). Changing global weather patterns, however, may already be impacting upon these highly adapted systems without being immediately obvious. Unusually Mn-tolerant plant communities could provide suitable ecological models for monitoring Mn toxicity-related oxidative stress by way of tracking ongoing imposition of climate change constraints. In contrast to the inherently Mn-tolerant ecosystems of eastern Australia, acid-rain-induced changes to soil chemistry in certain North American forests has raised their soil Mn bioavailability to comparatively low concentrations of around 95–150 μg g–1 d. wt, yet sufficient to induce Mn toxicity in maple (StClair and Lynch, 2005a).
HEAT EFFECTS ON PLANT Mn UPTAKE
There is ample evidence that elevating ambient temperature enhances foliar Mn uptake, at least in crop plants (Siman et al., 1974; Rufty et al., 1979; Heenan and Campbell, 1990), with similar information on natural systems scant. Some of these studies suggest that warmer growing conditions can render certain crop lines more Mn tolerant by expanding their capacity to accumulate shoot Mn without apparent physiological stress. So far, this has been interpreted as an enhancement of Mn tolerance. Interestingly, such conclusions have been from growth experiments utilizing artificial light well below the illumination levels of sunlight. Renewed understanding about latent Mn phytotoxicity that manifests only under certain environmental conditions calls for re-evaluation of such hypotheses. Combined with Mn phytotoxicity triggered by full sun exposure on Mn-accumulated leaves, rising global temperatures could (1) overextend the limits of Mn tolerance in naturally adapted systems; (2) increase stress on natural systems not adapted to high Mn; and (3) further compound Mn phytotoxicity in crops. ‘Normal’ foliar Mn concentrations in healthy crop plants lie within approx. 50–800 μg g–1 d. wt (Marschner, 2002); however, predictions of increased temperatures across many global regions have major implications for agronomic plant toxicity.
GENETIC VARIATION
Crop experiments highlighting interspecific and phenotypic variation in Mn tolerance originally confirmed the role of genetics as a key player in the mediation of excess Mn (Graham et al., 1988; Izaguirre-Mayoral and Sinclair, 2005). Sensitive and tolerant lines of wheat, soybean, cowpea, common bean, lupins, rice, etc. are now well described in the literature, and often occur multiply within species. Crop plants such as sunflower (Helianthus) and Macadamia are notable for their high tolerance to Mn, which accumulates foliarly via contrasting detoxifying sequestration mechanisms (Blamey et al., 1986; Fernando et al., 2006). In sunflower, excess foliar Mn is deposited around trichomes in non-photosynthetic dermal tissues, whereas large photosynthetic cell vacuoles serve as sinks for soluble foliar Mn in Macadamia. Transporters associated with the vacuolar sequestration of excess foliar Mn have also been implicated in certain Mn-tolerant Fabaceae (González and Lynch, 1999a; Delhaize et al., 2003). The former described dermal vacuolar Mn sequestration in Phaseolus vulgaris, while the latter identified the vacuolar Mn transporter ShMTP1 in Stylosanthes hamata.
Natural habitats exposed to excess Mn in eastern Australia and North America provide differing examples of genetic variability in Mn tolerance. In the first scenario, native plants evolved on highly Mn-enriched soils tolerate Mn by regulating its excessive uptake, ranging from exclusion through to overaccumulation and safe sequestration. There is marked heterogeneity in the Mn-accumulative abilities of sympatric sister species of the eastern Australian rain forest tree genus Gossia, which includes several ‘Mn hyperaccumulators’ so defined by their extraordinary abilities to accumulate foliar Mn (Fernando et al., 2009b). In the second scenario where anthropogenically induced Mn phytotoxicity is contributing to overall physiological stress in Pennsylvanian forest trees, it is clear that its extent varies at least at the species level (StClair et al., 2005). Investigation of the sympatric native Acer species sugar maple and red maple established that while both species experience elevated foliar Mn concentrations, their physiological stress levels differed markedly. By virtue of its greater genetic tolerance to excess Mn, red maple is the stronger competitor in establishment, growth and overall tree health. It is noteworthy that innate overaccumulation of foliar metals can be regarded as a form of chemical defence against herbivory, yet anthropogenically induced overaccumulation appears to compound susceptibility to insect attack (Dean, 2007).
MOLECULAR STUDIES INTO PLANT Mn SENSITIVITY AND TOLERANCE
Predicting, evaluating and addressing plant Mn stress in a practical sense largely hinges on visible symptoms, including dark leaf speckling widely inferred as indicative of Mn toxicity-related processes, which according to one line of argument predominates in the apoplast (Horst and Marschner, 1978; Wissemeier and Horst, 1992; Fecht-Christoffers et al., 2003, 2006). To date, the primary focus of molecular research aimed at resolving Mn sensitivity and tolerance via description of Mn-specific transporters in root and shoot tissues has been on symplastic components, commonly vacuolar and cytoplasmic membranes (Thomine et al., 2000; Delhaize et al., 2003, 2007; Peiter et al., 2007; Cailliatte et al., 2010; Ishimaru et al., 2012; Podar et al., 2012; Sasaki et al., 2012). A recent review by Socha et al. (2014) provides a useful summary of the current state of knowledge about the molecular basis of Mn uptake and transport, mostly based on arabidopsis and rice models. While conceding that ‘little is known about transporters essential for Mn uptake and storage in the cell’, it comprehensively evaluates literature on the following gene transporter families implicated in Mn transport: NRAMP (natural resistance associated macrophage protein), YSL (yellow stripe-like), ZIP [zinc regulated transporter/iron-regulated transporter (ZRT/IRT1)-related protein], CAX (cation exchanger), CCX (calcium cation exchangers), CDF/MTP (cation diffusion facilitator/metal tolerance protein), P-type ATPases and VIT (vacuolar iron transporter). The function and synthesis of iron-binding proteins have been linked to iron regulation and protection against oxidative stress (Briat et al., 2010). Similarly, elevated concentrations of Mn-chelating compounds and/or free-radical-mitigating enzymes in the presence of excess tissue Mn have been interpreted as Mn tolerance responses, including prevention of Mn uptake by roots via malate efflux as observed in transgenic plants (Yao et al., 2012; Chen et al., 2015).
CLIMATE CHANGE
Shifting global weather patterns are giving rise to induced environmental phenomena whose tangible and predicted effects are extremely concerning (IPCC, 2014). It is clear that rising temperatures, acid rain, extended periods of warm sunny weather, elevated atmospheric CO2 and ozone levels, depletion of permanent ice, flooding and wildfire are altering plant life on Earth.
The projected effects of global climate change are likely to work synergistically to exacerbate Mn toxicity over the next several decades. Taken individually, flooding that leads to hypoxic soils and increased Mn phytoavailability, acid rain that also releases Mn for plant uptake, drought and rising ambient temperatures are all likely to contribute cumulatively to Mn overaccumulation in plant tissues. This is as a direct result of changing climate patterns. In reality, these variables interact to greater detrimental effect than individually, further compounding Mn phytoavailabilty and accelerating soil nutritional imbalance and weathering (Tomlinson, 2003; Lynch and StClair, 2004; StClair and Lynch, 2010). Atmospheric ozone and bright sunshine delivering photosynthetic and UV radiation generate a range of free radicals in planta. As discussed here, their important role in triggering Mn phytotoxicity has been recognized only relatively recently. Increases in ozone levels and lengthier periods of sunshine are further contributing to the rising risk of wide-scale plant Mn stress associated with changing global weather patterns. Climate variables individually capable of inducing Mn phytotoxicity at the above- and below-ground levels can also interactively drive complex plant–soil effects that further compound Mn phytotoxicity. Evidence presented here highlights the urgency of the Mn phytotoxicity issue, particularly on the basis that Mn is ubiquitous in soil and on predictions of future climate scenarios favouring increased plant Mn uptake and toxicity stress triggers.
FUTURE DIRECTIONS
In drawing attention to a currently neglected yet increasingly important problem, this synthesis has re-visited long-standing and recent hypotheses about the mediation of Mn phytotoxicity. These discussions highlight likely ramifications that cannot be underestimated, particularly in the face of changing weather patterns. Addressing this issue will require going beyond present reliance on visual symptoms of overexposure to Mn that have traditionally been interpreted as indicating phytotoxicity. A broad-scale evaluative approach incorporating additional parameters is called for, coupled with improved awareness of the potential of apparently non-phytotoxic excess foliar Mn to become phytotoxic under certain climatic conditions that serve as potent triggers. Regular data gathering by monitoring soil and vegetation chemistry to ascertain variation in parameters such as soil pH and Eh, foliar Mn and enzyme concentrations may be useful in tracking plant response patterns across a range of variables including climatic conditions and genetic drivers. Present and future climate variables and overall plant health are important considerations for routine monitoring strategies. It is essential, therefore, that existing practices be expanded to achieve more realistic predictions so as to enable recognition of the true extent of stress events associated with Mn overexposure, including those not immediately obvious. Crop breeding and genetics should be prioritized as part of future research aimed at improving plant management and monitoring. It is clear from the literature that the exacerbation of Mn phytoxicity by climate change is highly plausible and merits renewed attention.
ACKNOWLEDGEMENTS
D.F. gratefully acknowledges funding from the Australian Research Council (grant no. DE120100510). The authors wish to thank Robert Snyder, US Forestry Services staff Joe Gomola and Ernie Wiltsie, and the PA Department of Conservation and Natural Resources for their invaluable assistance with fieldwork.
LITERATURE CITED
- Bernier B, Brazeau M. 1988. Magnesium deficiency symptoms associated with sugar maple dieback in a Lower Laurentians site in southeastern Quebec. Canadian Forest Research 18: 1265–1269. [Google Scholar]
- Blamey FPC, Joyce DC, Edwards DG, Asher CJ. 1986. Role of trichomes in Sunflower tolerance to manganese toxicity. Plant and Soil 91: 171–180. [Google Scholar]
- Briat JF, Ravet K, Arnaud N, et al. 2010. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Annals of Botany 105: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliatte R, Schikora A, Briat J-F, Mari S, Curie C. 2010. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. The Plant Cell 22: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Sun L, Liu P, Liu G, Tian J, Liao H. 2015. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiology 167: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM. 2007. Chemical ecology of plant–microbe interactions and effects on insect herbivores. PhD thesis, Pennsylvania State University, State College, PA. [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR. 2003. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. The Plant Cell 15: 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber B, Pittman JK, et al. 2007. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. The Plant Journal 51: 198–210. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Lawrence GB, Bulger AJ, et al. 2001. Acid deposition in the northeastern United States: sources and inputs; ecosystem effects, and management strategies. BioScience 51: 180–198. [Google Scholar]
- Drohan PJ, Stout SL, Peterson GW. 2002. Sugar maple (Acer saccharum Marsh.) decline during 1979–1989 in northern Pennsylvania. Forest Ecology Management 170: 1–17. [Google Scholar]
- Ducic T, Polle A. 2005. Transport and detoxification of manganese and copper in plants. Brazilian Journal of Plant Physiology 17: 103–112. [Google Scholar]
- Elamin OM, Wilcox GE. 1986. Manganese toxicity development in muskmelons as influenced by nitrogen form. Journal of the American Horticultural Society 111: 323–327. [Google Scholar]
- El-Jaoual T, Cox DA. 1998. Manganese toxicity in plants. Journal of Plant Nutrition 21: 353–386. [Google Scholar]
- Fecht-Christoffers MM, Braun H-P, Lemaitre-Guillier C, VanDorsselaer A, Horst WJ. 2003. Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiology 133: 1935–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecht-Christoffers MM, Fuhrs H, Braun H-P, Horst WJ. 2006. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiology 140: 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando DR, Bakkaus EJ, Perrier N, et al. 2006. Manganese accumulation in the leaf mesophyll of four tree species: a PIXE/EDAX localization study. New Phytologist 171: 751–758. [DOI] [PubMed] [Google Scholar]
- Fernando DR, Baker AJM, Woodrow IE. 2009a. Physiological responses in Macadamia integrifolia on exposure to Mn treatment. Australian Journal of Botany 57: 406–413. [Google Scholar]
- Fernando DR, Guymer G, Reeves RD, Woodrow IE, Baker AJ, Batianoff GN. 2009b. Foliar Mn accumulation in eastern Australian herbarium specimens: prospecting for ‘new’ Mn hyperaccumulators and its potential application in taxonomy. Annals of Botany 103: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy CD. 1984. Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In: Adams F, ed. Soil acidity and liming. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. 1994. Photooxidative stress in plants. Physiologia Plantarum 92: 696–717. [Google Scholar]
- González A, Lynch J. 1999a. Subcellular and tissue compartmentation in bean leaves under Mn toxicity stress. Australian Journal of Plant Physiology 26: 811–822. [Google Scholar]
- González A, Lynch J. 1999b. Tolerance of tropical common bean genotypes to manganese toxicity: performance under different growing conditions. Journal of Plant Nutrition 22: 511–512. [Google Scholar]
- González A, Steffen KL, Lynch JP. 1998. Light and excess manganese. Plant Physiology 118: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RD, Hannam RJ, Uren NC. 1988. Manganese in soils and plants. In: Graham RD, Hannam RJ, Uren NC, eds. International Symposium on Manganese in Soils and Plants. Glen Osmond, South Australia: Kluwer Academic Press. [Google Scholar]
- Heenan DP, Campbell LC. 1990. The influence of temperature on the accumulation and distribution of manganese in two cultivars of soybean Glycine max L. Merr. Australian Journal of Agricultural Research 41: 835–844. [Google Scholar]
- Horiguchi T. 1987. Mechanism of manganese toxicity and tolerance of plants II. Deposition of oxidized manganese in plant tissues. Soil Science and Plant Nutrition 33: 595–606. [Google Scholar]
- Horiguchi T. 1988. Mechanism of manganese toxicity and tolerance of plants. 7. Effect of light-intensity on manganese-induced chlorosis. Journal of Plant Nutrition 11: 235–246. [Google Scholar]
- Horsley SB, Long RP, Bailey SW, Halett RA, Hall TJ. 2000. Factors associated with the decline disease of sugar maple on the Allegheny Plateau. Canadian Forest Research 30: 1365–1378. [Google Scholar]
- Horst WJ, Marschner H. 1978. Symptome von mangan-uberschuB bei bohnen (Phaseolus vulgaris). Zeitschrift für Pflanzenernährung und Bodenkunde 141: 129–142. [Google Scholar]
- IPCC . 2014. IPCC, 2014: Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, et al., eds. Climate Change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York USA: Cambridge University Press. [Google Scholar]
- Ishimaru Y, Takahashi R, Bashir K, et al. 2012. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Scientific Reports 2: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre-Mayoral ML, Sinclair TR. 2005. Soybean genotypic difference in growth, nutrient accumulation and ultrastructure in response to manganese and iron supply in solution culture. Annals of Botany 96: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, StClair SB. 2004. Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Fields Crops Research 90: 101–115. [Google Scholar]
- Marschner H. 2002. Mineral nutrition of higher plants. London: Academic Press. [Google Scholar]
- Moraghan JT. 1985. Manganese nutrition of flax as affected by FeEDDHA and soil air drying. Soil Science Society of America 49: 668–671. [Google Scholar]
- Peiter E, Montanini B, Gobert A, et al. 2007. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proceedings of the National Academy of Sciences, USA 104: 8532–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar D, Scherer J, Noordally Z, Herzyk P, Nies D, Sanders D. 2012. Metal selectivity determinants in a family of transition metal transporters. Journal of Biological Chemistry 5: 3185–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty TW, Miner GS, Raper CD. 1979. Temperature effects on growth and manganese tolerance in tobacco. Agronomy Journal 71: 638–644. [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, FMa J. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. The Plant Cell 24: 2155–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman A, Cradock FW, Hudson AW. 1974. The development of manganese toxicity in pasture legumes under extreme climatic conditions. Plant and Soil 41: 129–140. [Google Scholar]
- Socha AL, Guerinot ML. 2014. M-neuvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Frontiers in Plant Science 5: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow LA, Uren NC. 1987. The role of manganese toxicity in crop yellowing on seasonally waterlogged and strongly acidic soils in north-eastern Victoria. Australian Journal of Experimental Agriculture 27: 303–307. [Google Scholar]
- Sparrow LA, Uren NC. 2014. Manganese oxidation and reduction in soils: effects of temperature, water potential, pH and their interactions. Soil Research 52: 483–494. [Google Scholar]
- StClair SB, Lynch JP. 2004. Photosynthetic and antioxidative enzyme responses of sugar maple and red maple sedlings to excess manganese in contrasting light environments. Functional Plant Biology 31: 1005–1014. [DOI] [PubMed] [Google Scholar]
- StClair SB, Lynch JP. 2005a. Differences in the success of sugar maple and red maple seedlings on acid soils are influenced by nutrient dynamics and light environment. Plant, Cell and Environment 28: 874–885. [Google Scholar]
- StClair SB, Lynch JP. 2005b. Element accumulation patterns of deciduous and evergreen tree seedlings on acid soils: implications for sensitivity to manganese toxicity. Tree Physiology 25: 85–92. [DOI] [PubMed] [Google Scholar]
- StClair SB, Lynch JP. 2010. The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant and Soil 335: 101–115. [Google Scholar]
- StClair SB, Carlson JE, Lynch JP. 2005. Evidence for oxidative stress in sugar maple stands growing on acidic, nutrient imbalanced forest soils. Oecologia 145: 258–269. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. 2000. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proceedings of the National Academy of Sciences, USA 97: 4991–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson GH. 2003. Acidic deposition, nutrient leaching and forest growth. Biogeochemistry 65: 51–81. [Google Scholar]
- Wissemeier AH, Horst WJ. 1992. Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea Vigna unguiculata L. walp. Plant and Soil 143: 299–309. [Google Scholar]
- Yao Y, Xu G, Mou D, Wang J, Ma J. 2012. Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemospere 89: 150–157. [DOI] [PubMed] [Google Scholar]