Abstract
Background
Managing patients with polypharmacy is a challenging issue in primary care. The aim of this study is to determine whether a patient-centered systematic review leads to more appropriate medication use in patients without negatively affecting quality of life and the course of the disease.
Methods/Design
The trial is a two-armed, double blinded cluster-randomized controlled trial. Primary care physicians (PCPs) will be randomly assigned to the intervention or control group. Physicians in the intervention group undergo training with instruction of the algorithm. The control group is given a lecture on multimorbidity and instructions for collecting data in a usual care manner.
PCPs will approach patients aged 60 years or older who are taking 5 or more drugs. The study period is 1 year.
The primary outcome measure is the change in the number of drugs 12 months after the algorithm was applied by the PCP during consultation with the patient. Secondary outcomes are: change in the number of drugs immediately after the encounter and 6 months later, reason for a change of the medication, discrepancy in the decision to change between PCP and patient, number of drugs for which the patient is suggesting a change, number of drugs the patient is taking that are not known to the PCP, time consumption of the intervention, disease-specific variables to evaluate the course of the disease(s) for which the patient is being treated , quality of life, barriers against using the algorithm, numbers of drugs readopted due to an unfavorable course of the disease, and numbers of drugs which have been started.
Discussion
Answering the four questions of the algorithm requires a weighing-up of risks and benefits and contains a shared-decision-making approach: a prioritization of the treatment goals is necessary. This can only be done in collaboration with the patient. The majority of patients with multimorbidity are treated in the primary care setting. This underlines the significance of our study carried out in this setting: given the high prevalence of adverse drug events in patients with multimorbidity an intervention like ours has a large potential to reduce drug-related morbidity.
Trial registration
ISRCTN16560559 13 November 2014
Keywords: Multimorbidity, Polypharmacy, Shared-decision--making, Prioritization, Adverse drug events
Background
The intake of multiple drugs (polypharmacy), overuse and misuse of drugs are increasing problems in the care of people with a number of health conditions (multimorbid) as well as older people [1, 2]. One of the problems in polypharmacy is the danger of adverse drug reactions [3]. This leads to an increase in morbidity, hospital admissions [4, 5], health-related costs [6] and number of deaths [7, 8]. Paradoxically, a clear relationship between polypharmacy and underuse of indicated drugs has been shown [9–11]. Up to now, several clinical tools and approaches to guide appropriate prescribing of drugs are available [11-14]. Deprescribing is a complex process that is required for the safe and effective cessation of inappropriate medications [3]. Barriers to and enablers of deprescribing from the point of view of the patients as well as from primary care physicians (PCPs) have been investigated [3, 15–18]. A recently published study using a newly developed algorithm for a systematic reduction of medication (Good Palliative Geriatric Practice, GPGP) showed that deprescribing resulted in a significant positive effect on health in older patients [19, 20]. In our study, we slightly adapted the GPGP algorithm and pilot-tested the algorithm in a primary care setting to assess feasibility and practicability [21]. The algorithm enforces a systematic drug review considering patients’ perspective and preferences. As primary care physicians (PCPs) take care of the majority of patients with multimorbidity, our tool has a potentially high impact on polypharmacy in primary care settings. Previous studies, investigating interventions to reduce polypharmacy, have taken place in an older persons care setting. In primary care settings results are still lacking. This study intends to bridge this gap.
Study hypothesis
The implementation of an algorithm adapted to the GPGP leads to a reduction of polypharmacy among patients with multimorbidity (60 years and older) in a primary care setting.
Furthermore, this implementation does not worsen the quality of life or the course of the disease for which the drug was originally prescribed for (safety issues of the intervention).
Methods/Design
We will conduct a cluster-randomized controlled trial (randomization on PCP-practice level) with primary care physicians in the northern part of Switzerland (see Fig. 1). The design and methodology of our study is based on the experiences of a small pilot study.
Fig. 1.
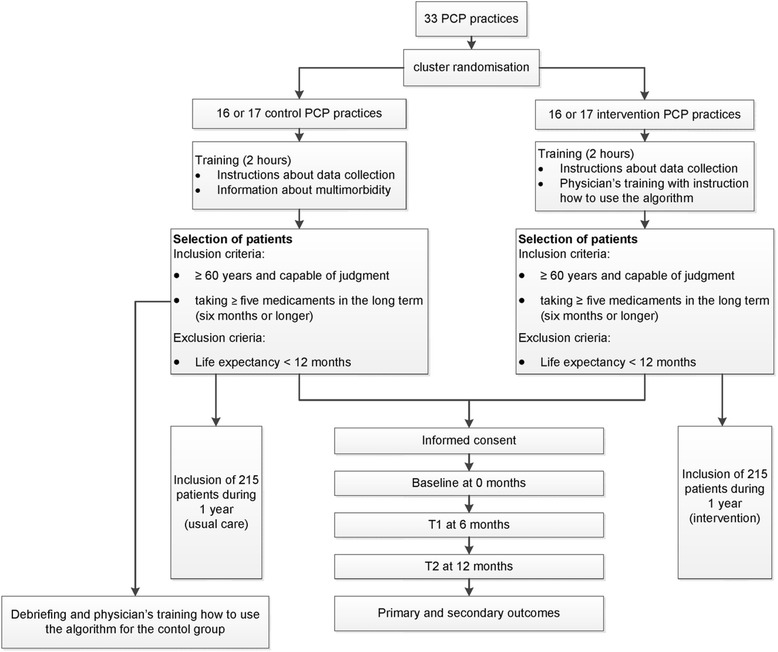
Study flow chart
Pilot study
The pilot study [21] was conducted with 14 primary care physicians. An evaluation was conducted to test the logistics, baseline measurements and the feasibility of the study. The pilot study resulted in minor changes in the questionnaire and an important element of shared-decision-making was added: beside the four major clinical problems defined by the PCP the four most important complaints in a patient’s perspective were added. In order to detect potential undertreatment a special section was added entitled “medication started”.
Recruitment and eligibility of primary care physicians
PCPs are eligible for participation if they provide care in the routine primary setting to unselected patients. About 1700 PCPs in the Canton of Zurich will be invited by a formal letter of the Institute of Primary Care of the University of Zurich.
PCPs who finally agree to participate will be listed in alphabetical order. If two PCPs of the same practice agree to participate, the first name in the alphabet receives a number which is valid also for the second one in order to avoid contamination between groups. Randomization will be stratified according to the practice size (e.g. single-handed versus group practice). The randomization scheme will be generated by using the website Randomization.com (http://www.randomization.com) and group assignment will be performed by a study nurse not involved in further data analysis. The PCPs with the corresponding numbers will be randomly allocated to the intervention and control group, respectively.
PCPs will be informed only after completion of the study about the group they have been randomized to (intervention or control group) in a debriefing session. At this time, an education session on how to use the algorithm will be offered to the control group.
If the number of the required 33 (see power calculation below) participating PCPs cannot be reached, the randomization will be done with the interested PCPs and the recruiting will be expanded to other Cantons of the northern area of Switzerland, followed by another randomization process.
Each PCP in the intervention and control groups will receive a financial allowance.
Patient recruitment
All PCPs are asked to approach, consecutively and regardless of the reason for the current consultation, patients aged 60 or older who are taking 5 or more long-term drugs.
Patient inclusion criteria
Be aged at least 60 years.
Be taking 5 or more long-term drugs (6 months or longer).
Patient exclusion criteria
Life expectancy less than 12 months.
Intervention
Blinding of PCPs is not possible in this study design and even participation in a study addressing a certain topic can influence a physician’s behavior. To achieve a degree of blinding, PCPs are not provided with specific information at invitation. They will receive a lecture (length: 2 hours) about multimorbidity in general and instructions for collecting data in a usual care group. PCPs from the control group will be told that the study purpose is to investigate best practices for physician-patient communication.
PCPs in the intervention group will undergo a physicians’ training with instruction on how to use the algorithm including a communication skills training. The adapted GPGP-algorithm used in our trial consists of four steps and is a patient-centered process. First, the drugs are listed together with the four main diagnoses as well as the four main disorders from the patient’s perspective. Then the following steps will be systematically followed (see Fig. 2):
Indication of the drug in this patient (identifying undertreatment or overtreatment)
Do the known possible adverse reactions of the drug outweigh the possible benefits? (Potential adverse events)
Can the dosing rate be reduced with no significant risk? (Dosing problems)
Is there another drug that may be superior to the one in question?
Fig. 2.
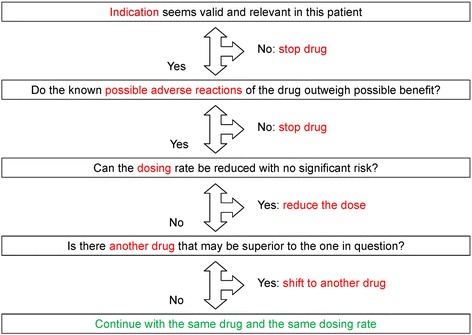
Improving drug therapy in primary care (adapted from [20] and [21])
Finally, the result of this analysis will be discussed together with the patient. For each drug the PCP will note whether the patient agrees with the recommendation.
After obtaining informed consent from the patient, a practice nurse or the PCP creates a list of the patient’s present medication. Then, the PCP defines the four major clinical problems and, together with the patient, the four most important complaints from the patients’ perspective. Physicians in the intervention group then decide, for every drug listed, whether the indication is correct, whether there are side effects, whether an alternative treatment would be suitable or whether a change in the dosage is indicated (key questions of the algorithm). After discussion with the patient, the PCP and patient decide together whether to stop a drug, to change the dosage of a drug or to switch to an alternative drug, with the option to restart if symptoms should increase or the disease deteriorate.
Primary outcomes measures
Change in the number of drugs (deprescribing rate) 12 months after applying the tool.
The primary outcome will be calculated on the basis of the medication list, provided by the practice nurse or the PCP at baseline (before the intervention) and 12 months afterwards.
Secondary outcome measures
Table 1 displays the secondary outcomes with the appropriate measuring method.
Table 1.
Secondary outcomes and the appropriate measuring method
| Secondary outcomes | Measuring method |
|---|---|
| 1. Change in the number of drugs immediately after the encounter and 6 months later | Medication list at baseline and after 6 months |
| 2. Reason for a change, categorized in the 4 options of the algorithm, number of drugs in each category 12 months after applying the tool | Medication list of the changed medications after the intervention, with the reason for a change |
| 3. Discrepancy in the decision to quit, change or continue the drug between PCP and patient | Medication list after intervention with any suggestion from the PCP for a change; medication list after the intervention and after shared-decision-making |
| 4. Number of drugs for which the patient is suggesting a change | Medication lists at baseline, after 6 and 12 months |
| 5. Number of drugs the patient is taking not known to the PCP | Medication lists at baseline, after 6 and 12 months |
| 6. Time consumption of the intervention | Measurement of the time through the practice nurse and the PCP |
| 7. Disease-specific variables to evaluate the course of the disease(s) for which the patient is being treated | Symptom scores and measurements of biometric analysis (e.g. blood pressure monitoring, serum glucose) and VAS scales (e.g. pain). Event rates (hospitalization, death) and unexpected adverse event rates |
| 8. Number of drugs readopted due to an unfavorable course of the disease(s) (readoption rate) | Medication lists at baseline, after 6 and 12 months |
| 9. Quality of life (QoL) | Patient rating on a 5-point Likert scale |
| EQ-5D-3 L at baseline, after 6 and 12 months | |
| 10. Barriers perceived by patients and PCPs against the approach/algorithm | Phone interview with the patients 13 months after intervention |
| 11. Number of drugs which have been started | Medication lists at baseline, after 6 and 12 months |
EQ-5D-3 L EuroQol health status measure, PCP primary care practitioner, VAS visual analogue scale
After the baseline assessment (including gender and living situation) systematic follow-up measurements will take place after 6 and 12 months.
Patient-reported outcomes
Quality of life (QoL) will be assessed using the EuroQol (EQ-5D-3 L). This is a generic preference-based health status measure that has been shown to be valid and reliable in a variety of populations and patients groups [22–25].
Furthermore, patients are asked to determine their QoL on a Likert scale from −2 to +2. They are asked about their main complaint and they have to indicate the intensity (no complaint to unsupportable) on a visual analogue pain scale.
Disease-specific parameters
PCPs of both groups measure the following disease-specific parameters at baseline, after 6 and after 12 months:
Weight and blood pressure
Glycated hemoglobin (HbA1c), for patients with diabetes only
Thyroid-stimulating hormone (TSH), for patients with thyroid hormone substitution only
Hemoglobin, for patients with iron substitution or vitamin B12 substitution only
Furthermore, the PCP evaluates the course of the main diseases (stable, improvement, aggravation).
Safety outcomes
Adverse events have to be reported by the PCP at the study center and the safety board. The following definition for adverse events is used:
Complication of an existing disease (e.g. myocardial infarction in a coronary heart disease patient)
Acute illness newly diagnosed
Hospitalization or death
In case of adverse events the safety board can consult the medical history of the patient and, together with the PCP, it decides whether there is a plausible connection between intervention and adverse event.
The safety board is composed of a clinical ethicist, biostatistician and a geriatrician.
Data collection procedures
Patients will receive detailed written information on the aim of the study. After giving their written informed consent data will be collected by means of paper documents. For every patient a dossier with the different case report forms will be created. The forms are encoded; the code is stored at each primary care physicians’ practice. If a case-tracking is needed (in case of adverse events) it can be decoded. The transfer from paper to electronic data will be carried out through a research associate and controlled by a second research associate. Grouping of diagnoses and drugs will be organized by standardised databases.
Statistical analysis
The primary data analysis will follow the intent-to-treat (ITT) approach. This means that all available data from all individuals will be analyzed according to treatment group assignment, regardless of whether or not each individual actually received the assigned treatment.
The primary outcome, change in the number of drugs 12 months after applying the deprescribing tool, will be compared by using a t test for independent groups comparison. Hierarchical regression will be used to consider the cluster-design for potential confounder. Determinants associated with a change of the medication are investigated by exploratory, multivariate regression analysis.
For secondary outcomes, parametric (t test) or non-parametric tests (chi-square and Wilcoxon test) will be used where appropriate.
Descriptive statistics will be used to describe the study population. Drop-out and loss to follow-up will be described.
In case of study discontinuation the collected data that are hitherto available will be anonymized and evaluated.
The last observation carried forward (LOCF) method will be used for dealing with the missing data for patients who have dropped out. The drop-out rate and the causes of drop-outs will be compared between the two groups to investigate the possible influence of the intervention. To estimate the last observation carried forward-effect a per-protocol (PP) analysis will be applied.
Timeframe of the study
The recruitment of the PCP is planned over 2 months between March and May 2015.
Patients’ eligibility screening and patient inclusion is projected within a period of 4 months. The aim is to include 1 patient/week per PCP.
Ethics approval
The study protocol is approved by the Ethics Committee of the Canton of Zurich (reference KEK-ZH-number 2015–0595).
Patient informed consent
Previous to study participation, patients receive written and verbal information about the content and extent of the planned study from the PCPs. In case of acceptance, they sign the informed consent form.
Data security/disclosure of original documents
The patient names and all other confidential information fall under medical confidentiality rules and are treated according to appropriate Federal Data Security Laws. The patient names are not accessible to the study staff.
Sample size calculation
Assuming a 0.05 2-sided significance level, 30 clusters (on PCP-level) including 13 patients each would have an 80 % power to detect a difference of 1 drug, which we consider to be clinically relevant.
Based on previous studies [14] we assumed an intracluster correlation coefficient (ICC) of 0.02 for the primary outcome and a standard deviation of 2.8 [21]. In consideration of a drop-out rate of 10 %, 33 PCPs (16 respectively 17 PCPs with 215 patients in each arm) are needed.
Discussion
The aim of this study is to determine whether a systematic review with a specific algorithm leads to more appropriate medication use in patients with multimorbidity (60 years and older) without worsening their quality of life and the course of the disease. Up to now, similar interventions have been tested in older populations but not in a primary care setting. In this setting, it is crucial, that an intervention is time-saving for enabling implementation in the daily clinical practice. In the pilot study the expenditure of time was 15 minutes (median; interquartile range (IQR): 10–30). The feasibility and acceptability was rated with a 5-point Likert scale in different items (means: 3.2–4.2, SD 0.83–1.3). Other studies concerning improving appropriate prescription in (mostly older) patients receiving complex polypharmacy present a more time-consuming algorithm or method than usual care [26]. The time aspect might be a strength of our approach in comparison to other ongoing studies [14, 15, 24].
A special feature of our study is the investigation of overtreatment as well as undertreatment. After piloting the study, an extension was made: beside the list of medications in use a special section was added with “medication started.”
Prioritization of patient’s problems is a crucial issue in the shared-decision-making process. To foster this approach and discussions between physician and patient on this issue, we added a list of the four most important complaints from the patient’s perspective to our tool in in parallel with the list of the four major clinical problems defined by the PCP.
Based on other studies [24], measurements of EQ-5D-3 L might even enable an economic evaluation of the danger of adverse drug reactions.
Finally, our study contains an exploratory part, as patient’s barriers and enablers against the approach will be inquired systematically 13 months after intervention. This has the potential to optimize the algorithm or further adapt it to the specific needs of primary care.
Trial status
Patient recruitment had not started at the first submission in April 2015.
Acknowledgments
This study is funded by Gottfried und Julia Bangerter-Rhyner-Stiftung, Basel (Switzerland).
Abbreviations
- GPGP
Good Palliative Geriatric Practice
- HbA1c
glycated hemoglobin
- ICC
intracluster correlation coefficient
- IQR
interquartile range
- ITT
intent-to-treat
- LOCF
last observation carried forward
- PCP
primary care physician
- PP
per-protocol
- QoL
quality of life
- SD
standard deviation
- TSH
thyroid-stimulating hormone
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SN is the trial sponsor, he was the initiator for this study, organized the recruitment and helped to draft the manuscript and to develop the case report forms. OS conceived of the study, participated in the design of the study and performed the statistical analysis. TR participated in the coordination of the study. SH made the applications for ethical approval of the study, developed the case report forms, organized the recruitment and wrote and revised the final manuscript. All authors read and approved it.
Contributor Information
Susann Hasler, Email: susann.hasler@ksw.ch.
Oliver Senn, Email: oliver.senn@usz.ch.
Thomas Rosemann, Email: thomas.rosemann@usz.ch.
Stefan Neuner-Jehle, Email: sneuner@bluewin.ch.
References
- 1.Gallagher P, Lang PO, Cherubini A, Topinkova E, Cruz-Jentoft A, Montero Errasquin B, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67(11):1175–88. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 2.Wise J. Polypharmacy: a necessary evil. BMJ. 2013;347:f7033. doi: 10.1136/bmj.f7033. [DOI] [PubMed] [Google Scholar]
- 3.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. doi: 10.1007/s40266-013-0106-8. [DOI] [PubMed] [Google Scholar]
- 4.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 5.Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77(6):1073–82. doi: 10.1111/bcp.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reich O, Rosemann T, Rapold R, Blozik E, Senn O. Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakkarainen KM, Hedna K, Petzold M, Hagg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions – -a meta-analysis. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 9.Kuijpers MA, van Marum RJ, Egberts AC, Jansen PA, Group OS. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol. 2008;65(1):130–3. doi: 10.1111/j.1365-2125.2007.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63(2):187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher PF, O’'Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 12.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 13.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 14.Jager C, Freund T, Steinhauser J, Joos S, Wensing M, Szecsenyi J. A tailored implementation intervention to implement recommendations addressing polypharmacy in multimorbid patients: study protocol of a cluster randomized controlled trial. Trials. 2013;14:420. doi: 10.1186/1745-6215-14-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altiner A, Schafer I, Mellert C, Loffler C, Mortsiefer A, Ernst A, et al. Activating GENeral practitioners’ dialogue with patients on their Agenda (MultiCare AGENDA) study protocol for a cluster randomized controlled trial. BMC Fam Pract. 2012;13:118. doi: 10.1186/1471-2296-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuling J, Gebben H, Veehof LJ, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56. doi: 10.1186/1471-2296-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthys J, Elwyn G, Van Nuland M, Van Maele G, De Sutter A, De Meyere M, et al. Patients’ ideas, concerns, and expectations (ICE) in general practice: impact on prescribing. British J Gen Pract. 2009;59(558):29–36. doi: 10.3399/bjgp09X394833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahler C, Hermann K, Jank S, Haefeli WE, Szecsenyi J. Can a feedback report and training session on medication counseling for general practitioners improve patient satisfaction with information on medicines? Patient Preference Adherence. 2012;6:179–86. doi: 10.2147/PPA.S27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9(6):430–4. [PubMed] [Google Scholar]
- 20.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 21.Neuner-Jehle S, Krones T, Senn O. Systematic elimination of prescribed medicines is acceptable and feasible among polymorbid family medicine patients. Praxis. 2014;103(6):317–22. doi: 10.1024/1661-8157/a001591. [DOI] [PubMed] [Google Scholar]
- 22.EuroQol G. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 23.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 24.Willeboordse F, Hugtenburg JG, van Dijk L, Bosmans JE, de Vries OJ, Schellevis FG, et al. Opti-Med: the effectiveness of optimised clinical medication reviews in older people with ‘geriatric giants’ in general practice; study protocol of a cluster randomised controlled trial. BMC Geriatr. 2014;14:116. doi: 10.1186/1471-2318-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greiner W, Claes C, Busschbach JJ, von der Schulenburg JM. Validating the EQ-5D with time trade off for the German population. European J Health Econ. 2005;6(2):124–30. doi: 10.1007/s10198-004-0264-z. [DOI] [PubMed] [Google Scholar]
- 26.Drenth-van Maanen AC, van Marum RJ, Knol W, van der Linden CM, Jansen PA. Prescribing optimization method for improving prescribing in elderly patients receiving polypharmacy: results of application to case histories by general practitioners. Drugs Aging. 2009;26(8):687–701. doi: 10.2165/11316400-000000000-00000. [DOI] [PubMed] [Google Scholar]


