Abstract
Context:
Systemic conditions, especially chronic infections, have a direct impact on the general health and well-being of an individual. Similarly, the long-standing inflammatory changes seen during periodontitis have been associated with the altered diabetic control, preterm, low birth weight infants, and cardiovascular disease. Being a low-grade infection, the signs may not be as severe as seen in other systemic conditions, but they definitely cannot be ignored.
Aims:
The present study was designed to compare clinical, hematological, and systemic inflammatory markers in patients with chronic periodontitis.
Subjects and Methods:
A total of 90 chronic periodontitis patients were selected for the present study from the outpatient department of the Department of Periodontology, and the various clinical and hematological parameters were then assessed.
Statistical Analysis Used:
Z-test was used to compare the probing depth, clinical attachment loss, hematological parameter, and interleukin-6 values between Group A and Group B. Mann–Whitney U-test was used to compare gingival index, plaque index, and bleeding on probing between Group A and Group B.
Results:
The results of the study were based on the comparison of the clinical, hematological, and systemic inflammatory markers in smokers and nonsmokers with chronic periodontitis and came out to be statistically highly significant.
Conclusions:
With the resurgence of emphasis on significance of oral diseases related to systemic health, the medical professionals also need to familiarize themselves with the oral cavity and the oral-systemic inter-relationships to treat or reduce the morbidity of the underlying medical condition. Furthermore, the oral health care professionals must reach out to the medical community and the general public to improve patient care through education and communication about the oral health-systemic health link.
Keywords: Chronic periodontitis, clinical, hematological, nonsmokers, smokers, systemic inflammatory markers
Introduction
Periodontal diseases are initiated by consortia of oral bacteria that elicit local inflammatory responses that lead to bleeding on probing (BOP), loss of periodontal attachment, bone, and eventual tooth loss.[1] They have been linked to systemic conditions including heart disease, diabetes, obesity, and complex metabolic syndrome. The association between periodontal diseases and these systemic conditions seems to be due to a low-grade inflammatory burden that links them through a common pathophysiological mechanism. Conceivably, locally secreted cytokines and periodontal pathogens can enter the bloodstream and contribute to damage elsewhere in the body, and there has been a substantial evidence to support this hypothesis.[2]
These associations suggest that periodontal diseases have systemic effects. In addition, some studies have found that periodontal infection elicits systemic blood chemistry changes.[3] For thousands of years, blood has been regarded as the ultimate body fluid that could indicate the progression of a disease process and before that the presence of a pathological state. In the past decade, there has been a renewed interest to study the effect of periodontitis on changes in the cellular and molecular components of peripheral blood. The relationship of periodontitis with leukocytes, thrombocytes, C-reactive protein, interleukin-6 (IL-6), fibrinogen, erythrocyte sedimentation rate (ESR), Von Willebrand factor, and red blood cells has been investigated in a large number of studies.[4]
Anemia of chronic disease (ACD) has been described in the literature and seems to be one of the most common forms of anemia observed in clinical medicine. ACD is defined as the anemia occurring in chronic infections, chronic inflammatory processes, or tumor formation that is not due to dysfunction of bone marrow cells or other diseases, and occurring despite the presence of adequate iron stores and vitamins.[5] It is characterized by hypoferramia with adequate reticuloendothelial iron stores, normal to elevated ferritin concentration and is seen as a frequent complication of chronic inflammatory conditions. A characteristic finding of the disorders associated with ACD was the increased production of the cytokines that mediates the immune or inflammatory response such as Tumor necrosis factor α (TNF-α), IL-1, and interferons. All the processes involved in the development of ACD can be attributed to these cytokines including shortened red cell survival, blunted erythropoietin response to anemia, impaired erythroid colony formation in response to erythropoietin, and abnormal mobilization of the reticuloendothelial iron stores.[6]
Hutter et al.,[7] suggested that periodontitis has chronic and systemic effects and that periodontitis may tend toward anemia. Tumor necrosis factor α (TNF-α) and IL-6 are key cytokines in the initiation and maintenance of systemic inflammation which have been implicated in progression and severity of periodontitis.[8] These cytokines are also released by periodontal tissues in response to bacterial infection, which suggests that periodontitis like other chronic disease may cause ACD. In addition, higher serum levels of these cytokines have been observed in patients diagnosed with chronic periodontitis than in periodontally healthy individuals.[9]
Smoking and tobacco consumption, to add on, further comprise a major environmental factor for periodontal disease. Numerous functions of oral or peripheral neutrophil are negatively affected by smoking or nicotine exposure, including phagocytosis, as well as decreased production of superoxide, hydrogen peroxide, and protease inhibitors. Alterations in gingival crevicular fluid and cytokines in smokers, tipping the balance in favor of tissue breakdown, have been noted. Smoking as it represents a significant confounder in the inter-relationship between periodontal disease and its systemic squeal, therefore, needs to be investigated in specific population with regard to how it impacts this relationship. Considering the above factors, the need of the study was felt to evaluate the influence of smoking on clinical parameters, hematologic signs of ACD, and marker of systemic inflammation (IL-6) in patients with chronic periodontitis.
Subjects and Methods
A total of 90 patients were selected for the present study from the outpatient department of the Department of Periodontology. These patients were further divided into two groups with Group A having 45 nonsmokers and Group B having 45 smokers with moderate to severe chronic periodontitis [Figure 1], with plaque highlighted with the help of disclosing solutions [Figure 2]. In the present study, smokers and nonsmokers were selected as per the criteria established by the Centre for Disease Control and Prevention.[10] Smokers were considered as “current smokers” if they had smoked 100 or more cigarettes over their lifetime and smokers at the time of interview. Nonsmokers were the ones who had not smoked 100 or more cigarettes in their lifetime.
Figure 1.
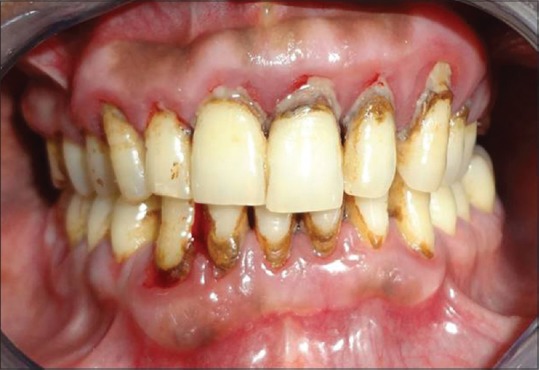
Chronic generalized periodontitis
Figure 2.
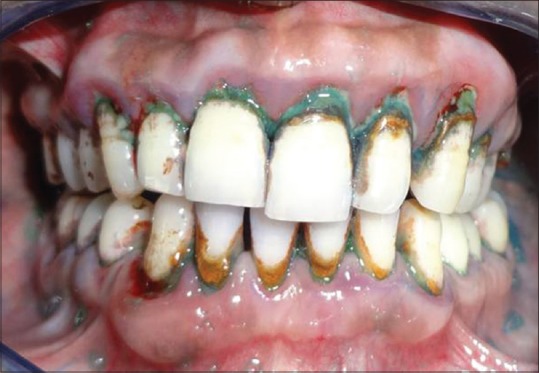
Chronic generalized periodontitis highlighted with disclosing solution
The inclusion criteria for the study were:
Patients who were willing to be a part of the study
Male patients within age group of 30–65 years
Patients with no other systemically illness
Patients with body mass index within 18–28 kg/m2
Previously periodontally untreated patients
Presence of at least 20 teeth and
Patients with moderate to severe chronic generalized periodontitis including ≥30 % sites with probing depth (PD) ≥ 4 mm [Figure 3] and clinical attachment loss (CAL) ≥ 3 mm [Figure 4] (as per AAP criteria, 1999).
Figure 3.
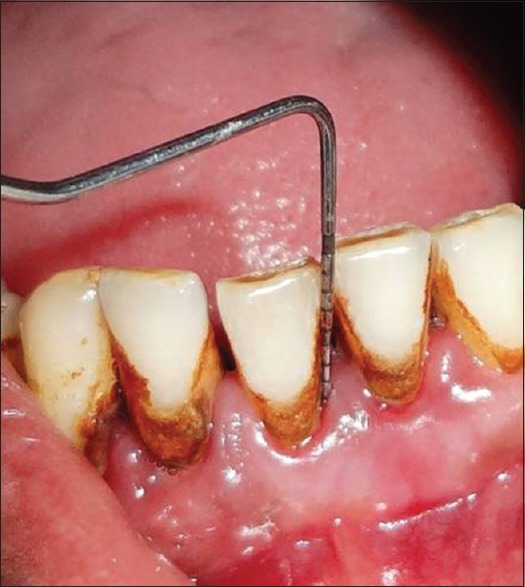
Measurement of probing depth
Figure 4.
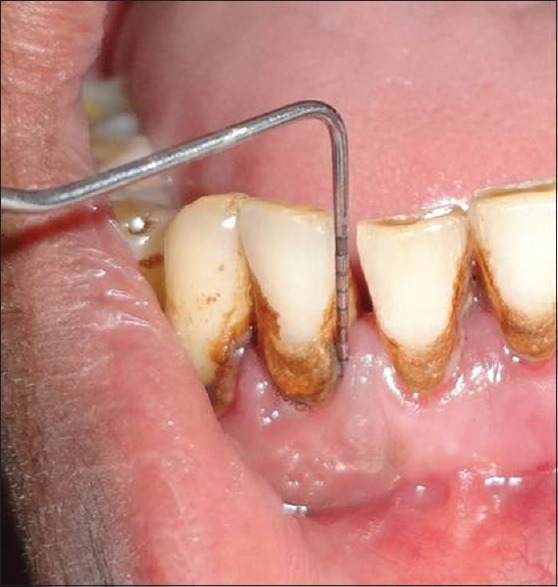
Measurement of clinical attachment level
The exclusion criteria for the study were:
Patients who were not willing to be a part of the study
Chronically alcoholic patients
Patients with history of use of vitamin or iron supplementation within last 6 months
Patients on anti-inflammatory or antimicrobial therapy within last 6 months
Patients with a history of trauma and/or recent tooth extractions within last 6 months.
A written informed consent was obtained from all. A detailed case history was then taken followed by a complete clinical examination. The following clinical parameters were recorded:
Gingival index (GI), (Loe and Silness, 1963)
Plaque index (PI), (Silness and Loe, 1964)
BOP, (Ainamo and Bay, 1975)
PD, (at six sites of teeth with UNC15 probe)
CAL, (CEJ-Bottom of sulcus)
Percentage sites of pockets with CAL ≥ 3 mm and PD ≥ 4 mm (as per AAP criteria, 1999).
Hematological analysis
Under aseptic conditions, 5 ml of venous blood sample was obtained between 8.30 and 11.00 am by venipuncture in the antecubital fossa [Figure 5] using 5 ml syringe. It was divided equally into two bulbs-one containing ethylenediaminetetraacetic acid (EDTA) and the other, a plain bulb [Figure 6]. The EDTA bulbs were then transported to the clinical laboratory for complete hemogram analysis. Plain tubes were immediately put on ice, centrifuged [Figure 7], and serum isolated [Figure 8] within 2 h. Serum samples were stored in Eppendorf tubes [Figure 9] in a storage vial box [Figure 10] containing a dry ice pack, before being transferred and stored in a freezer at −20°C till the time of evaluation.
Figure 5.
Collection of blood from antecubital vein
Figure 6.
Blood being transferred in vial
Figure 7.
Centrifuged blood in vial
Figure 8.
Separation of serum being carried out
Figure 9.
Separated serum in Eppendorf tube
Figure 10.

All serum samples in storage box
Following parameters were assessed with the help of a standardized procedure:
Complete hemogram:
Hemoglobin concentration (Hb %)
Erythrocyte count (red blood cell [RBC] count)
Hematocrit value (packed cell volume [PCV])
ESR
Mean corpuscular volume (MCV)
Mean corpuscular hemoglobin (MCH) and
MCH concentration (MCHC).
Sera assessment for IL-6 was done with the help of Diaclone Elisa Kit in the Department of Biochemistry [Figures 11–17].
Figure 11.

Microplate washer
Figure 17.
Readings on microplate reader
Figure 12.

Microplate reader
Figure 13.
Coated walls for interleukin-6
Figure 14.
Samples added to coated walls for interleukin-6
Figure 15.
Samples during incubation
Figure 16.
Samples after incubation and completion of procedure
Results
The results of the study were based on the comparison of the clinical, hematological, and systemic inflammatory markers in smokers and nonsmokers with chronic periodontitis. All the measurements were subjected to statistical analysis with the help of SPSS version 19 software.
Statistical analysis used
Z-test was used to compare the PD, CAL, hematological parameter, and IL-6 values between Group A and Group B. Mann–Whitney U-test was used to compare GI, PI, and BOP between Group A and Group B.
Clinical results
Mean full mouth gingival score: Mean gingival score in smokers was 2.29 ± 0.24 and in nonsmokers was 2.09 ± 0.12. Comparison between smokers and nonsmokers showed statistically significant results as seen in Table 1 (P < 0.0001).
Table 1.
Comparison of GI, BOP, and PI in smoker and nonsmoker groups
Mean full mouth bleeding on probing score
Mean BOP score in smokers was 0.88 ± 0.06 and in nonsmokers was 0.87 ± 0.07. Comparison between these two groups showed a P > 0.05 which was statistically not significant [Table 1].
Mean full mouth plaque score
Mean plaque score in smokers was 2.18 ± 0.18 and in non-smokers was 1.18 ± 0.14. Comparison between these two groups showed a P < 0.0001 which was statistically significant [Table 1].
Mean full mouth PD score: Mean PD score in smokers was 5.99 ± 0.33 and in nonsmokers was 4.98 ± 0.35. Comparison between these two groups showed a P < 0.0001 which was statistically significant [Table 2].
Table 2.
Comparison of PD and CAL in smoker and nonsmoker groups
Mean full mouth clinical attachment level score
Mean clinical attachment level (CAL) score in smokers was 8.41 ± 0.35 and in nonsmokers was 7.02 ± 0.52. Comparison between these two groups showed a P < 0.0001 which was statistically significant [Table 2].
Percentage of sites with pockets with PD ≥ 4 mm (as per AAP criteria, 1999): Percentage of sites with pockets with PD ≥ 4 mm (as per AAP criteria, 1999) was calculated for both the groups.
Percentage of sites with pockets with
probing depth (PD) ≥ 4 mm

Mean % of sites with pockets with PD ≥ 4 mm (as per AAP criteria, 1999) in smokers was 72.28 ± 7.80 and in nonsmokers was 53.46 ± 3.56. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 3].
Table 3.
Comparison of percentage of sites with PD and CAL as per AAP criteria, 1999 in smoker and nonsmoker groups
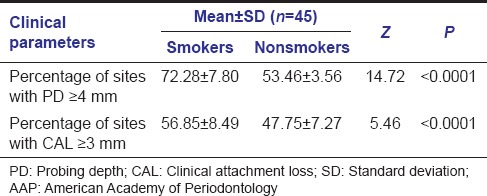
Percentage of sites with pockets with CAL ≥ 3 mm (as per AAP criteria, 1999): Percentage of sites with pockets with CAL ≥ 3 mm (as per AAP criteria, 1999) was calculated for both the groups.
Percentage of sites with pockets with
clinical attachment loss (CAL) ≥ 3 mm

Mean % of sites with pockets with CAL ≥ 3 mm (as per AAP criteria, 1999) in smokers was 56.85 ± 8.49 and in nonsmokers was 47.75 ± 7.27. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 3].
Hematological results
Mean interleukin-6 level
Mean IL-6 level in smokers was 15.47 ± 4.43 and in nonsmokers was 5.38 ± 0.49. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 4].
Table 4.
Comparison of IL-6 levels in smoker and nonsmoker groups

Mean hemoglobin level
Mean Hb level in smokers was 11.9 ± 0.91 and in nonsmokers was 13.67 ± 0.53. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 5].
Table 5.
Comparison of hematological parameters in smoker and nonsmoker groups
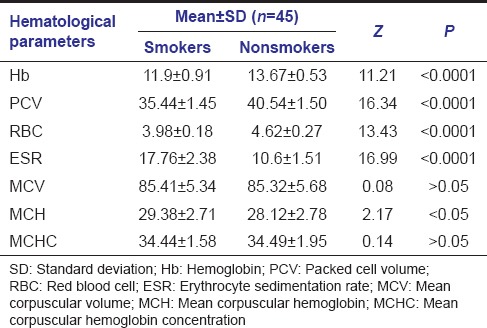
Mean packed cell volume
Mean PCV level in smokers was 35.44 ± 1.45 and in nonsmokers was 40.54 ± 1.50. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 5].
Mean red blood cell count
Mean RBC count in smokers was 3.98 ± 0.18 and in nonsmokers was 4.62 ± 0.27. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 5].
Mean erythrocyte sedimentation rate
Mean ESR level in smokers was 17.76 ± 2.38 and in nonsmokers was 10.6 ± 1.51. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant [Table 5].
Mean corpuscular volume
Mean MCV level in smokers was 85.41 ± 5.34 and in nonsmokers was 85.32 ± 5.68. Comparison between these two groups showed a P > 0.05 which was statistically insignificant [Table 5].
Mean corpuscular hemoglobin
Mean MCH level in smokers was 29.38 ± 2.71 and in nonsmokers was 28.12 ± 2.78. Comparison between these two groups showed a P < 0.05 which was statistically significant [Table 5].
Mean corpuscular hemoglobin concentration
Mean MCHC level in smokers was 34.44 ± 1.58 and in nonsmokers was 34.49 ± 1.95. Comparison between these two groups showed a P > 0.05 which was statistically insignificant [Table 5].
Discussion
Mouth is the mirror of health and disease, as an assessable model for the study of other tissues or organs and as a potential source of pathology affecting other systems and organs. The concept of periodontal diseases as localized entities affecting only the teeth and supporting apparatus has been revised, as it has been seen that rather being confined to the periodontium, periodontal diseases have wide-ranging systemic effects. Periodontal disease has a potential relationship with several other systemic conditions such as cardiovascular diseases, diabetes mellitus, adverse pregnancy outcomes, obesity, and stroke.[10,11,12,13,14,15] Substantial scientific data indicate that the localized infections characteristic of periodontitis can have a significant impact on the systemic health of both humans and animals.[3] Just as the periodontal tissues mount an immune-inflammatory response to bacteria and their products, the systemic challenge with these agents also induces a major vascular response. This host response may offer explanatory mechanisms for the interactions between periodontal infection and a variety of other potential systemic disorders.[16] One of the lesser documented associations has been the inter-relationship between periodontal disease and anemia.
ACD is an immune driven process in which cytokines result in decreased erythropoietin production, impaired proliferation of erythroid progenitor cells, and disturbed iron homeostasis.[17,18] This normocytic and normochromic anemia has been described in many chronic diseases such as rheumatoid arthritis, renal failure, bacterial and parasitic infections, and chronic periodontitis, among others. Long-standing chronic inflammation can lead to anemia. Similarly, such chronic diseases as bacterial, fungal, and parasitic infections have also been reported to show signs of anemia. Anemia seen in chronic diseases is collectively termed as “ACD.”[19,20] The association of anemia and periodontitis has been explored since the early 20th century. Earlier reports have suggested anemia to be a cause, and not a consequence, of destructive periodontitis. Lainson[21] was one of the first authors to implicate anemia as a systemic cause of periodontitis. Chawla et al.[22] suggested that anemia is an important factor in the etiology or pathogenesis of periodontal disease. Seigel[23] reported a depression in the number of erythrocytes apparently secondary to the presence of periodontal disease. Hutter et al.[7] evaluated the blood parameters in patients with chronic periodontitis and concluded that these patients show signs of anemia. The literature regarding the effect of a chronic periodontal inflammatory burden on ACD is sparse, especially in Asian countries with a high incidence of anemia.
Cigarette smoking is the major preventable risk factor in the incidence as well as a progression of periodontal disease[24,25] and also impacts red blood cells. Females were excluded as they undergo physiological blood loss and cyclic hormonal imbalance, which is responsible for an altered and exaggerated response to local factors. Gokhale et al.,[26] reported that in India, anemia is more prevalent in females due to poor nutrition, increased menstrual losses, high incidence of tropical and intestinal infections, and other miscellaneous factors. Furthermore, adiposity has well-recognized effects on the systemic host response. Therefore, in this study, the BMI measures were also analyzed. Nishida et al.,[27] suggested that the immunological disorders or inflammation might be the reason that obese smokers tend to exhibit escalating poor periodontal status relative to nonobese and nonsmoking individuals. Hence, obese patients were excluded from the study.
In the present study, mean plaque score in smokers was 2.18 ± 0.18 and in nonsmokers was 1.18 ± 0.14 which is in agreement with the studies conducted by Linden et al.,[28] Preber et al.,[29] and Zambon et al.,[30] who interpreted the effect of cigarette smoking on the periodontium to be indirect and due to inadequate levels of oral hygiene and increased plaque accumulation among smokers relative to nonsmokers. The mean gingival score in smokers was 2.29 ± 0.24 and in nonsmokers was 2.09 ± 0.12 which was again in accordance with the study conducted by Haber et al.[31] The statistically insignificant mean BOP score in smokers of 0.88 ± 0.06 and in nonsmokers of 0.87 ± 0.07 was congruent with the reports regarding the disputed effects of nicotine on blood flow with some observers claiming a reduced[32] while a few others, an increased[33] or largely unchanged[34] blood flow. Mean PD score in smokers came out to be 5.99 ± 0.33 while in nonsmokers, came out to be 4.98 ± 0.35. Comparison between these two groups showed a P < 0.0001 which was statistically significant. Mean CAL score also came out to be statistically significant with values of 8.41 ± 0.35 in smokers while 7.02 ± 0.52 in nonsmokers. In the present study, PD and CAL values were higher in smokers than in nonsmokers in accordance with the previous literature.[28,30,35,36,37,38] A significant positive correlation has been shown between smoking and CAL values.[35,39] The reason of increased PD and CAL values in smokers may depend on the accumulation of dental plaque and poor oral hygiene.[29,30]
Mean % of sites with pockets with PD ≥ 4 mm (as per AAP criteria, 1999) in smokers was 72.28 ± 7.80 and in nonsmokers was 53.46 ± 3.56 while the mean % of sites with pockets with CAL ≥ 3 mm (as per AAP criteria, 1999) in smokers was 56.85 ± 8.49 and in nonsmokers was 47.75 ± 7.27. Comparison between these two groups showed a P < 0.0001 which was statistically highly significant. These findings were in support of smoking's role in increased severity of periodontal disease.[24,25]
Smoking affects the immune system and microflora of the patient leading to deeper PDs[40,41] and greater clinical attachment[42] and bone loss.[43,44] Neutrophil functions such as phagocytosis,[45] superoxide production,[46] and protease inhibitor production[47] are hampered by exposure to nicotine. In addition, tobacco products may modify the production of cytokines. Smoking has a greater impact on the release of cytokines from neutrophils than periodontal disease. Smoking also affects erythrocytes and other blood parameters.[48] According to a study by Erdemir et al.,[49] smokers with chronic periodontitis had a lower number of erythrocytes, a lower value of Hb, and lower hematocrit and iron compared to nonsmokers with chronic periodontitis. Our findings supported this observation of the previous studies as indicated by the lower mean Hb level in smokers of 11.9 ± 0.91 and in nonsmokers of 13.67 ± 0.53. Similarly, the mean RBC count in smokers came out to be 3.98 ± 0.18 while in nonsmokers came out to be 4.62 ± 0.27. Mean PCV level in smokers was 35.44 ± 1.45 and in nonsmokers was 40.54 ± 1.50, while mean MCV level in smokers was 85.41 ± 5.34 and in nonsmokers was 85.32 ± 5.68. A mean MCH level in smokers of 29.38 ± 2.71 and in nonsmokers of 28.12 ± 2.78 while a mean MCHC level in smokers of 34.44 ± 1.58 and in nonsmokers of 34.49 ± 1.95 were also in agreement with the symptoms of ACD. MCV levels are the main determinants of the some kinds of anemia. A depressed level of MCV (microcytosis) relates anemia to iron deficiency while an elevated level of MCV (macrocytosis) relates anemia to vitamin deficiency.[50,51] In our study, MCV levels were between the reference values, as mostly seen in ACD and called as normocytosis.
Tobacco components may also modify the production of cytokines or inflammatory mediators. An imbalance in cytokine production seems to occur in smokers. Elevated concentrations of IL-6 as observed in the present study with a mean IL-6 level in smokers of 15.47 ± 4.43 while in nonsmokers of 5.38 ± 0.49 were in accordance with the previous studies which revealed an elevated IL-6 level in the plasma of smokers,[51] as well as in the alveolar cells of healthy donors stimulated by tobacco glycoprotein.[47] Nicotine also, one of the most deleterious products of cigarette, has been shown to increase the release of IL-6 by cultured murine osteoblasts.[52,53] Giannopoulou et al.[54] indicated that smoking interferes with cytokine production. It has also been reported that the release of cytokines from peripheral neutrophils and various parameters of inflammation in plasma seem to be affected more by cigarette smoking than periodontal disease.[55]
It has been proposed that hepcidin is a primary factor in the pathogenesis of the ACD, a cytokine-mediated anemia commonly encountered in clinical practice and characterized by hypoferremia with adequate reticuloendothelial iron stores.[56] Previous studies indicated that IL-6 mediates an increase in hepcidin levels and consequent hypoferremia during inflammation.[57] This also suggested that hepcidin could be the pathogenic mediator of ACD. Kemna et al.[58] showed the importance of IL-6-hepcidin axis in the development of hypoferremia in inflammation and highlighted the rapid responsiveness of this iron regulatory system. Nemeth et al.[59] found that patients with ACD due to inflammatory disorders or infections had markedly increased excretion of urinary hepcidin. In vitro stimulation of fresh human hepatocytes with a panel of cytokines showed strong induction of hepcidin mRNA by IL-6, but not by TNF-α and other potential cytokines, indicating that IL-6 may be the mediator of hepcidin induction by inflammation. Although there are not many studies about the relationship between hepcidin and periodontal diseases or the effect of smoking on hepcidin, it is well-known that pro-inflammatory cytokines and mediators are significantly elevated with gingival inflammation during the destructive phase of periodontitis.[60,61,62,63,64,65]
To brief, our findings imply that smokers with chronic periodontitis manifest an increased systemic inflammatory burden as evidenced by higher IL-6 levels when compared to nonsmoker subjects with chronic periodontitis owing to dual effects of smoking and periodontal inflammation on the cytokine response. This is congruent with hematological parameters suggestive of ACD in this group. Taken together, the present study demonstrates a positive association of concurrent periodontal disease and smoking status on ACD. Future studies are needed to verify the causal link by use of larger sample sizes, prospective study designs, and investigation of hepcidin levels. Potential drawbacks of the study were that no female patients were included in the study; all parameters were recorded at baseline with no evaluation of change in level was done post periodontal therapy and that the data obtained were specific to individuals suffering from chronic generalized periodontitis and were not compared with healthy individuals.
Conclusion
The following conclusions were arrived at from this study:
Smoking increases severity of periodontal disease
Chronic generalized periodontitis being a long standing infection resulted in greater depression in the values of hematological parameters. The reduction of values was more for smokers than for nonsmokers
Highly significant values were observed with respect to Hb, PCV, RBC, ESR, IL-6, PD, CAL, GI, PI, percentage of PD, and percentage of CAL in the smokers with chronic generalized periodontitis than the nonsmokers
Statistically significant changes in the values of MCH while nonsignificant changes in the values of MCV and MCHC indicated that the decreased red cell counts were not due to any vitamin or mineral deficiency, but secondary to the inflammatory changes induced by periodontal disease
No significant changes were observed with respect to BOP.
The emerging field of periodontal medicine offers new insights into the concept of the oral cavity as the different systems in the body are interconnected with each other. If this notion is found to be accurate, we need to assume a larger responsibility apart from diagnosing and treating the periodontal infections, and also educate the public about the importance of oral health in the overall systemic well-being of the individual. With the resurgence of emphasis on significance of oral diseases related to systemic health, the medical professionals also need to familiarize themselves with the oral cavity and the oral-systemic inter-relationships to treat or reduce the morbidity of the underlying medical condition. Furthermore, the oral health care professionals must reach out to the medical community and the general public to improve patient care through education and communication about the oral health-systemic health link.
Acknowledgment
To all the patients who contributed in the study without whom this study would not have been feasible.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–76. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 2.Nibali L, D’Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: A case-control study. J Clin Periodontol. 2007;34:931–7. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 3.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 4.Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontitis. Periodontol 2000. 1997;14:173–201. doi: 10.1111/j.1600-0757.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee GR. The anemia of chronic disease. Semin Hematol. 1983;20:61–80. [PubMed] [Google Scholar]
- 6.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 7.Hutter JW, van der Velden U, Varoufaki A, Huffels RA, Hoek FJ, Loos BG. Lower numbers of erythrocytes and lower levels of hemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol. 2001;28:930–6. doi: 10.1034/j.1600-051x.2001.028010930.x. [DOI] [PubMed] [Google Scholar]
- 8.Ejeil AL, Gaultier F, Igondjo-Tchen S, Senni K, Pellat B, Godeau G, et al. Are cytokines linked to collagen breakdown during periodontal disease progression? J Periodontol. 2003;74:196–201. doi: 10.1902/jop.2003.74.2.196. [DOI] [PubMed] [Google Scholar]
- 9.Graves DT. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999;28:482–90. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 10.Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: An overview. Ann Periodontol. 2001;6:91–8. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: A state-of-the-science review. Ann Periodontol. 2001;6:9–15. doi: 10.1902/annals.2001.6.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C. Poor oral health is associated with coronary heart disease and elevated systemic inflammatory and haemostatic factors. J Clin Periodontol. 2004;31:25–9. doi: 10.1111/j.0303-6979.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 13.Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. 2004;83:151–5. doi: 10.1177/154405910408300213. [DOI] [PubMed] [Google Scholar]
- 14.Agueda A, Ramón JM, Manau C, Guerrero A, Echeverría JJ. Periodontal disease as a risk factor for adverse pregnancy outcomes: A prospective cohort study. J Clin Periodontol. 2008;35:16–22. doi: 10.1111/j.1600-051X.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- 15.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Mealey BL, Klokkevold PR. Periodontal medicine. In: Newman MG, Takei HH, editors. Carranza's Clinical Periodontology. Philadelphia: Saunders; 2002. p. 232. [Google Scholar]
- 17.Yamamoto T, Tsuneishi M, Furuta M, Ekuni D, Morita M, Hirata Y. Relationship between decrease of erythrocyte count and progression of periodontal disease in a rural Japanese population. J Periodontol. 2011;82:106–13. doi: 10.1902/jop.2010.100211. [DOI] [PubMed] [Google Scholar]
- 18.Nissenson AR, Goodnough LT, Dubois RW. Anemia: Not just an innocent bystander? Arch Intern Med. 2003;163:1400–4. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 19.Barrett-Connor E. Anemia and infection. Am J Med. 1972;52:242–53. doi: 10.1016/0002-9343(72)90073-3. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs D, Hausen A, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, et al. Immune activation and the anaemia associated with chronic inflammatory disorders. Eur J Haematol. 1991;46:65–70. doi: 10.1111/j.1600-0609.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 21.Lainson PA, Brady PP, Fraleigh CM. Anemia, a systemic cause of periodontal disease? J Periodontol. 1968;39:35–8. doi: 10.1902/jop.1968.39.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Chawla TN, Kapoor KK, Teotia SP, Singh NK. Anaemia and periodontal disease – A correlative study. J Indian Dent Assoc. 1971;43:67–78. [PubMed] [Google Scholar]
- 23.Seigel EH. Total erythrocyte, leucocyte and differential white cell counts of blood in chronic periodontal disease. J Dent Res. 1945;24:270. [PubMed] [Google Scholar]
- 24.Bergström J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65:545–50. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 25.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale SR, Sumanth S, Padhye AM. Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia. J Periodontol. 2010;81:1202–6. doi: 10.1902/jop.2010.100079. [DOI] [PubMed] [Google Scholar]
- 27.Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, et al. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. J Periodontol. 2005;76:923–8. doi: 10.1902/jop.2005.76.6.923. [DOI] [PubMed] [Google Scholar]
- 28.Linden GJ, Mullally BH. Cigarette smoking and periodontal destruction in young adults. J Periodontol. 1994;65:718–23. doi: 10.1902/jop.1994.65.7.718. [DOI] [PubMed] [Google Scholar]
- 29.Preber H, Kant T, Bergström J. Cigarette smoking, oral hygiene and periodontal health in Swedish army conscripts. J Clin Periodontol. 1980;7:106–13. doi: 10.1111/j.1600-051x.1980.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 30.Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67:1050–4. doi: 10.1902/jop.1996.67.10s.1050. [DOI] [PubMed] [Google Scholar]
- 31.Haber J, Wattles J, Crowley M, Mandell R, Joshipura K, Kent RL. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. 1993;64:16–23. doi: 10.1902/jop.1993.64.1.16. [DOI] [PubMed] [Google Scholar]
- 32.Clarke NG, Carey SE. Etiology of chronic periodontal disease: An alternative perspective. J Am Dent Assoc. 1985;110:689–91. doi: 10.14219/jada.archive.1985.0398. [DOI] [PubMed] [Google Scholar]
- 33.Baab DA, Oberg PA. The effect of cigarette smoking on gingival blood flow in humans. J Clin Periodontol. 1987;14:418–24. doi: 10.1111/j.1600-051x.1987.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 34.Meekin TN, Wilson RF, Scott DA, Ide M, Palmer RM. Laser Doppler flowmeter measurement of relative gingival and forehead skin blood flow in light and heavy smokers during and after smoking. J Clin Periodontol. 2000;27:236–42. doi: 10.1034/j.1600-051x.2000.027004236.x. [DOI] [PubMed] [Google Scholar]
- 35.van der Weijden GA, de Slegte C, Timmerman MF, van der Velden U. Periodontitis in smokers and non-smokers: Intra-oral distribution of pockets. J Clin Periodontol. 2001;28:955–60. doi: 10.1034/j.1600-051x.2001.028010955.x. [DOI] [PubMed] [Google Scholar]
- 36.Machuca G, Rosales I, Lacalle JR, Machuca C, Bullón P. Effect of cigarette smoking on periodontal status of healthy young adults. J Periodontol. 2000;71:73–8. doi: 10.1902/jop.2000.71.1.73. [DOI] [PubMed] [Google Scholar]
- 37.Feldman RS, Bravacos JS, Rose CL. Association between smoking different tobacco products and periodontal disease indexes. J Periodontol. 1983;54:481–7. doi: 10.1902/jop.1983.54.8.481. [DOI] [PubMed] [Google Scholar]
- 38.Stoltenberg JL, Osborn JB, Pihlstrom BL, Herzberg MC, Aeppli DM, Wolff LF, et al. Association between cigarette smoking, bacterial pathogens, and periodontal status. J Periodontol. 1993;64:1225–30. doi: 10.1902/jop.1993.64.12.1225. [DOI] [PubMed] [Google Scholar]
- 39.Grossi SG, Genco RJ, Machtei EE, Ho AW, Koch G, Dunford R, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66:23–9. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- 40.Haffajee AD, Socransky SS. Relationship of cigarette smoking to attachment level profiles. J Clin Periodontol. 2001;28:283–95. doi: 10.1034/j.1600-051x.2001.028004283.x. [DOI] [PubMed] [Google Scholar]
- 41.Bergström J. Tobacco smoking and risk for periodontal disease. J Clin Periodontol. 2003;30:107–13. doi: 10.1034/j.1600-051x.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 42.Axelsson P, Paulander J, Lindhe J. Relationship between smoking and dental status in 35-, 50-, 65-, and 75-year-old individuals. J Clin Periodontol. 1998;25:297–305. doi: 10.1111/j.1600-051x.1998.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 43.Bergström J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71:1338–47. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 44.MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992;63:908–13. doi: 10.1902/jop.1992.63.11.908. [DOI] [PubMed] [Google Scholar]
- 45.Ryder MI, Fujitaki R, Johnson G, Hyun W. Alterations of neutrophil oxidative burst by in vitro smoke exposure: Implications for oral and systemic diseases. Ann Periodontol. 1998;3:76–87. doi: 10.1902/annals.1998.3.1.76. [DOI] [PubMed] [Google Scholar]
- 46.Persson L, Bergström J, Ito H, Gustafsson A. Tobacco smoking and neutrophil activity in patients with periodontal disease. J Periodontol. 2001;72:90–5. doi: 10.1902/jop.2001.72.1.90. [DOI] [PubMed] [Google Scholar]
- 47.Tappia PS, Troughton KL, Langley-Evans SC, Grimble RF. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci (Lond) 1995;88:485–9. doi: 10.1042/cs0880485. [DOI] [PubMed] [Google Scholar]
- 48.Ernst E. Haemorheological consequences of chronic cigarette smoking. J Cardiovasc Risk. 1995;2:435–9. doi: 10.1177/174182679500200508. [DOI] [PubMed] [Google Scholar]
- 49.Erdemir EO, Nalcaci R, Caglayan O. Evaluation of systemic markers related to anemia of chronic disease in the peripheral blood of smokers and non-smokers with chronic periodontitis. Eur J Dent. 2008;2:102–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Ward PC. Investigation of macrocytic anemia. Postgrad Med. 1979;65:203–7. doi: 10.1080/00325481.1979.11715063. 209, 212. [DOI] [PubMed] [Google Scholar]
- 51.Samson D. The anaemia of chronic disorders. Postgrad Med J. 1983;59:543–50. doi: 10.1136/pgmj.59.695.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francus T, Romano PM, Manzo G, Fonacier L, Arango N, Szabo P. IL-1, IL-6, and PDGF mRNA expression in alveolar cells following stimulation with a tobacco-derived antigen. Cell Immunol. 1992;145:156–74. doi: 10.1016/0008-8749(92)90320-o. [DOI] [PubMed] [Google Scholar]
- 53.El-Ghorab N, Marzec N, Genco R, Dziak R. Effect of nicotine and estrogen on IL-6 release from osteoblasts. J Dent Res. 1997;76:341. [Google Scholar]
- 54.Giannopoulou C, Cappuyns I, Mombelli A. Effect of smoking on gingival crevicular fluid cytokine profile during experimental gingivitis. J Clin Periodontol. 2003;30:996–1002. doi: 10.1034/j.1600-051x.2003.00416.x. [DOI] [PubMed] [Google Scholar]
- 55.Fredriksson M, Bergström K, Asman B. IL-8 and TNF-alpha from peripheral neutrophils and acute-phase proteins in periodontitis. J Clin Periodontol. 2002;29:123–8. doi: 10.1034/j.1600-051x.2002.290206.x. [DOI] [PubMed] [Google Scholar]
- 56.Means RT. Hepcidin and cytokines in anaemia. Hematology. 2004;9:357–62. doi: 10.1080/10245330400018540. [DOI] [PubMed] [Google Scholar]
- 57.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–6. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 59.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 60.Kinane DF, Adonogianaki E, Moughal N, Winstanley FP, Mooney J, Thornhill M. Immunocytochemical characterization of cellular infiltrate, related endothelial changes and determination of GCF acute-phase proteins during human experimental gingivitis. J Periodontal Res. 1991;26:286–8. doi: 10.1111/j.1600-0765.1991.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 61.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–42. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 62.Ranney RR. Immunologic mechanisms of pathogenesis in periodontal diseases: An assessment. J Periodontal Res. 1991;26:243–54. doi: 10.1111/j.1600-0765.1991.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 63.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64:456–60. [PubMed] [Google Scholar]
- 64.Ebersole JL, Singer RE, Steffensen B, Filloon T, Kornman KS. Inflammatory mediators and immunoglobulins in GCF from healthy, gingivitis and periodontitis sites. J Periodontal Res. 1993;28:543–6. doi: 10.1111/j.1600-0765.1993.tb02121.x. [DOI] [PubMed] [Google Scholar]
- 65.Tonetti MS, Freiburghaus K, Lang NP, Bickel M. Detection of interleukin-8 and matrix metalloproteinases transcripts in healthy and diseased gingival biopsies by RNA/PCR. J Periodontal Res. 1993;28:511–3. doi: 10.1111/j.1600-0765.1993.tb02114.x. [DOI] [PubMed] [Google Scholar]


