Abstract
Background:
Today, most approaches to caries viewed that catastrophic change in normal plaque biofilm is responsible for the disease. The behavior and composition of the biofilm are a reflection of the oral environment; the caries is a reflection of adverse changes occurring in that environment. Thus, it is important to identify the pathogenicity of the plaque biofilm so as to predict the caries risk. The recently developed three-tone plaque disclosing agent was used to test its ability in identifying the pathogenicity of plaque.
Aim:
To assess the efficacy of three-tone plaque disclosing agent in identifying the plaque pathogenicity and correlate with the clinical caries status and microbiological findings.
Materials and Methods:
Sixty children of 6–13 years age group of both sexes were clinically examined for caries and plaque scores, and then disclosing agent was applied; the color stained plaque samples were collected and cultured for microbiological assessment, and the data were analyzed based on the caries status of the children.
Results:
There was a significant difference between the pathological plaque of caries active and caries free group (P < 0.05). The pathological plaque scores and the total colony counts, Streptococcus counts and mutans streptococci counts increased with the increase in caries.
Conclusion:
Three-tone plaque disclosing agent was effective in identifying pathological plaque and can be used as one of the chairside adjuvants in caries risk assessment.
Keywords: Caries microflora, caries risk, three-tone plaque disclosing agent
Introduction
Caries risk assessment is the first step in caries management. There are now multiple lines of incidence which indicate that dental caries is a multipathogenic disease and mutans streptococci are no longer regarded as sole or necessarily dominant pathogens in dental caries. The risk of dental caries at any one point of time may be low, moderate, or high, but it is never zero.[1] It suggests that the assessment of dental plaque biofilm should be based on parameters such as acid production by fermentation under conditions of substrate challenge[2,3] and perhaps by bacterial growth and survival under conditions of low pH (ecological plaque hypothesis). The behavior and composition of the biofilm are a reflection of the oral environment, and the caries is a reflection of the adverse changes occurring in that environment. Thus, it is important to identify the pathogenicity of the plaque biofilm so as to predict the caries risk.
The new technology available to achieve this diagnostic objective, literally a visible Stephan's curve was the first generation “GC Plaque Check + pH” fermentation test, which was developed based on the association between caries activity and the production of strong acids from plaque in response to sucrose. The recent development to this was the second generation technology (GC Tri Plaque ID Gel™) test that takes the same concept of acid production but incorporates the assessment of plaque ecology at multiple sites simultaneously rather than separate steps. It relies on the pH selective response of different dyes (rose Bengal, brilliant blue, FCF) included in a glucose containing disclosing liquid, to show intraorally both the plaque age and plaque acid production following a substrate challenge within 2 min. In case of new plaque, the plaque biofilm is sparse, and the blue pigment is easily washed off and this gives the new plaque a pink/red color. Old plaque (> 48 h plaque) biofilm is matured and its structure is dense, so both the blue and red pigments are trapped and it gives it a blue/purple color. In case of extra high-risk plaque, the sucrose in three-tone plaque disclosing gel (GC Tri Plaque ID Gel™) will be metabolized by acidogenic bacteria within the plaque biofilm. The resulting acid produced lowers the plaque pH (< pH 4.5) and this makes the red pigment disappear and gives it a light blue color.
Considering these facts, the present study was done to assess the efficacy of the recently developed three-tone plaque disclosing agent in identifying plaque pathogenicity and to correlate it with clinical and microbiological findings, with an objective of its application as a chair side adjuvant to predict the caries risk.
Materials and Methods
Sixty children aged 6–13 years of both genders registered for dental care in the Department of Pediatric Dentistry were randomly selected for this study. Children with good health without systemic diseases who had not used mouth rinses or any medication for the past 6 months that might have influenced their oral hygiene condition, children without any orthodontic or prosthetic appliances which may have the ability to modify surface characteristics, Children with no history of oral habits and who were willing to participate with informed parental consent were included in the study.[4]
Study design
All human subject protocols, consent forms, and specimen collections were reviewed and approved by the Institutional Review Board. Caries experience was assessed with DMFT and deft indices separately, and the average was calculated. Plaque index score was first completed for each participant without the use of disclosing solution, using Silness and Loe plaque index (PII).[5] Materials and instruments used in the study are illustrated in Figure 1.
Figure 1.
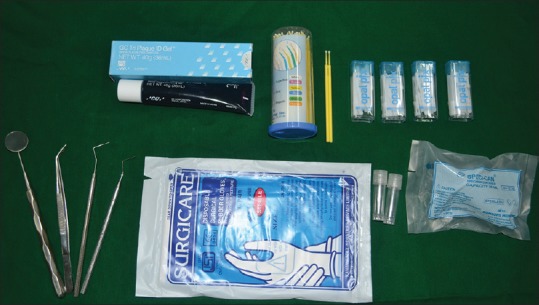
Materials and instruments used in the study
Assessment of plaque pathogenicity with three-tone plaque disclosing gel
After completing visual plaque scoring the three-tone disclosing gel (GC Tri Plaque ID Gel™) was applied [Figure 2] with a micro brush on all the tooth surfaces and left undisturbed for 2 min; with the help of water spray and high volume suction, the tooth surfaces are then gently rinsed for 30 s and the plaque color changes were then observed [Figure 3]. The color stained plaque was scored separately.
Figure 2.
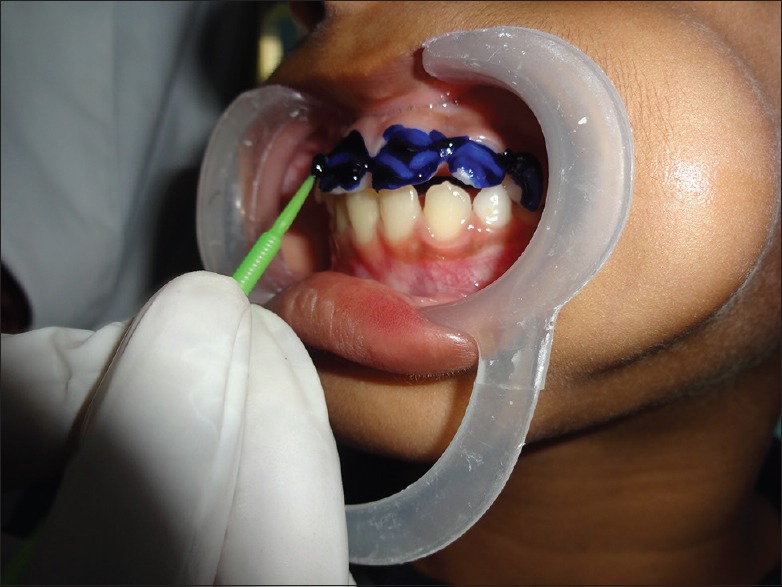
Application of the disclosing agent
Figure 3.
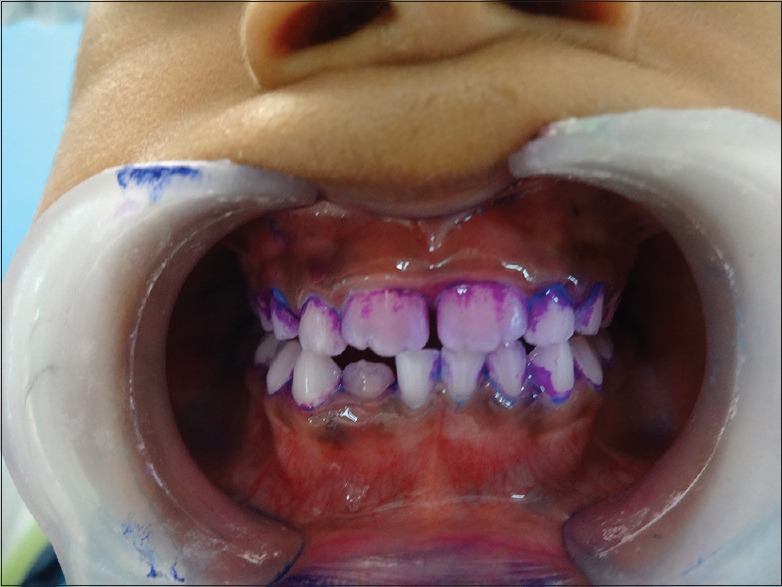
Observed color changes
Plaque specimens collected from specific teeth
Each colored plaque sample was collected from all children with the help of sterile disposable picks (Opalpix, Ultra Dent Products, Inc., South Jordan, and Utah). Samples were collected from one of the available teeth; sites based on the plaque abundance, maxillary right first molar, mandibular left molar, maxillary incisor or mandibular incisor, mandibular left premolar or second primary molar were selected as they represent all four quadrants of the mouth. The collection sites were chosen from the first preference site to the next available preferred site. The collection was conducted by a sweeping action across the entire chosen tooth surface and was not placed subgingivaly. Each sample picked was then placed into sterile containers that had an anonymous coding and were sealed for transport to the laboratory.
Culture methods and microbiological identification of plaque bacteria
Each plaque specimen was suspended in one ml of phosphate buffered saline (PBS) with the addition of glass beads, and dispersed by vigorous agitation on a rocker platform (37°C for 5 min). Dispersed plaque samples were subjected to 10-fold serial dilutions in PBS and then plated on enriched blood agar for total colony count, mitis salivarius agar for Streptococcus count and mitis salivarius bacitracin agar for mutans streptococci counts. The agar plates were incubated at 37°C in a candle jar environment for 48 h. After 2 days, the number of microorganisms were counted and recorded, and the results were tabulated.
Confirmatory tests
The organisms were confirmed using smear examination – grams staining and biochemical tests (starch, sorbitol, mannitol, inorbital, trehalose).
Based on the child's caries status, the data were analyzed under the following groups:[6]
Group I: One to two caries lesions (n = 15)
Group II: Three to five caries lesions (n = 15)
Group III: More than five caries lesions (n = 15)
Group IV: Caries free group (n = 15) (control group).
Results
Tables 1 and 2 show the mean plaque scores and the caries status of the study population, respectively. The study results show that [Table 3 - one-way ANOVA analysis] pathological (blue) plaque scores were significantly higher in Group II and Group III (P = 0.000) compared new (pink) and mature (purple) plaque scores. Whereas new (pink) plaque scores were significantly higher than pathological (blue) plaque score in Group I and Group IV (P = 0.002) (P = 0.000).
Table 1.
Plaque scores of study population
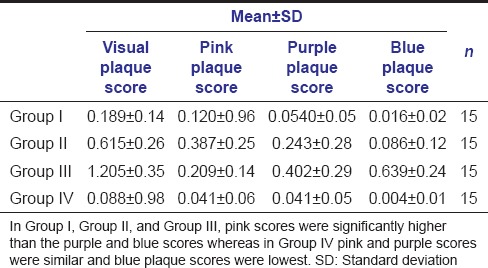
Table 2.
Caries status of study population
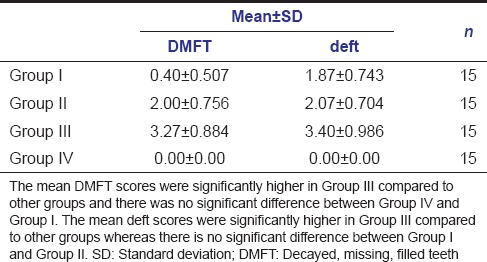
Table 3.
Plaque pathogenicity among the study groups
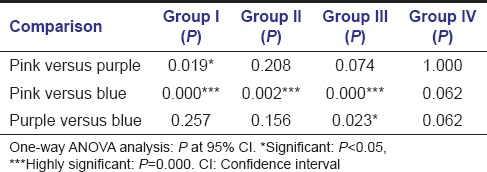
Table 4 shows the distribution of mean plaque scores and microbiological status of the study groups; as caries score increases, there was increase in the mature (purple) and pathological plaque scores (blue), total colony forming units, Streptococcus counts and mutans streptococci counts from Group IV to Group III.
Table 4.
Association between caries, plaque and microbial status of the study groups
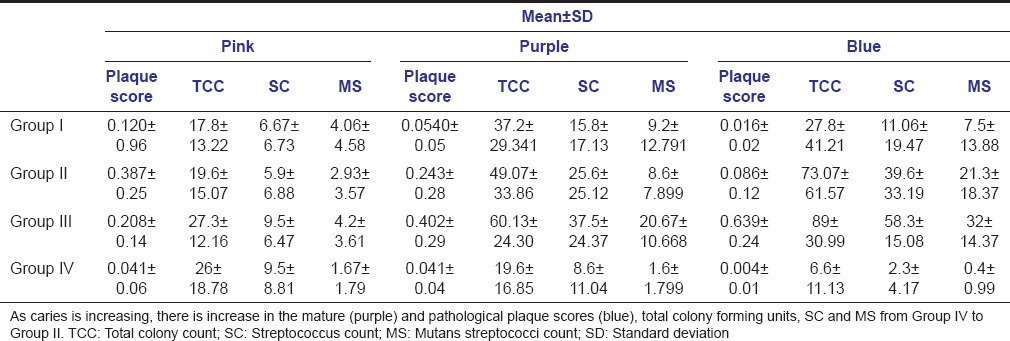
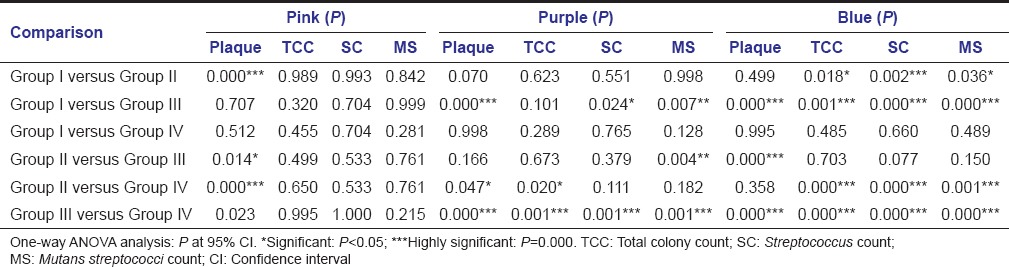
There was no significant difference in Streptococcus counts and mutans streptococci counts between new (pink) and pathological (blue) plaque samples in Group I and Group IV (caries free group) whereas in Group II and Group III Streptococcus counts and mutans streptococci counts were significantly higher in pathological (blue) plaque samples compared to new (pink) and mature (purple) plaque samples [Table 5 - one-way ANOVA analysis].
Table 5.
Comparison of plaque pathogenicity and microbial status (TCC, SC, and MS counts) among the study groups
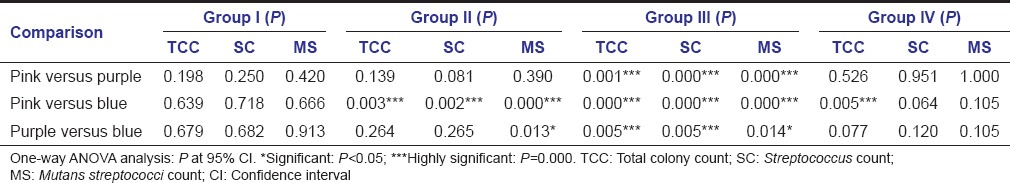
There was significant increase in plaque pathogenicity, Streptococcus counts and mutans streptococci counts in high caries group (Group III and Group II) compared to low caries group (Group I) and caries free group (Group IV) [Table 6 - one-way ANOVA analysis].
Table 6.
Comparison of plaque pathogenicity and microbial status (TCC, SC, and MS counts) between the study groups
Discussion
Caries is recognized as a complex disease, and so it is necessary to redefine the method of diagnosis and treatment planning in an effective and efficient manner. Effective prevention can be achieved only by long-term sustainable changes to the oral environment. Hence, it is important to identify the risk associated environment and the changes taking place in it from time to time.[7]
The risk factors associated are visible bacterial plaque and biofilm behavior (plaque thickness, maturity, acid production and levels of mutans streptococci), presence of active caries and microbial loads in each child, all of which have influence on the oral environment that is plaque biofilm as explained by ecological plaque hypothesis.[2,3] The demineralization/remineralization cycle can be modified by manipulating the physical, ionic, and metabolic factors that modulate the properties of the biofilm and thus provide a powerful approach to caries prevention and control. A number of clinical chairside tools have been developed to assess the caries risk such as adenosine triphosphate driven bioluminescence system,[4] saliva check, plaque check and traffic light matrix,[8] wheel of misfortune,[9] caries screen, pH meter,[10] and radioactive sucrose.[11] Despite their ready availability, tests in the form of commercial kits are still expensive; no one test is an adequate predictor of caries risk, and the specificity and sensitivity of the tests are not reliably diagnostic for an individual. Hence, the entire concept of what plaque is and what it does to the tissues must be made vital and important to the patient by visualization as it never fails to generate in patients a sense of motivation that results in a concern for its removal. Now with the advent of disclosing agents, the concept of visualization is made possible. These agents have been used since the early 20th century with the introduction of iodine by Skinner in 1914, various organic solutions by Berwick in 1920, Easlick in 1935, Merchurome solutions by Raybin in 1945, and King in 1951, and the recent plaque disclosing agents are based upon approved food colorants like basic fuchsin and erythrosine and their variants, fluorescein disclosing agents (plak check), and plaque probe. In the present study, the three-tone disclosing agent (GC Tri Plaque ID Gel™) was used which identifies both the location and the type of plaque.
The present study was undertaken to assess the efficacy of three-tone plaque disclosing gel, and the study sample was divided according to the severity of the caries to assess the effectiveness of three-tone plaque disclosing gel in identifying the pathogenicity of the plaque.[5]
Caries was assessed using DMFT and deft indices individually according to WHO criteria as it is universally accepted and commonly used in research studies.[12] Silness and Loe plaque index (PII) was used to assess the plaque score due its ease of use. In the present study, all the colored plaque samples were collected and assessed irrespective of caries activity from either side of specified teeth and sites of each child to rule out the influence of variables on results of plaque pathogenicity and as they reflect the variations in plaque fermentation. Teeth such as the maxillary right first molar and mandibular left molar were chosen because of their difficulty to brush for most patients, and also because of the close proximity to a salivary duct for one tooth and the distance away from the duct of the other tooth. A maxillary incisor was chosen because of its susceptibility to show enamel demineralization and significant plaque accumulation in children. Finally, a mandibular incisor was chosen because of its close proximity to a salivary gland and the tongue's enhanced cleansing properties. Similar teeth and sites were selected in the study done by Fazilat et al.[4] but in studies done by Igarashi et al.[10] plaque samples were taken from sound enamel, incipient white spot lesions, and cavities to compare the acidogenic potential of plaque in these sites and He stated that the plaque samples from the white spot lesions showed faster decrease in pH and significantly greater quantities of acid were produced in comparison to the control plaque. Walsh and Tsang[1] recommended that sampling should include those sites most at risk for development of dental caries as plaque varies regionally in the oral cavity because of site-specific effects of saliva.
In the present study, total bacterial profile was studied by culturing on enriched blood agar. Total Streptococcus counts were assessed using mitis salivarius agar and mutans streptococci counts were identified using mitis salivarius bacitracin agar as followed in the study done by Fazilat et al.[4] In our study, both the total bacterial load and the cariogenic bacteria were evaluated as the ecological hypothesis states that caries results from a shift in the balance of resident microflora driven by modification in local environmental conditions.
The present study results showed that there was an increase in plaque scores with increase in caries scores. The association between plaque and caries has been confirmed in studies done by Alaluusua, and Malmivitra,[13] and Roeters et al.[14] and stated that visible plaque on the labial surfaces of maxillary incisors was strongly associated with caries development, and the best indicator of caries risk was visible plaque compared to other potential indicators, including the use of a nursing bottle, mother's caries prevalence, and mother's salivary level of mutans streptococci. Wendt et al.[15] also stated that children with no visible plaque at 2 years of age had greater chances of remaining caries free until 3 years of age, compared to children with visible plaque. Although in some studies supragingival plaque accumulation has not been highly correlated with caries experience,[16,17,18] studies by Lindhe et al.[19] and Poulsen et al.[20] showed that professional plaque removal could prevent caries, thus establishing dental plaque as a significant and probable risk factor for dental caries.[21]
Further, it is seen in this study [Table 1] that pink plaque scores were higher in Group IV and Group I, whereas purple and blue scores were higher in Group II and Group III suggesting that with the increase in mature (purple) and pathological plaque (blue) scores caries also increased. Similar findings were reported in studies done by Block et al.,[22] Igarashi et al.[10]
The present study results showed that as caries was increasing there was an increase in total colony forming units, Streptococcus counts and mutans streptococci counts from Group IV to Group III. This finding confirms the pathogenicity of the different colored plaques and its association with cariogenic bacteria and occurrence of caries. The study findings of Duchin and van Houte[23] showed that plaque from the caries lesions showed markedly elevated levels of Streptococcus mutans compared to plaque from sound enamel. Boyar and Bowden[24] and Milnes and Bowden[25] demonstrated that the levels of S. mutans and lactobacilli were at higher levels at sites where caries developed. Igrashi et al.[10] concluded that enhanced production of acid observed in the plaque from white spot lesions reflected the presence of highly acidogenic cariogenic bacteria such as S. mutans and lactobacilli.
Thus, the present study findings confirmed the relation between caries, plaque, and cariogenic microorganisms. With increase in caries status, there was an increase in the mature and pathological scores, and mutans streptococci counts showed the efficacy of the three-tone plaque disclosing gel in identifying the caries risk associated with plaque. However, further longitudinal studies are required to support this observation, taking into account factors like age and gender variations along with assessment of anaerobic microorganisms such as lactobacilli which is also responsible for caries progression. Since the plaque pathogenicity can be performed and observed very quickly; with three-tone disclosing gel (GC Tri Plaque ID Gel™), it can be used as part of caries susceptibility testing for sites in a child's dentition known to be prone for development of carious lesions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Walsh LJ, Tsang AK. Chairside testing for cariogenic bacteria: Current concepts and clinical strategies. Int Dent S Afr. 2008;10:50–65. [Google Scholar]
- 2.Walsh LJ. A System for Total Environmental Management (STEM) of the oral cavity, and its application to dental caries control. Int Dent S Afr. 2008;10:26–41. [Google Scholar]
- 3.Walsh LJ. Dental plaque fermentation and its role in caries risk assessment. Int Dent S Afr. 2006;1:4–13. [Google Scholar]
- 4.Fazilat S, Sauerwein R, McLeod J, Finlayson T, Adam E, Engle J, et al. Application of adenosine triphosphate-driven bioluminescence for quantification of plaque bacteria and assessment of oral hygiene in children. Pediatr Dent. 2010;32:195–204. [PubMed] [Google Scholar]
- 5.Soben P. 5th ed. Arya Medi Publishing house; 2013. Indices in dental epidemiology: Essentials of public health dentistry; pp. 422–3. [Google Scholar]
- 6.Saxena S, Pundir S, Aena J. Oratest: A new concept to test caries activity. J Indian Soc Pedod Prev Dent. 2013;31:25–8. doi: 10.4103/0970-4388.112400. [DOI] [PubMed] [Google Scholar]
- 7.Sakeenabi B, Hiremath SS. Dental caries experience and salivary Streptococcus mutans, lactobacilli scores, salivary flow rate and salivary buffering capacity among 6 year old Indian school children. J Clin Exp Dent. 2011;3:412–7. doi: 10.4103/2231-0762.97697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngo H, Gaffney S. Risk assessment in the diagnosis and management of caries. Pediatr Dent. 2007;2:61–82. [Google Scholar]
- 9.Walsh LJ. New paradigms for assessing caries risk and lesion activity. Auxillary. 2011;5:28–33. [Google Scholar]
- 10.Igarashi K, Hamada Y, Nishimaki H, Sakurai S, Kamiyama K. The acidogenic potential of plaque from sound enamel, white spot lesions, and cavities in children. Pediatr Dent. 1987;9:212–5. [PubMed] [Google Scholar]
- 11.Minah GE, Loesche WJ. Sucrose metabolism in resting-cell suspensions of caries associated and non-caries-associated dental plaque. Infect Immun. 1977;17:43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.3rd ed. Geneva, Switzerland: WHO; 1987. WHO. Oral Health Surveys: Basic Methods. [Google Scholar]
- 13.Alaluusua S, Malmivirta R. Early plaque accumulation – A sign for caries risk in young children. Community Dent Oral Epidemiol. 1994;22:273–6. doi: 10.1111/j.1600-0528.1994.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 14.Roeters J, Burgersdijk R, Truin GJ, van’t Hof M. Dental caries and its determinants in 2-to-5-year-old children. ASDC J Dent Child. 1995;62:401–8. [PubMed] [Google Scholar]
- 15.Wendt LK, Hallonsten AL, Koch G, Birkhed D. Analysis of caries-related factors in infants and toddlers living in Sweden. Acta Odontol Scand. 1996;54:131–7. doi: 10.3109/00016359609006019. [DOI] [PubMed] [Google Scholar]
- 16.Koch G, Lindhe J. The state of the gingivae and caries increment in school children during and after withdrawal of various prophylactic measures. In: McHugh WD, editor. Dental Plaque. Edinburgh, UK: Livingstone; 1970. pp. 271–81. [Google Scholar]
- 17.Franz FE, Baume LJ. Statistical correlation between oral hygiene and dental caries tested in Haitian and Hamburg children. SSO Schweiz Monatsschr Zahnheilkd. 1983;93:1183–8. [PubMed] [Google Scholar]
- 18.McHugh WD. Role of supragingival plaque in oral disease initiation and progression. In: Loe H, Kleinman DV, editors. Dental Plaque Control Measures and Oral Hygiene Practices. Washington, DC: IRL Press; 1986. pp. 1–12. [Google Scholar]
- 19.Lindhe J, Axelsson P, Tollskog G. Effect of proper oral hygiene on gingivitis and dental caries in Swedish schoolchildren. Community Dent Oral Epidemiol. 1975;3:150–5. doi: 10.1111/j.1600-0528.1975.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 20.Poulsen S, Agerbaek N, Melsen B, Korts DC, Glavind L, Rölla G. The effect of professional toothcleansing on gingivitis and dental caries in children after 1 year. Community Dent Oral Epidemiol. 1976;4:195–9. doi: 10.1111/j.1600-0528.1976.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 21.Leverett DH, Featherstone JD, Proskin HM, Adair SM, Eisenberg AD, Mundorff-Shrestha SA, et al. Caries risk assessment by a cross-sectional discrimination model. J Dent Res. 1993;72:529–37. doi: 10.1177/00220345930720021001. [DOI] [PubMed] [Google Scholar]
- 22.Block PL, Lobene RR, Derdivanis JP. A two-tone dye test for dental plaque. J Periodontol. 1972;43:423–6. doi: 10.1902/jop.1972.43.7.423. [DOI] [PubMed] [Google Scholar]
- 23.Duchin S, van Houte J. Relationship of Streptococcus mutans and lactobacilli to incipient smooth surface dental caries in man. Arch Oral Biol. 1978;23:779–86. doi: 10.1016/0003-9969(78)90155-3. [DOI] [PubMed] [Google Scholar]
- 24.Boyar RM, Bowden GH. The microflora associated with the progression of incipient carious lesions of children living in a water-fluoridated area. Caries Res. 1985;19:298–306. doi: 10.1159/000260859. [DOI] [PubMed] [Google Scholar]
- 25.Milnes AR, Bowden GH. The microflora associated with developing lesions of nursing caries. Caries Res. 1985;19:289–97. doi: 10.1159/000260858. [DOI] [PubMed] [Google Scholar]


