Abstract
Introduction
A key problem in stable coronary artery disease (CAD) is non-invasive identification of patients with severe multivessel CAD. Determination of biomarkers that have pro-inflammatory properties (C-reactive protein – hsCRP) and indicate heart muscle ischemia (high-sensitive troponin T – hsTnT) can contribute to the improvement of stratification in this regard.
The aim of the study
The aim of the study was to identify factors associated with the presence of multivessel CAD in clinically stable men.
Material and methods
The study included 92 symptomatic men (mean age 64.05 ± 9.42 years) with preserved left ventricular function, scheduled for elective coronary angiography. Patients were divided and analyzed in two groups: with multivessel coronary artery disease (2-3-vessel disease, n = 46) vs. without multivessel coronary artery disease (n = 46).
Results
Patients with multivessel CAD had significantly higher levels of hsTnT (0.01 vs. 0.007, p = 0.0021) and fasting glucose (6.0 vs. 5.45, p = 0.0112). Based on the drawn ROC curves, the cut-off points were determined for hsTnT ≥ 0.0085 ng/ml and fasting plasma glucose ≥ 5.85 mmol/l. From multivariate analysis only hsTnT in concentration higher than the cut-off point enhanced the risk of multivessel CAD (OR 4.286, 95% CI: 1.79-10.263, p = 0.001).
Conclusions
In men with stable CAD, preserved systolic left ventricular function and non-high cardiovascular risk determined from the initial concentration of hsCRP, elevated level of hsTnT was independently associated with the risk of multivessel coronary artery disease.
Keywords: stable CAD, high-sensitive troponin T
Abstract
Wstęp
Kluczowym problemem w stabilnej chorobie wieńcowej (coronary artery disease – CAD) pozostaje nieinwazyjna identyfikacja pacjentów z zaawansowaną wielonaczyniową chorobą wieńcową. Oznaczanie biomarkerów, które wykazują właściwości prozapalne (białko C-reaktywne – hsCRP) czy wskazują na niedokrwienie (wysokoczuła troponina T – hsTnT), może przyczynić się do poprawy stratyfikacji w tym zakresie.
Cel pracy
Identyfikacja zmiennych powiązanych z występowaniem wielonaczyniowej CAD u mężczyzn ze stabilnym obrazem klinicznym.
Materiał i metody
Do badania włączono 92 mężczyzn (średni wiek 64,05 ± 9,42 roku) z zachowaną funkcją skurczową lewej komory zakwalifikowanych do planowej koronarografii. Wykonano podstawowe badania laboratoryjne, w tym oznaczenie stężeń biomarkerów: hsCRP i hsTnT. Chorych analizowano w dwóch grupach: z chorobą wieńcową wielonaczyniową (2–3-naczyniową, n = 46) vs pacjenci bez zmian wielonaczyniowych w koronarografii (n = 46).
Wyniki
U pacjentów z wielonaczyniową chorobą wieńcową stwierdzono istotnie większe stężenia hsTnT (Med. 0,01 vs Med. 0,007, p = 0,0021) oraz glukozy na czczo (Med. 6,0 vs Med. 5,45, p = 0,0112). Na podstawie krzywych ROC wyznaczono punkty odcięcia dla hsTnT ≥ 0,0085 ng/ml i glukozy na czczo ≥ 5,85 mmol/l. Analiza wieloczynnikowa wykazała, że ryzyko wystąpienia choroby wieńcowej wielonaczyniowej niezależnie zwiększała jedynie hsTnT w stężeniach większych niż punkt odcięcia (OR 4,286; 95% CI: 1,79–10,263, p = 0,001).
Wnioski
U mężczyzn ze stabilną chorobą wieńcową, zachowaną funkcją skurczową i niewysokim ryzykiem sercowo-naczyniowym określanym na podstawie wyjściowego stężenia hsCRP zwiększone stężenie hsTnT niezależnie wiąże się z ryzykiem wystąpienia wielonaczyniowej choroby wieńcowej.
Introduction
A key problem in stable coronary artery disease (CAD) is non-invasive identification of patients with multivessel CAD. Current guidelines for the management of stable angina emphasize the significant role of clinical assessment of the pre-test probability (PTP) of coronary artery disease, as the main criterion in the selection of diagnostic strategies [1]. Although there are many non-invasive imaging and exercise tests to confirm coronary artery disease in symptomatic patients, there is still no simple screening test which could be helpful in identification of the severity of CAD and in avoiding unnecessary diagnostic procedures [1, 2]. The best tool of noninvasive non-imaging identification of the severity of CAD seems to be biomarkers, which could be used as an aid in the clinical assessment of patients [3]. Determination of biomarkers that have pro-inflammatory properties (C-reactive protein – CRP) and indicate heart muscle ischemia (high-sensitive troponin T – hsTnT) can contribute to the improvement of risk stratification in this regard.
Many of the guidelines emphasize the role of predictive determination of inflammatory biomarkers in CAD [4–7]. Well known as a coronary risk biomarker is high-sensitive CRP (hsCRP), which can indicate the reactions of the acute phase but also processes of chronic inflammation, such as atherosclerosis [8, 9]. It is obvious that CRP concentration has continuous associations with the risk of developing coronary heart disease, ischemic stroke or vascular mortality [10]. On the basis of analysis of the initial concentration of hsCRP a group of experts of the Centers for Disease Control and Prevention and the American Heart Association identified three groups of cardiovascular risk patients: low risk (hsCRP < 1.0 mg/l), moderate (hsCRP 1.0-3.0 mg/l) and high risk (hsCRP > 3.0 mg/l) [11].
Determination of troponin, which is an indicator of myocardial ischemia, generally used in the diagnosis of acute coronary syndromes, may also contribute to the improvement of stratification in stable CAD. It was recently demonstrated that using a highly sensitive assay for TnT can reveal the presence of detectable levels in the population of patients with stable CAD [12, 13].
The aim of the study was to identify factors associated with the presence of multivessel CAD in clinically stable men.
Material and methods
The study comprised consecutive, symptomatic, stable and non-diabetic 92 men (mean age 64.05 ± 9.42 years), who had undergone elective coronary angiography and had preserved left ventricular systolic function. All patients had typical angina.
The inclusion criteria were: male sex, stable angina and established qualification for coronary angiography (PTP high or moderate with > 10% induced ischemia in non-invasive tests). The hsCRP level ≥ 10 mg/l, impaired left ventricular function, acute coronary syndrome and diabetes mellitus were exclusion criteria.
Thirty-five data sets were analyzed, including: data from medical history (age, body mass index, family history, smoking status, CCS class), twelve-lead ECG results (heart rate, elevated heart rate ≥ 70 bpm), basic laboratory tests results (serum lipid levels, cholesterol ratio LDL/HDL, creatinine, hsTnT, hsCRP, fasting glucose level), results of coronary angiography, results of echocardiography (standard M-mode, 2-dimensional and Doppler) and the presence of concomitant cardiovascular diseases (arterial hypertension, previous stroke and peripheral artery disease).
Plasma concentration of cardiac hsTnT was quantitatively determined using the high-sensitivity assay (Roche Diagnostics) in a Cobas e 411 immunoanalyzer based on electrochemiluminescence technology (Roche Diagnostics) with detection limit 0.003 ng/ml.
Conventional coronary arteriography was performed using a radial or femoral approach. Stenosis ≥ 50% of the left main and > 75% of the major coronary arteries (left anterior descending artery, left circumflex artery and right coronary artery and their branches) were considered significant and identified as 1-, 2- or 3-vessel disease.
Patients were divided and analyzed related to the severity of CAD: multivessel (2- and 3-vessel disease, n = 46) vs without multivessel CAD (1-vessel disease, 50-70% lesions or marginal changes, n = 46).
The present study was in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee of Medical University of Lodz (RNN/163/10/KE).
Statistical analysis
Statistical analyses were performed using STATISTICA PL software, version 9.0 and SPSS software, version 19. Continuous variables are presented as mean values and standard deviation (SD) or medians and interquartile range depending on normality of distribution. Nominal variables are presented as number of observations (N) and percentages (%).
To check normality, the Shapiro-Wilk test was used.
To study the relationship between qualitative variables the following tests were used: the χ2 test for independence or χ2 test with Yates’ correction, and where appropriate the χ2 test for trend.
Differences between two independent samples for continuous data were analyzed using Student's t-test (since data distribution was normal) and the Mann-Whitney U test (in the absence of normal distribution).
In the analysis two independent groups for quantitative variables that significantly affected the group membership, were drawn the ROC curves and determined the optimal cut-points that have been assigned the sensitivity, specificity, positive and negative predictive value (PPV and NPV). It also provides odds ratios (OR) with 95% strength confidence interval (95% CI). Similar measures are given for qualitative variables.
Variables significant in univariate analysis (significance level p < 0.10) were used for the construction of multivariate stepwise logistic forward regression models for the identification of factors associated with multivessel coronary artery disease.
The results were considered statistically significant at p < 0.05.
Results
The baseline characteristics of patients are presented in Table I. Most of the studied patients had arterial hypertension (82.61%), more than half of the population had dyslipidemia (56.04%), 34.78% were obese, and 7.61% were current smokers. In respect of risk determined by hsCRP level, the studied population belonged to the non-high cardiovascular risk group.
Tab. I.
Characteristics of the studied population
| Age (years) | 64.05 ± 9.42 (43-84), Med. 63 (58-70) |
| Angina pectoris (CCS class) | |
| 1 | 1 (1.18) |
| 2 | 35 (41.18) |
| 3 | 42 (49.41) |
| 4 | 7 (8.24) |
| Positive family history | 36 (39.13) |
| Previous MI | 25 (27.17) |
| Previous PCI | 20 (21.74) |
| Current smoking | 7 (7.61) |
| PAD | 11 (11.96) |
| BMI [kg/m2] | 28.73 ± 4.47 (19.82-42.61), Med. 28.37 (25.66-31.14) |
| Ejection fraction [%] | 61.68 ± 7.36 (46-79), Med. 61.0 (56-67) |
| Heart rate [bpm] | 67.57 ± 0.36 (44-105), Med. 65.0 (60-75) |
| Heart rate ≥ 70 bpm | 35 (38.46) |
| Creatinine [mmol/l] | 0.83 ± 0.34 (0.50-3.1), Med. 0.74 (0.64-0.90) |
| hsCRP [mg/l] | Med. 1.1 (0.6-2.4) |
| hsTnT [ng/ml] | 0.013 ± 0.019 (0.001-0.120), Med. 0.008 (0.005-0.012) |
| Glucose [mmol/l] | 6.26 ± 1.73 (4.2-12.6), Med. 5.7 (5.2-6.6) |
Data are expressed as N (%), mean ± SD and range, median and interquartile range (IQR)
MI – myocardial infarction, PCI – percutaneous coronary intervention, BMI – body mass index, hsTnT – high-sensitivity troponin T, hsCRP – high-sensitivity C-reactive protein, PAD – peripheral artery disease
The results of the main laboratory tests and ECG did not differentiate analyzed groups (Table II). There were also no differences in the burden of co-morbidities such as arterial hypertension, dyslipidemia, diabetes mellitus, peripheral artery disease or current smoking between the studied groups (for all p > 0.05).
Tab. II.
Patients’ characteristics related to coronary angiography results
| Parameters | Patients with multivessel coronary artery disease (n = 46) | Patients without multivessel coronary artery disease (n = 46) | P |
|---|---|---|---|
| Age [years] | 65.52 ± 9.70 | 62.59 ± 9.0 | 0.1360 |
| BMI [kg/m2] | 28.10 ± 3.92 | 28.73 (21.97-42.6)* | 0.3655 |
| BPs [mmHg] | 135.33 ± 16.98 | 130 (105-180)* | 0.2621 |
| BPd [mmHg] | 80.0 (60-105)* | 80.0 (65-100)* | 0.2830 |
| HA | 41 (89.13) | 35 (76.09) | 0.1690 |
| Dyslipidemia | 28 (60.87) | 23 (51.11) | 0.3484 |
| PAD | 6 (13.04) | 5 (10.87) | 0.7478 |
| Ejection fraction [%] | 61.61 ± 7.48 | 61.76 ± 7.31 | 0.9248 |
| Heart rate [bpm] | 65 (53-105)* | 68.09 ± 10.97 | 0.2638 |
| Heart rate ≥70 bpm | 15 (32.61) | 20 (44.44) | 0.2459 |
| Total cholesterol level [mmol/l] | 4.40 ± 1.03 | 4.25 (2.5-8.3)* | 0.8661 |
| LDL-cholesterol [mmol/l] | 2.1 (0.8-5.6)* | 2.37 ± 1.09 | 0.5664 |
| HDL-cholesterol [mmol/l] | 1.36 ± 0.38 | 1.34 (0.62-2.47)* | 0.4733 |
| Triglycerides [mmol/l] | 1.37 (0.36-5.35)* | 1.24 (0.57-7.47)* | 0.8222 |
| LDL/HDL index | 1.58 (0.56-4.67)* | 1.67 (0.40-4.60)* | 1.0000 |
| Creatinine [µmol/l] | 0.76 (0.54-1.88)* | 0.73 (0.49-3.10)* | 0.3426 |
| Glucose [mmol/l] | 6.0 (4.9-11.20)* | 5.45 (4.20-12.60)* | 0.0112 |
| hsCRP [mg/l] | 1.1 (0.1-9.3)* | 1.10 (0.20-6.70)* | 0.9191 |
| hsTnT [ng/ml] | 0.010 (0.001-0.120)* | 0.007 (0.003-0.012)* | 0.0021 |
Data are expressed as N (%), mean ± SD and range or median (Med) and interquartile range (IQR) in variables with nonnormal distribution (*)
BMI – body mass index, BPs – systolic blood pressure, BPd – diastolic blood pressure, HA – arterial hypertension, PlGF – placental growth factor, hsCRP – high-sensitivity C-reactive protein, hsTnT – high-sensitivity troponin T, CAD – coronary artery disease, PAD – peripheral artery disease
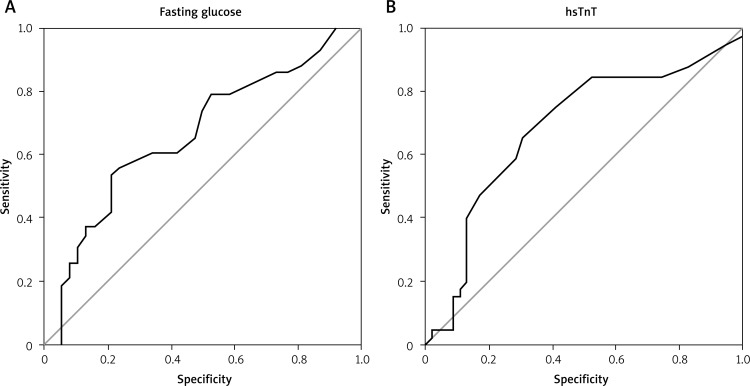
Opposite to patients without multivessel CAD, those with multivessel coronary artery disease were in higher CCS class [Med. 2 (IQR 2-3) vs. Med. 3 (IQR 2-3), p = 0.017] and had significantly higher hsTnT and fasting glucose levels (Table II). Based on the drawn ROC curves, the optimal cut-off points for fasting glucose were ≥ 5.85 mmol/l (AUC 0.664 ± 0.061, 95% CI: 0.545-0.783, sensitivity 89.7%, specificity 54.8%, PPV 64.8%, NPV 85.2%) and for hsTnT ≥ 0.0085 ng/ml (AUC 0.685 ± 0.058, 95% CI: 0.573-0.798, sensitivity 68.2%, specificity 58.3%, PPV 60.0% and NPV 66.7%) (Fig. 1).
Fig. 1.
ROC curve for fasting glucose and hsTnT for presence of multivessel coronary artery disease
Multivariate stepwise logistic forward regression analysis revealed that the risk of multivessel CAD was associated only with the hsTnT concentration. This variable independently increased that risk more than 4-fold (OR 4.286, 95% CI: 1.79-10.263, p = 0.001).
Discussion
The main finding of our study was that in symptomatic men without high cardiovascular risk determined from the initial concentration of hsCRP, elevated level of hsTnT was independently associated with the risk of multivessel CAD. So far only a few publications have examined the relationship between hsTnT level and severity of CAD.
In 2011, Ndreppa et al. also revealed the association between hsTnT and severity of CAD based on results of coronary angiography. In their study they examined whether this association is independent of conventional risk factors, and other biomarkers such as N-terminal pro-brain natriuretic peptide and C-reactive protein. Their study included 1,316 patients (904 with coronary artery disease proven by coronary angiography and 412 with angina but without significant changes in coronary artery disease in angiography) [14]. In the Ndreppa study there took part both male and female patients, at a mean age of respectively 63.3 (54.1-70.1) and 68.5 (62.1-75.8). Similar to our population, all patients in their study had preserved left ventricular function, and most of them had arterial hypertension and non-high cardiovascular risk determined from the initial concentration of CRP and were of similar age. Opposite to our results, in the Ndreppa study patients with 3-vessel coronary artery disease had significantly more often diabetes (p < 0.001), hypercholesterolemia (p < 0.001), smoking status (p = 0.014), lower glomerular filtration rate (p < 0.001) and higher concentrations of N-terminal pro-brain natriuretic peptide (p < 0.001).
In patients without significant coronary artery disease (< 25% coronary artery lumen obstruction) and in those with 1-, 2- and 3-vessel disease, the hsTnT levels increased with increasing severity of coronary artery disease (respectively 0.005 µg/l vs. 0.006 µg/l vs. 0.008 µg/l vs. 0.010 µg/l, p < 0.001). They revealed that hsTnT was an independent predictor of CAD presence (OR 1.30, 95% CI: 1.07-1.59, p = 0.009), irrespective of traditional cardiovascular risk factors, NT-pro-BNP and CRP concentrations.
Similar to our results, researchers of the Ndreppa group confirmed that in symptomatic patients with stable CAD there is an association between hsTnT level and severity of angiographically proved CAD, independently of cardiovascular risk factors, NT-pro-BNP and CRP [14].
In the next article, Laufer et al. investigated the relationship between hsTnT and coronary atherosclerotic plaque burden in 615 patients, who were suspected of having CAD and were referred for cardiac computed tomographic angiography (CCTA) [15]. The group studied by Laufer consisted of male and female patients at the mean age 57 ± 11 years. All patients were also at non-high cardiovascular risk determined by hsCRP. There were also no differences in diabetes or blood pressure values.
To assess coronary plaque burden 3 scoring systems were used: the CT plaque burden score, the Segment-Based Score and the Plaque Involvement Score. There was also calculated the Agatston score – quantification of coronary artery calcium using computed tomography. Based on the results of CCTA and CT plaque burden score, the form of coronary artery disease was classified as mild (50% lesion), moderate (50% to 70% lesion), severe (70% lesion), or multivessel CAD (multiple 70% lesions). The study showed the progressive increase (p < 0.01) of hsTnT level according to the extent of CAD assessed as CT plaque burden score: mild (median 4.5 ng/l), moderate (median 5.5 ng/l), severe (median 5.7 ng/) and multivessel (median 8.6 ng/l) compared with patients without CAD (median 3.7 ng/l). The study revealed a positive correlation between hsTnT concentrations and CT plaque burden score (r = 0.293, p < 0.001) or Agatston score (r = 0.353, p < 0.001). For the involvement score and the segment-based score, similar correlations were found.
Similar to our results, Laufer and collaborators did not find an association between hsCRP level and extent of coronary artery disease [15]. Opposite to Laufer, who assessed the extent of coronary artery disease using CCTA, in our study, the severity of coronary artery disease was evaluated on the results of coronary angiography. Our study was performed on a male population, in contrast to Ndreppa and Laufer, who examined both women and men, and their populations were more numerous.
What is also important, depending on the used reagents and measurements, there exist differences in units of hsTnT. Currently they should be mostly reported in ng/l (as in the Laufer study) rather than pg/ml, ng/ml (which was used by Ndreppa and in our measurements) or µg/l.
The results of the main laboratory tests and ECG did not differentiate analyzed groups (Table II). There were also no differences in the burden of co-morbidities such as arterial hypertension, dyslipidemia, diabetes mellitus, peripheral artery disease or current smoking between the studied groups (for all p > 0.05).
Compared to patients without multivessel CAD, those with multivessel coronary artery disease were in higher CCS class and had higher fasting glucose levels. Several studies have evaluated the impaired glucose metabolism in patients with CAD confirmed by coronary arteriography. In 2001 Kowalska et al. reported a significant positive correlation between disturbances of glucose metabolism (fasting and postload insulin concentrations) and the number of involved vessels in coronary angiography. In a population of 363 nondiabetic, consecutive male patients referred for coronary angiography, those with two- and three-vessel CAD had significantly more pronounced disturbances with glucose metabolism [16]. Opposite to our study, the fasting glucose levels did not differ between the studied groups for severity of CAD. In another study, Dong et al. assessed the relationship between fasting plasma glucose levels and the prevalence and severity of angiographic coronary artery disease, and they revealed that the prevalence of angiographic CAD as well as the severity of CAD increased correspondingly with the fasting plasma glucose level [17].
Our study also showed that there is no correlation between hsCRP level and the severity of CAD. There have been many works highlighting the role of CRP as a predictor of cardiovascular events in patients with stable coronary artery disease, but the role of the correlation between hsCRP and severity of coronary artery disease is still unclear [1].
Determination of the high-sensitivity troponin level seems to contribute to the improvement of stratification in stable CAD. There are many mechanisms responsible for releasing very low levels of cardiac troponin in patients suffering for stable CAD. Among many processes, there are distinguished transient, clinically silent ischemic episodes and small-vessel occlusions, inflammatory processes or cardiomyocyte apoptosis [18]. In times of ongoing discussions regarding which patients with stable CAD need invasive diagnostic tests, a simple determination of the concentration the hsTnT in the serum appears to be a very useful tool in risk stratification of severity of changes in coronary arteries and could help to avoid serious cardiovascular events.
Despite many studies investigating the role of biomarkers in stable CAD, there still do not exist optimal non-invasive methods for predicting the severity of CAD.
It is worth noting that, although there exist reports about the prognostic value of determination of hsCRP and hsTnT in patients with stable coronary artery disease, they have not enough independent prognostic value to be able to recommend the systematic determination of ambulatory care of patients with stable CAD [1].
Searching for new biomarkers reflecting the severity of coronary artery atherosclerosis used as an initial diagnostic tool would be of vital importance in a new approach for early identification of patients when it is necessary to consider the decision for implementation of invasive diagnostic tests.
Limitations
The main limitation of the study was the small number of patients and absence of a control group. There is a need for further investigations in a larger population of patients. There is also a lack of prospective evaluation of occurrence of cardiovascular events. The visual assessment of lesions by coronary angiography did not include quantitative analysis and fractional flow reserve. The group of patients without multivessel changes in coronary arteriography was very heterogeneous, being composed of patients with 1-vessel disease, 50-70% changes and marginal changes. However, the main objective of this study was the identification of patients with multivessel CAD. An important element would be a multimarker strategy in risk stratification of coronary artery disease severity. In our study we focused on identification of factors routinely assayed at admission to the hospital, and finding the association between them and the presence of multivessel CAD in clinically stable men.
Conclusions
In men with stable coronary artery disease with preserved systolic left ventricular function and non-high cardiovascular risk determined from the initial concentration of hsCRP, elevated level of hsTnT was independently associated with the risk of multivessel coronary artery disease.
Acknowledgements
The study was supported from a Medical University Lodz grant (Nr 502-03/1-005-02/502-14-091).
Disclosure
Authors report no conflict of interest.
References
- 1.Task Force Members. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, ESC Committee for Practice Guidelines. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers. Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 2.Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, ESC Committee for Practice Guidelines. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, EACTS Clinical Guidelines Committee. Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Zamorano JL. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardiothorac Surg. 2014;46:517–592. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS. Biomarkers of cardiovascular disease. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 5.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, Couture P, Dufour R, Fodor G, Francis GA, Grover S, Gupta M, Hegele RA, Lau DC, Leiter L, Lewis GF, Lonn E, Mancini GB, Ng D, Pearson GJ, Sniderman A, Stone JA, Ur E. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol. 2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NACB LMPG Committee Members. Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, Grundy SM, Labarthe DR, Levy D, Rifai N, Wilson PW. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–384. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW, American College of Cardiology Foundation; American Heart Association 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Zebrack JS, Anderson JL, Maycock CA, Horne BD, Bair TL, Muhlestein JB, Intermountain Heart Collaborative (IHC) Study Group Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am J Cardiol. 2002;89:145–149. doi: 10.1016/s0002-9149(01)02190-7. [DOI] [PubMed] [Google Scholar]
- 9.Arroyo-Espliguero R, Avanzas P, Quiles J, Kaski JC. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis. 2009;204:239–243. doi: 10.1016/j.atherosclerosis.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP, CDC; AHA CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease Application to Clinical and Public Health Practice: Report From the Laboratory Science Discussion Group. Circulation. 2004;110:e545–549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 12.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Røsjø H, Šaltytė Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E, PEACE Investigators Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;6:1240–1249. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Bernard J, Gersh BJ, Phil D, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndrepepa G, Braun S, Schulz S, Mehilli J, Schömig A, Kastrati A. High-sensitivity troponin T level and angiographic severity of coronary artery disease. Am J Cardiol. 2011;108:639–643. doi: 10.1016/j.amjcard.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Laufer EM, Mingels AM, Winkens MH, Joosen IA, Schellings MW, Leiner T, Wildberger JE, Narula J, Van Dieijen-Visser MP, Hofstra L. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol. 2010;30:1269–1275. doi: 10.1161/ATVBAHA.109.200394. [DOI] [PubMed] [Google Scholar]
- 16.Kowalska I, Prokop J, Bachórzewska-Gajewska H, Telejko B, Kinalskal I, Kochman W, Musial W. Disturbances of glucose metabolism in men referred for coronary arteriography. Postload glycemia as predictor for coronary atherosclerosis. Diabetes Care. 2001;24:897–901. doi: 10.2337/diacare.24.5.897. [DOI] [PubMed] [Google Scholar]
- 17.Dong X, Zhou L, Zhai Y, Lu B, Wang D, Shi H, Luo X, Fan W, Hu R. Impaired fasting glucose and the prevalence and severity of angiographic coronary artery disease in high-risk Chinese patients. Metabolism. 2008;57:24–29. doi: 10.1016/j.metabol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]