Abstract
Oral direct inhibitors of thrombin and activated factor Xa are approved as new anticoagulant drugs. In contrast to vitamin K antagonists (VKA) and heparins, the new agents have single targets in the coagulation cascade and more predictable pharmacokinetics, but they lack validated and available antidotes. Unlike VKA, they do not require routine monitoring of coagulation. However, the measurement of their pharmacologic effects might be of value in selected patients. They interfere with the routine coagulation tests, which should be interpreted with caution. Specific tests exist and can be used in case of emergencies. Adequate supportive care and temporary removal of all antithrombotic agents constitute the basis for management of serious bleeding complications. The administration of coagulation factors, such as fresh frozen plasma, prothrombin complex concentrates or recombinant activated FVII, can benefit in life-threatening bleeding or emergency surgery. Specific antidotes for non-vitamin K oral anticoagulants are in clinical development.
This review aims at answering in a brief and simplified manner some clinical questions.
Keywords: anticoagulation, rivaroxaban, dabigatran, apixaban
Abstract
Nowe leki przeciwzakrzepowe (inhibitor trombiny – dabigatran; inhibitory czynnika Xa – riwaroksaban, apiksaban, edoksaban) są coraz powszechniej stosowane w praktyce klinicznej. W przeciwieństwie do antagonistów witaminy K oraz heparyny, nowe doustne leki przeciwzakrzepowe charakteryzują się bardziej przewidywalną farmakokinetyką i farmakodynamiką oraz mniejszą liczbą interakcji ze składnikami diety. Główną ich zaletą jest brak potrzeby rutynowego monitorowania terapii. Obecnie nie istnieje swoiste antidotum dla dabigatranu, riwaroksabanu i apiksabanu. Sposób postępowania w powikłaniach krwotocznych w trakcie leczenia nowymi doustnymi antykoagulantami zależy od nasilenia i umiejscowienia krwawienia. W przypadku ciężkiego krwawienia oraz pilnego zabiegu operacyjnego należy w pierwszej kolejności odstawić lek, a następnie rozważyć zastosowanie świeżego osocza, rekombinowanego aktywnego czynnika VII bądź koncentratu aktywowanych czynników zespołu protrombiny. Przy krwawieniach podczas stosowania nowych antykoagulantów nie ma uzasadnienia dla podawania siarczanu protaminy, witaminy K czy desmopresyny.
Introduction
Vitamin K antagonists (VKA) were the only class of oral anticoagulants available to clinicians. VKA are economical and very well characterized, but they have important limitations that can outweigh these advantages, such as slow onset of action, a narrow therapeutic window and an unpredictable anticoagulant effect [1]. VKA-associated dietary precautions, monitoring and dosing adjustments to maintain the international normalized ratio (INR) within the therapeutic range, and bridging therapy, are inconvenient for patients, expensive, and may result in inappropriate use of VKA therapy. This can lead to increased bleeding risk or reduced anticoagulation and increased risk of thrombotic events [2]. The side effects of conventional anticoagulants have prompted research into novel drugs. Several non-vitamin K oral anticoagulants (NOACs) with more stable pharmacokinetic and pharmacodynamics profiles have been licensed for clinical practice [3–6]. Currently, dabigatran (a direct thrombin inhibitor), rivaroxaban and apixaban (a direct factor Xa inhibitor) are the most extensively evaluated novel anticoagulant agents [3–6]. NOACs have little interaction with food or drugs and can therefore be prescribed in a fixed dose without the requirement of frequent monitoring [7]. They have a rapid onset of action, a relatively predictable pharmacokinetic profile, and a relatively short plasma half-life, making initiation, maintenance, and discontinuation of anticoagulant therapy considerably easier than with VKA (Table I) [7]. They have been shown to be effective and safe in various large-scale clinical trials [4–6]. Despite the many advantages, physicians should exercise caution in prescribing these medications to patients, especially patients who are elderly, have impaired renal function or liver dysfunction, low body weight or have a history of bleeding [7]. Monitoring of coagulation is not required, but patients should be followed up regularly to detect conditions that may lead to changes in the expected efficacy or safety [7]. Moreover, patients should be warned that reduced adherence or nonadherence to the treatment regimen could be fatal due to a thromboembolism event.
Tab. I.
Absorption and metabolism of the different non-vitamin K oral anticoagulants (NOAC) [7]
| Dabigatran | Apixaban | Rivaroxaban | Edoxaban | |
|---|---|---|---|---|
| Bio-availability | 3-7% | 50% | 66% without food Almost 100% with food | 62% |
| Prodrug | YES | NO | NO | NO |
| Plasma protein binding | 35% | 87% | > 90% | 55% |
| Non-renal/renal clearance of absorbed dose | 20%/80% | 73%/27% | 65%/35% | 50%/50% |
| Liver metabolism: CYP3A4 involved | NO | YES | YES | Minimal (< 4% of elimination) |
| Absorption with food | NO EFFECT | NO EFFECT | Increase of 39% more | 6–22% more |
| Intake with food recommended | NO | NO | Mandatory | NO |
| Gastro-intestinal tolerability | Dyspepsia | No problem | No problem | No problem |
| Elimination half-life | 12-17 h | 12 h | 5-9 h (young) 11-13 h (elderly) | 9-11 h |
NOACs have been approved in many countries for the prevention of venous thromboembolism after hip or knee arthroplasty (dabigatran, rivaroxaban, and apixaban), the treatment of deep vein thrombosis or pulmonary embolism (rivaroxaban), and for stroke prevention in nonvalvular atrial fibrillation (dabigatran, rivaroxaban, and apixaban) [4–6]. Rivaroxaban has also been approved in Europe for secondary prophylaxis after acute coronary syndromes, but clinical use in this setting is limited [5]. Recently edoxaban, as another factor X inhibitor, was approved by the US Food and Drug Administration (FDA) to reduce the risk of thromboembolic events in patients with non-valvular atrial fibrillation. The FDA also approved edoxaban for treating patients with deep vein thrombosis (DVT) and pulmonary embolism (PE), who have already been treated with a parenteral anticoagulant for 5 to 10 days [8].
Coagulation assays
In contrast to VKA, NOACs do not require routine monitoring of coagulation. However, the measurement of their pharmacologic effects might be of value in selected patients, such as those with excessive bleeding risk due to worsening renal insufficiency, frailty, drug interactions, and overdoses [7]. Furthermore, laboratory monitoring might help in checking patient compliance or in revealing over-anticoagulation [9]. There is at present no consensus on the best methodology for assessing NOAC activity in vivo and hence guiding dosage. Unlike with VKA, traditional coagulation tests cannot be used to assess or adjust dosing [10–12]. The prothrombin time (PT), international normalized ratio (INR) and activated partial thromboplastin time (aPTT) are prolonged during NOAC treatment [7, 13]. More characteristics are summarized in Table II. However, these tests are relatively insensitive and suffer from a high degree of variability, especially at high drug concentrations (typically greater than 200 ng/ml). The sensitivity also depends on the different reagents used [10–12].
Tab. II.
Interpretation of coagulation assays in patients treated with different non-vitamin K oral anticoagulants (NOAC) [7]
| Dabigatran | Apixaban | Rivaroxaban | Edoxaban | |
|---|---|---|---|---|
| Plasma peak level | 2 h after ingestion | 1-4 h after ingestion | 2-4 h after ingestion | 1-2 h after ingestion |
| Plasma trough level | 12-24 h after ingestion | 12-24 h after ingestion | 16-24 h after ingestion | 12-24 h after ingestion |
| PT | Cannot be used | Cannot be used | Prolonged: may indicate excess bleeding | Prolonged |
| INR | Cannot be used | Cannot be used | Cannot be used | Cannot be used |
| aPTT | At trough: > 2 × ULN suggests excess bleeding risk | Cannot be used | Cannot be used | Prolonged |
| dTT | At trough: > 200 ng/ml or 65 s excess bleeding risk | Cannot be used | Cannot be used | Cannot be used |
| ECT | At trough: > 3 × ULN excess bleeding risk | Not affected | Not affected | Not affected |
PT – prothrombin time, aPTT – activated partial thromboplastin time, dTT – diluted thrombin time, INR – international normalized ratio, ECT – ecarin clotting time, ULN – upper limit of normal
Activated partial thromboplastin time
aPTT is an effective qualitative method widely available for determining the presence or absence of an anticoagulant effect in patients receiving dabigatran, but cannot be used to determine the drug level. In the RE-LY trial, median trough aPTT in patients receiving a 150 mg dose of dabigatran was 52 (IQR 40-76) seconds [14]. Timing of the last dose of dabigatran and timing of blood sampling need to be considered when interpreting this result. An aPTT of ≥ 1.5 times the control level is strongly suggestive of a therapeutic drug level [15]. If the aPTT level at trough 10-16 h after the last dose still exceeds two times the upper limit of normal, it is indicative of a high bleeding risk [15]. The aPTT also shows a curvilinear response to rivaroxaban, edoxaban and apixaban but is less sensitive compared to dabigatran [16].
More appropriate assays for dabigatran may be diluted thrombin time (dTT), thrombin time (TT) and the ecarin clotting time (ECT). In contrast to PT, the dPT assay utilizes a similar thromboplastin reagent that has been diluted. This dilution has been shown to increase the sensitivity of the assay, resulting in more accurate prediction of the coagulation state [16].
Thrombin time
The thrombin time assay measures the activity of thrombin in plasma. The TT shows a linear concentration response to dabigatran, but the results are highly dependent on the reagents and coagulometer used, and most TT assays will be too sensitive. Their only use may be as a sensitive method for determining if any dabigatran is present, which will be excluded by a normal TT [15, 16]. In contrast to the direct thrombin inhibitors (DTIs), rivaroxaban and apixaban make TT an undesirable assay to measure these anticoagulants [17]. Analysis of TT can be used to differentiate between different NOACs in emergency situations in which patients are unconscious. In the case of dabigatran, the TT will be prolonged, while in the case of rivaroxaban or apixaban, the TT will be within the normal range [16].
Ecarin clotting time
The ecarin clotting time (ECT) assay provides a direct measure of the activity of DTIs, but may not be readily available. In this assay, coagulation is initiated with ecarin, a type of snake venom. Ecarin activates prothrombin, which in turn stimulates the thrombin precursor meizothrombin [18]. The ability of dabigatran to inhibit the activity of meizothrombin and subsequent clot formation results in the prolongation of ECT [15, 19]. When dabigatran is used, with twice daily dosing, ≥ 3 times elevated ECT at trough is associated with a higher risk of bleeding [19]. Factor Xa inhibitors have no impact on the ECT [16, 17].
International normalized ratio (INR)
Regardless of the reagent used, dabigatran has little effect on the PT and INR at clinically relevant plasma concentrations [15]. Although use may be associated with an increase in INR, this increase does not relate to the effectiveness of therapy or provide a linear correlation of concentration and effect that is seen when measuring warfarin levels [10]. In addition, a normal INR does not guarantee that the patient is no longer fully anticoagulated [15]. Dabigatran should not be adjusted to achieve an INR of 2 to 3, as is the practice for warfarin. In healthy volunteers on standard doses of dabigatran the INR is typically mildly elevated (about 1.2-1.8) [15]. From the literature there are known reports of high INR values as high as over 8 in point-of-care readings during treatment with dabigatran, particularly in elderly patients with impaired renal function [20, 21]. Thus, serum creatinine should be measured for every patient before dabigatran is prescribed. Afterwards, creatinine levels should be monitored periodically in those with unstable renal function, and at least annually in the elderly [4].
Rivaroxaban has also increased INR. This has been shown on a small group of patients undergoing elective arthroplasty who were anticoagulated with 10 mg of rivaroxaban [22]. On the first day after rivaroxaban was commenced, 67.9% of patients recorded a high INR value (above 1.4) while 7.1% of patients had a normal value of INR value (0.8-1.2). However, the number of patients with a normal value of INR increased over the next 2 days. On the third day 32.1% of the patients had an elevated INR value while 35.7% of the patients recorded a normal INR value [22]. All patients in this study had a normal coagulation profile pre- and intra-operatively.
When interpreting the coagulation assay, it is essential to know when the NOAC was administered relative to the time of blood sampling. The maximum effect occurs at the same time as maximal plasma concentrations of the drug, which occurs approximately 2-4 hours after oral administration, with the maximal effect on coagulation being within 2 hours [4, 7, 15].
Prothrombin time
In contrast, a good correlation has been found between the prolongation of the PT time and the plasma concentration of rivaroxaban. At therapeutic concentrations, rivaroxaban has a relatively weak effect on PT [16]; however, the effect is more profound at higher concentrations [23]. According to the results of the studies, the PT should not be used to assess the level of rivaroxaban in the blood [24–26]. First, the PT assay is a global clotting test and may be prolonged for a number of reasons. In patients with hepatic impairment, sepsis, vitamin K deficiency, disseminated intravascular coagulation, various types of cancers, and other systemic diseases, the basal levels are increased before taking rivaroxaban [27, 28]. Second, the effect of rivaroxaban on PT is transient and changes over time after administration according to the half-life of 7 to 11 hours [5]. Finally, different thromboplastin reagents used in the PT assays showed variable sensitivities to rivaroxaban at the same concentration [16]. The coefficients of variation between different laboratories are too large to provide a precise measurement [29]. To achieve a doubling of PT, the concentration of rivaroxaban varied 2.6- to 8-fold between thromboplastin reagents [23]. For example, recombinant human Innovin is relatively unresponsive to rivaroxaban, while Recombiplastin and Neoplastin-Plus are among the more responsive thromboplastins [30]. This variability is not corrected by conversion of PT to INR values [16]. In fact, at higher concentrations of rivaroxaban, converting PT to the INR creates a wider range of variability compared with PT [23]. The INR was developed specifically for monitoring anticoagulation with VKA [1] and, therefore, should not be used for rivaroxaban [11, 12, 31]. Furthermore, the calculated INR values for different factor Xa inhibitors show markedly different results [31].
The standardization model used for VKA could also be applied for rivaroxaban with the use of specific thromboplastin. Evidence was provided that standardization across reagents is feasible by devising an international sensitivity index (ISI) based on plasma spiked with increasing doses of rivaroxaban (ISI-rivaroxaban). This index was successfully used to convert the PT ratio into INR-rivaroxaban and proved effective in minimizing between-thromboplastin results variability (Table III) [30].
Tab. III.
Validation of the efficacy of ISI-rivaroxaban in results normalization thromboplastins [28]
| PT ratio | INR VKA | INR rivaroxaban | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| Mean | 1.27 | 1.76 | 2.67 | 1.34 | 2.01 | 3.39 | 1.33 | 1.96 | 3.23 |
| CV% | 5.5 | 12.1 | 18.1 | 10.4 | 24.6 | 39 | 2.1 | 3.3 | 1.9 |
| Overall CV | 14.0 | 29.6 | 2.1 | ||||||
A, B and C refer to the test plasmas with 0.1 µg ml−1, 0.3 µg ml−1 and 0.7 µg ml−1 rivaroxaban.
CV – between-thromboplastin coefficient of variation, INR – international normalized ratio; PT – prothrombin time, ISI – international sensitivity index. Mean PT values were obtained with different thromboplastins and different ways of expressing the results. PT ratio, INR VKA and INR rivaroxaban refer to the ratio of the clotting time (test plasma-to-normal plasma), and the INRs valid for vitamin K antagonists and rivaroxaban, respectively.
For apixaban and edoxaban, as other selective direct inhibitors of factor Xa, a concentration-dependent prolongation of PT is also found [32, 33]. However, the effect of apixaban on the PT assay has been reported to be small in comparison with rivaroxaban.
Measuring NOAC concentration can be done directly via high-performance liquid chromatography mass spectrometry in a pharmaceutical or reference laboratory [34, 35]. However, these methods are only available in certain highly specialized places and are rarely performed in clinical practice. Moreover, there are no data on a cut-off of these specific tests below which elective or urgent surgery is ‘safe’, and therefore their use in this respect cannot be recommended at this time [7].
Safety of anticoagulant therapy
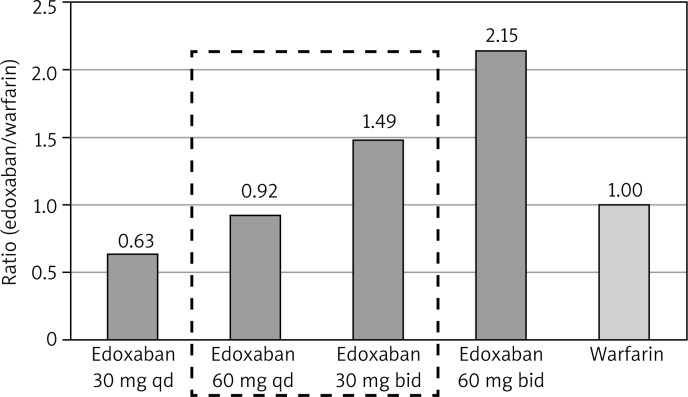
A once daily (qd) dosing regimen was related to greater adherence vs. a twice daily (bid) regimen in cardiovascular patients [36]. It is likely that also for NOACs a qd dosing regimen is the best from a compliance perspective. The observed clinical data from the phase II safety study of edoxaban (a specific direct inhibitor of factor Xa) compared with warfarin in AF patients demonstrated that bleeding (major, clinically relevant non-major, and minor) incidence was numerically higher for the bid dose regimens relative to the warfarin control arm, while the qd edoxaban dose regimens showed lower bleeding rates (Fig. 1) [37]. The pharmacokinetic analysis found that trough concentration (Cmin) of edoxaban was the most predictive parameter of the probability of having a bleeding event [37]. The reason for the discordance is not clear, but it may be related to the molecular pharmacokinetics, various patient populations and concomitant medications used to manage these different patients. Final recommendations will be further refined with data collected from the phase III study of edoxaban.
Fig. 1.
Bleeding (major, non-major clinically relevant and minor) rates by dosing regimen relative to warfarin bleeding rate in patients with atrial fibrillation [37]
qd – once daily dosing, bid – twice daily dosing
Non-vitamin K anticoagulants and antiplatelet drugs
The concomitant use of dual antiplatelet therapy (DAPT) and oral anticoagulation (OAC) is necessary in some patients with cardiovascular disease. This is referred to as triple oral antithrombotic therapy (TOAT). The most frequent indications are atrial fibrillation (AF) in combination with drug-eluting stent implantation and/or the presence of an acute coronary syndrome. The decision to initiate TOAT must take into consideration an increased rate of bleeding events compared to lesser antithrombotic therapy [38].
The most important recommendations of the European Cardiology Society in AF patients requiring antiplatelet therapy are listed below [38]:
In AF patients, stroke risk must be assessed using the CHA2DS2-VASc score, and bleeding risk assessed using the HAS-BLED score.
The period of triple therapy should be as short as possible, followed by OAC plus a single antiplatelet therapy (preferably clopidogrel 75 mg/day, or as an alternative, aspirin 75–100 mg/day).
The duration of triple therapy is dependent on a number of considerations: acute vs. elective procedures, bleeding risk, type of stent.
Newer generation drug-eluting stents should be preferred over bare metal stents in patients at low risk for bleeding.
Where a NOAC is used in combination with clopidogrel and/or low-dose aspirin, the lower tested dose for stroke prevention in AF (that is, dabigatran 110 mg b.i.d., rivaroxaban 15 mg o.d. or apixaban 2.5 mg b.i.d.) may be considered.
Novel P2Y12 receptor inhibitors (prasugrel and ticagrelor) should not be part of a triple therapy regimen in patients with AF.
Proton pump inhibitors should be considered in all patients, particularly where aspirin is used.
Recommendations on the management of AF patients with ACS and/or undergoing PCI/stenting are summarized in Table IV [38].
Tab. IV.
Recommended antithrombotic strategies following coronary artery stenting in patients with atrial fibrillation at moderate-to-high thromboembolic risk [36]
| Hemorrhagic risk | Stroke risk | Clinical setting | Recommendations |
|---|---|---|---|
| Low or moderate (HAS-BLED 0–2) | Moderate (CHA2DS2-VASC = 1 in males) or High (CHA2DS2-VASC ≥ 2) | Stable CAD |
At least 4 weeks (no longer than 6 months): triple therapy of OAC + aspirin + clopidogrel Up to 12 th month: OAC and clopidogrel (or alternatively aspirin) Lifelong: OAC |
| ACS |
6 months: triple therapy of OAC + aspirin + clopidogrel Up to 12 th month: OAC and clopidogrel (or alternatively aspirin) Lifelong: OAC |
||
| High (HAS-BLED ≥ 3) | Moderate (CHA2DS2-VASC = 1 in males) | Stable CAD |
12 months: OAC and clopidogrel Lifelong: OAC |
| ACS |
4 weeks: triple therapy of OAC + aspirin + clopidogrel Up to 12 th month: OAC and clopidogrel (or alternatively aspirin) Lifelong: OAC |
||
| High (CHA2DS2-VASC ≥ 2) | Stable CAD |
4 weeks: triple therapy of OAC + aspirin + clopidogrel Up to 12 th month: OAC and clopidogrel (or alternatively aspirin) Lifelong: OAC |
|
| ACS |
4 weeks: triple therapy of OAC + aspirin + clopidogrel Up to 12 th month: OAC and clopidogrel (or alternatively aspirin) Lifelong: OAC |
ACS – acute coronary syndrome, CAD – coronary artery disease, OAC – oral anticoagulation, either warfarin (INR: 2.0–2.5) or non-VKA oral anticoagulant at the lower tested dose in AF (dabigatran 110 mg b.i.d., rivaroxaban 15 mg o.d. or apixaban 2.5 mg b.i.d.).
Management of bleeding complications
Clinicians are increasingly facing patients who suffer from bleeding while being treated with direct oral anticoagulants such as rivaroxaban or dabigatran. Unfortunately, there is little evidence to guide the management of the anticoagulated and bleeding patient. Given the emergent nature of these events and their inherent unpredictability, it is unlikely that randomized trials or even large cohort studies will be performed. Currently, recommendations on bleeding management are based on experts’ opinions or laboratory endpoints [7]. There is still no proper method to neutralize their anticoagulant activity. Protamine sulfate and vitamin K have no effect on the anticoagulant effects of NOACs [7]. Fresh frozen plasma will not be of help to reverse anticoagulation, but may be indicated to expand plasma volume in patients who require massive transfusion [7].
In the case of recent acute ingestion of an overdose, the use of activated charcoal to reduce absorption may be considered for any NOAC [39, 40]. A reasonable approach would be to administer charcoal with a standard dosing scheme for adults of 30-50 g within 1–2 h of intake to limit or prevent absorption in the gastrointestinal tract. Beyond 2 h use of charcoal is not effective [15, 39, 40]. Further management depends on the severity of bleeding. When bleeding is not severe, in view of the relatively short elimination half-lives of NOACs, temporary drug withdrawal may be the only requirement [7]. After cessation of treatment, restoration of hemostasis is to be expected within 12-24 h after the last taken dose. For more severe bleeding, general treatment measures may be required such as mechanical compression, surgical hemostasis, fluid replacement, and other hemodynamic support. The time frame of drug elimination strongly depends on kidney function; therefore adequate hydration and diuresis must be maintained to accelerate this process [4, 7]. This is particularly important in patients receiving dabigatran, where renal excretion is the dominant elimination pathway. Up to 80% of circulating unchanged dabigatran and small amounts of dabigatran glucuronides are excreted via the kidneys [4]. In patients with impaired renal function, dialysis may be useful, although there is only limited experience with this [4]. In contrast to dabigatran [41], dialysis has not been shown to be an option in patients treated with any of the FXa inhibitors due to the high plasma binding of most FXa inhibitors [5, 6].
In the presence of major/life-threatening bleeding, a more aggressive approach is required. Life-saving therapies, such as mechanical ventilation and vasopressors, should be used when appropriate. Determining the mechanical causes of the bleeding may require invasive approaches, including endoscopy, surgery, or invasive radiologic procedures [7]. In general, the risks associated with such procedures will be much lower than the risks associated with untreated hemorrhage. There is some experimental evidence to support the role of general hemostatic agents such as prothrombin complex concentrates (PCC; concentrates of coagulation factors II, VII, IX, and X) or recombinant factor VIIa in reversing the anticoagulant activity of NOAC in cases of major bleeding [42, 43]. Some studies have confirmed that the infusion of PCC reversed the effect of rivaroxaban, but the results for dabigatran are not as clear cut [44]. PCC administration could start at a dose of 25 U/kg and can be repeated if clinically indicated. Studies in healthy volunteers showed that activated prothrombin complex concentrates (aPCC, i.e. similar to PCC but with activated factor VIIa) corrected more coagulation parameters than PCC alone [7, 45]. Recombinant activated factor VIIa is an approved potent procoagulant and general hemostatic agent that can initiate hemostasis at sites of bleeding by directly activating thrombin on the surface of platelets in the absence of tissue factor [46]. As a result, it has been proposed that this agent may have potential in reversing the effects of the new oral anticoagulants [46, 47]. Other new reversing compounds are currently in development, including monoclonal antibodies to neutralize the effect of non-vitamin K oral anticoagulants, reaching the stage of early-phase clinical trials in humans [48].
Perioperative management
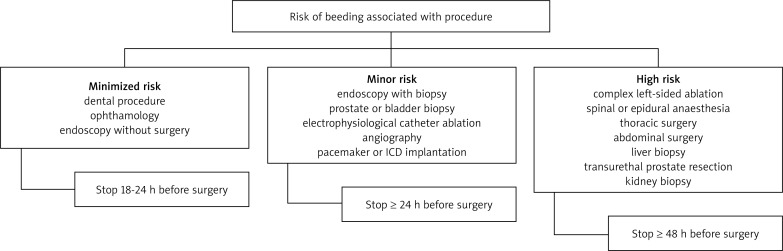
Temporary discontinuation of NOACs may be indicated in surgical patients, depending on the urgency of the procedure, level of bleeding risk and renal function [7]. The decision to stop treatment in non-urgent or elective surgery depends on the risk of bleeding versus the risk of thrombosis [7]. In surgical procedures with minor bleeding risk, the European Heart Rhythm Association recommends that the NOACS be discontinued 18-24 h before the procedure and then restarted 6 h later [7]. Longer delays may be needed for patients who undergo high bleeding risk surgery including major abdominal, cardiovascular, orthopedic or intracranial operations [7]. The management of NOACs before surgery is summarized in Table V and Fig. 2. Emergency surgical intervention should be deferred, if possible, until at least 12 h and ideally 24 h after the last dose [7]. Evaluation of common coagulation tests (aPTT, PT) or of a specific coagulation test (dTT, ECT) can be considered if there is concern about the pharmacokinetic waning of the anticoagulant effect. Nevertheless, such a strategy has never been evaluated, and therefore cannot be recommended and should not be used routinely [13]. In some cases of urgent major surgery, patients may require replacement of plasma or platelets in addition to red blood cells. PCC, aPCC or factor VIIa should be considered as a last resort in instances of life-threatening bleeding when conventional treatment methods have failed [7, 41].
Tab. V.
Last intake of drug before elective surgical intervention [7]
| Creatinine clearance (ml/min) | Dabigatran | Apixaban | Rivaroxaban | |||
|---|---|---|---|---|---|---|
| Low risk | High risk | Low risk | High risk | Low risk | High risk | |
| CrCl > 80 ml/min | ≥ 24 | ≥ 48 | ≥ 24 | ≥ 48 | ≥ 24 | ≥ 48 |
| CrCl 50-80 ml/min | ≥ 36 | ≥ 72 | ≥ 24 | ≥ 48 | ≥ 24 | ≥ 48 |
| CrCl 30-50 ml/min | ≥ 48 | ≥ 96 | ≥ 24 | ≥ 48 | ≥ 24 | ≥ 48 |
| CrCl 15-30 ml/min | Not indicated | Not indicated | ≥ 36 | ≥ 48 | ≥ 36 | ≥ 48 |
| CrCl < 15 ml/min | No official indication for use | |||||
Low risk – surgery with low risk of bleeding, high risk – surgery with high risk of bleeding
Fig. 2.
Algorithm for management with non-vitamin K oral anticoagulants around the time of surgery, for patients on rivaroxaban and apixaban with a creatinine clearance (CrCl) of more than 30 ml/min [7]
Conclusions
New oral anticoagulants have demonstrated their efficacy as an alternative to vitamin K antagonists. Although these agents do not require routine coagulation monitoring, assays to assess the level of anticoagulation may be of assistance in certain circumstances such as in case of overdose, in patients with a hemorrhagic or thromboembolic event during treatment, or to assess compliance. They cause significant prolongation of the clotting reaction, which in turn can produce altered and potentially misleading results in routine coagulation tests. Therefore, physicians will have to learn how to use these drugs effectively and safely in clinical practice.
Patients on NOACs pose multiple perioperative challenges for all medical disciplines involved. The individual risk for uncontrolled bleeding versus the urgency for surgery needs to be evaluated on an individual basis.
Biography

Disclosure
Authors report no conflict of interest.
References
- 1.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, American College of Chest Physicians Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 3.Beyer-Westendorf J, Ageno W. Benefit-risk profile of non-vitamin K antagonist oral anticoagulants in the management of venous thromboembolism. Thromb Haemost. 2014;16:113–128. doi: 10.1160/TH14-06-0484. [DOI] [PubMed] [Google Scholar]
- 4.Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2011. Pradaxa (dabigatran) package insert. [Google Scholar]
- 5.Bayer Pharma AG. Xarelto (Rivaroxaban) Xarelto® Summary of Product Characteristics – EU; 2013. Available at: http://www.xarelto.com/en/information-on-xarelto/summary-of-product-characteristics/ [Google Scholar]
- 6.2012. Eliquis Product Monograph Canada. [Google Scholar]
- 7.Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 8.FDA Advisory Committee Recommends Savaysa (edoxaban) for Reduction of Embolic Events in Non-Valvular Atrial Fibrillation. 2015 [Google Scholar]
- 9.Mismetti P, Laporte S. New oral antithrombotics: a need for laboratory monitoring. J Thromb Haemost. 2010;8:621–626. doi: 10.1111/j.1538-7836.2010.03764.x. [DOI] [PubMed] [Google Scholar]
- 10.Dager WE, Gosselin RC, Kitchen S, Dwyre D. Dabigatran effects on the international normalized ratio, activated partial thromboplastin time, thrombin time, and fibrinogen: a multicenter, in vitro study. Ann Pharmacother. 2012;46:1627–1236. doi: 10.1345/aph.1R179. [DOI] [PubMed] [Google Scholar]
- 11.Carreiro J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs. 2008;17:1937–1945. doi: 10.1517/13543780802528625. [DOI] [PubMed] [Google Scholar]
- 12.Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, Reeves RA, LaCreta F. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G, Favaloro EJ. Activated partial thromboplastin time: new tricks for an old dogma. Semin Thromb Hemost. 2008;34:604–611. doi: 10.1055/s-0028-1104539. [DOI] [PubMed] [Google Scholar]
- 14.Flaker G, Ezekowitz M, Yusuf S, Wallentin L, Noack H, Brueckmann M, Reilly P, Hohnloser SH, Connolly S. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854–855. doi: 10.1016/j.jacc.2011.10.896. [DOI] [PubMed] [Google Scholar]
- 15.van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate-a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 16.Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban – an oral, direct fac tor Xa inhibitor. Thromb Haemost. 2010;103:815–825. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 17.Wong PC, Crain EJ, Watson CA, Xin B. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost. 2009;7:1313–1320. doi: 10.1111/j.1538-7836.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 18.Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–847. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Yadava M, An IC, Sayeed A, Laird-Fick HS, Gourineni V, Abela GS. Coagulopathy and Extremely Elevated PT/INR after Dabigatran Etexilate Use in a Patient with End-Stage Renal Disease. Case Rep Med. 2013;2013:131395. doi: 10.1155/2013/131395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal Bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother. 2012;46:e10. doi: 10.1345/aph.1Q747. [DOI] [PubMed] [Google Scholar]
- 22.Yeo CH, Eranki V, Pillai A, Morrison G. Rivaroxaban and its Effect on International Normalised Ratio-A Prospective Study of 28 Hip and Knee Arthroplasty Patients. American Medical Journal. 2012;3:126–129. [Google Scholar]
- 23.Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 24.Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, Gent M, Haas S, Huisman MV, Kakkar AK, Kälebo P, Kwong LM, Misselwitz F, Turpie AG. Population pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct factor Xa inhibitor – in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47:203–216. doi: 10.2165/00003088-200847030-00006. [DOI] [PubMed] [Google Scholar]
- 25.Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–461. [PubMed] [Google Scholar]
- 26.Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct Factor Xa inhibitor – in healthy subjects. Int J Clin Pharmacol Ther. 2007;45:335–344. doi: 10.5414/cpp45335. [DOI] [PubMed] [Google Scholar]
- 27.Boks AL, Brommer EJ, Schalm SW, Van Vliet HH. Hemostasis and fibrinolysis in severe liver failure and their relation to hemorrhage. Hepatology. 1986;6:79–86. doi: 10.1002/hep.1840060115. [DOI] [PubMed] [Google Scholar]
- 28.Buccheri G, Ferrigno D, Ginardi C, Zuliani C. Haemostatic abnormalities in lung cancer: prognostic implications. Eur J Cancer. 1997;33:50–55. doi: 10.1016/s0959-8049(96)00310-3. [DOI] [PubMed] [Google Scholar]
- 29.Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–679. doi: 10.1097/FTD.0b013e3181f2f264. [DOI] [PubMed] [Google Scholar]
- 30.Tripodi A, Chantarangkul V, Guinet C, Samama MM. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011;9:226–228. doi: 10.1111/j.1538-7836.2010.04106.x. [DOI] [PubMed] [Google Scholar]
- 31.Tobu M, Iqbal O, Hoppensteadt D, Neville B, Messmore HL, Fareed J. Anti-Xa and anti-IIa drugs alter international normalized ratio measurements: potential problems in the monitoring of oral anticoagulants. Clin Appl Thromb Hemost. 2004;10:301–309. doi: 10.1177/107602960401000402. [DOI] [PubMed] [Google Scholar]
- 32.Douxfils J, Chatelain C, Chatelain B, Dogne JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110:283–294. doi: 10.1160/TH12-12-0898. [DOI] [PubMed] [Google Scholar]
- 33.Morishima Y, Kamisato C. Laboratory measurements of the oral direct factor xa inhibitor edoxaban: comparison of prothrombin time, activated partial thromboplastin time, and thrombin generation assay. Am J Clin Pathol. 2015;143:241–247. doi: 10.1309/AJCPQ2NJD3PXFTUG. [DOI] [PubMed] [Google Scholar]
- 34.Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis. 2013;36:195–202. doi: 10.1007/s11239-013-0923-y. [DOI] [PubMed] [Google Scholar]
- 35.Rohde G. Determination of rivaroxaban – a novel, oral, direct Factor Xa inhibitor – in human plasma by high-performance liquid chromatography – tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:43–50. doi: 10.1016/j.jchromb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Bae JP, Dobesh PP, Klepser DG, Anderson JD, Zagar AJ, McCollam PL, Tomlin ME. Adherence and dosing frequency of common medications forcardiovascular patients. Am J Manag Care. 2012;18:139–146. [PubMed] [Google Scholar]
- 37.Salazar DE, Mendell J, Kastrissios H, Green M, Carrothers TJ, Song S, Patel I, Bocanegra TS, Antman EM, Giugliano RP, Kunitada S, Dornseif B, Shi M, Tachibana M, Zhou S, Rohatagi S. mModelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost. 2012;107:925–936. doi: 10.1160/TH11-08-0566. [DOI] [PubMed] [Google Scholar]
- 38.Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS) Eur Heart J. 2014;35:3155–3179. doi: 10.1093/eurheartj/ehu298. [DOI] [PubMed] [Google Scholar]
- 39.Van Ryn J, Sieger P, Kink-Eiband M, et al. New Orleans, LA: 2009. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. Paper presented at 51st American Society of Hematology Annual Meeting and Exposition, (Abstract) [Google Scholar]
- 40.Suryanarayan D, Schulman S. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133:S158–S166. doi: 10.1016/S0049-3848(14)50026-6. [DOI] [PubMed] [Google Scholar]
- 41.Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172–2174. doi: 10.1182/blood-2011-11-393587. [DOI] [PubMed] [Google Scholar]
- 42.Bershad EM, Suarez JI. Prothrombin complex concentrates for oral anticoagulant therapy-related intracranial hemorrhage: a review of the literature. Neurocrit Care. 2010;12:403–413. doi: 10.1007/s12028-009-9310-0. [DOI] [PubMed] [Google Scholar]
- 43.Lachen: Octapharma; 2007. Octaplex product information. [Google Scholar]
- 44.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of Rivaroxaban and Dabigatran by Prothrombin Complex Concentrate. Circulation. 2011;124:1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 45.Marlu R, Hodaj E, Paris A, Albaladejo P, Crackowski JL, Pernod G. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–224. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 46.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 47.Van Ryn J, Ruehl D, Priepke H, et al. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. Haematologica. 2008:148. (Abstract) [Google Scholar]
- 48.Lu G, DeGuzman F, Karbarz MJ, et al. Paris: 2011. Reversal of rivaroxaban mediated anticoagulation in animal models by a recombinant antidote protein (r-antidote, PRT064445) Presented at the European Society of Cardiology Congress, (Abstract) [Google Scholar]