Abstract
Background
We explored the prevalence of and risk factors for type 2 diabetes in the adult population of Shanghai (China) with and without dyslipidemia.
Material/Methods
We conducted a cross-sectional survey including 14 385 adults (aged 16 to 88 years) in Shanghai using a stratified, multistage cluster sampling approach.
Results
Type 2 diabetes and hyperlipidemia were found in 1456 (10.1%) and 4583 (31.9%) subjects, respectively. Type 2 diabetes was more common in males (11.4%) than in females (9.2%, P<0.01), in the elderly (> or =65 years, 22.5%) than in younger (<55 years, <10%, P<0.01) individuals, and in urban (12.8%) than in rural populations (5.2%, P<0.01). Diabetes incidence was higher among patients with hyperlipidemia than in controls (16.9% vs. 7.0%, P<0.01; OR=2.72, 95% CI 2.44–3.03). Compared with controls, the risk for diabetes in subjects with isolated hypertriglyceridemia, isolated hypercholesterolemia, and mixed hyperlipidemia increased 1.75-fold (95% CI 1.53–1.99), 1.53-fold (95% CI 1.17–2.01), and 2.93-fold (95% CI 2.37–3.63), respectively. The fasting plasma glucose (FPG) and 2h-postprandial plasma glucose (2h-PG) increased with age in both sexes. The age- and sex-adjusted FPG and 2h-PG levels in hyperlipidemia were significantly higher than in controls (P<0.01).
Conclusions
A high prevalence of type 2 diabetes in hyperlipidemia patients exists in Shanghai. Hyperlipidemia is associated with elevated blood glucose levels and therefore requires prompt intervention for prevention and treatment of diabetes in patients with dyslipidemia.
MeSH Keywords: Diabetes Mellitus, Type 2; Hyperlipidemias; Risk Factors
Background
Type 2 diabetes is one of the most common chronic conditions and its prevalence has increased continuously over the past decades. China’s rapid economic growth, increased life expectancy, and altered lifestyle have significantly increased the prevalence of type 2 diabetes [1–3]. Dyslipidemia is a common comorbidity in diabetes [3]. In Asian and European epidemiological studies, hyperlipidemia is commonly associated with diabetes [3,4]. However, few, if any, epidemiological surveys have investigated the role of serum lipids in incident of type 2 diabetes in Shanghai, the biggest city of China [5].
Therefore, we carried out a subanalysis of dada of the Shanghai Dyslipidemia Study [6], using a cross-sectional survey to determine the prevalence of type 2 diabetes in patients with and without dyslipidemia. We assessed the risk odds of type 2 diabetes associated with different type of hyperlipidemia in the adult population of Shanghai.
Material and Methods
Study design and participants
The study is a stratified, multistage cluster sampling research involving 2 rural districts and 4 urban districts of Shanghai region. The study design, selection criteria, methodology, and participants’ baseline characteristics have been detailed elsewhere [6]. Inclusion in the study was restricted to adults aged at least 16 years and registered as local residents. Participants with known type 1 diabetes, serious diseases, or pregnancy were excluded. Anthropometric indexes, including body weight, height, and waist and hip circumference, were measured. Body mass index (BMI, weight/height2 [kg/m2]) and waist-to-hip ratio (WHR) were calculated from these measurements. The levels of serum triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were measured. Participants were administered a standard 2-h 75-g oral glucose tolerance test (OGTT).
The study protocol, which is in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical/Epidemiological Studies of the National Health and Family Planning Commission of the People’s Republic of China, received ethics approval from the institutional review boards of all the participating institutions. All enrolled patients provided written informed consent.
Individual data were forwarded to the Epidemiology Unit of Shanghai Jiao Tong University for data analysis.
Working definitions
Type 2 diabetes as defined by the American Diabetes Association, is characterized by fasting plasma glucose (FPG) level ≥7.0 mmol/L (126 mg/dl) or 2h-postprandial plasma glucose (2h-PG) level of at least 11.1 mmol/L (200 mg/dl) during OGTT. Other parameters were defined as follows. Isolated impaired fasting glucose (IFG): FPG level, 5.6mmol/L [100 mg/dl] −6.9 mmol/L [125 mg/dl], and 2h-PG level,<7.8 mmol/L [140 mg/dl]); Isolated impaired glucose tolerance (IGT): 2h-PG level, 7.8 mmol/L [140 mg/dl] −11.0 mmol/L [199 mg/dl] and FPG level,<5.6 mmol/L [100 mg/dl]); and Combined IFG and IGT: FPG level, 5.6 mmol/L [100 mg/dl] −6.9 mmol/L [125 mg/dl], and 2h-PG level, 7.8 mmol/L [140 mg/dl] −11.0 mmol/L [199 mg/dl]) [7]. Previously diagnosed diabetes was identified by a valid questionnaire. Impaired glucose regulation was defined as either impaired fasting glucose or impaired glucose tolerance.
According to the criteria set by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) [8], hyperlipidemia was classified into three phenotypes: isolated hypertriglyceridemia (TG ≥1.7 mmol/L and TC <6.2 mmol/L); isolated hypercholesterolemia (TC ≥6.2 mmol/l and TG <1.7 mmol/L); and mixed hyperlipidemia (TG ≥1.7 mmol/L and TC ≥6.2 mmol/L).
Statistical analysis
All analyses were performed using SPSS for Windows (version 13.0; SPSS Inc., Chicago, IL, USA). The significance of univariate differences was assessed by chi-squared and Student’s t- tests. Logistic regression analysis was performed to estimate the odds ratio (OR) of type 2 diabetes and 95% confidence intervals (CIs). We used logistic regression models to estimate the adjusted odds ratios (ORs) and 95% confidence intervals (CI) for incident type 2 diabetes comparing three phenotypes of hyperlipidemia categories to the normal group. The data were adjusted for the multiple covariates. In the multivariate models, we included variables that might confound the relationship between hyperlipidemia and type 2 diabetes, which include: age (per 10-yr increment), sex, urban residence, waist circumference, BMI and phenotypes of hyperlipidemia. A P-value<0.05 was considered statistically significant and all values were two-sided.
Results
Sample status
A total of 17 526 individuals were invited to participate in the study, of which 14 401 participants completed the study, with an overall response of 82.2%. After the exclusion of 12 subjects with missing plasma lipid profile, 14 385 (42.8% men) were included in the final analysis. Of these, 14 342 (99.7%) were ethnic Han Chinese, 5084 subjects were from rural and 9301 were from urban areas, aged 16–88 years, living in Shanghai.
Prevalence of type 2 diabetes and impaired glucose regulation
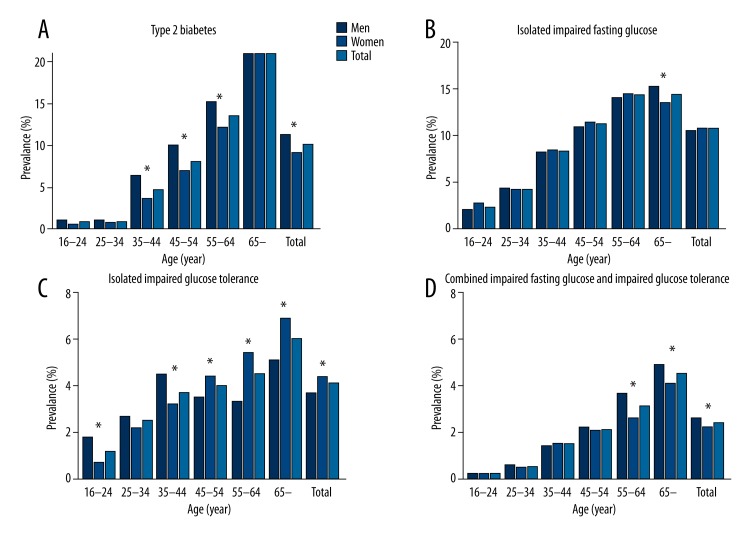
There were 1456 subjects (10.1%) with type 2 diabetes, including 699 (11.4%) men and 757 (9.2%) women (Figure 1). The prevalence of isolated IGF, isolated IGT, and combined IFG and IGT was 10.8%, 4.1% and 2.4%, respectively. After age and sex standardization based on the 2000 China census data, the type 2 diabetes prevalence was 7.0% (7.6% for men and 6.7% for women) (data not shown). Men showed a significantly higher prevalence of diabetes than women (χ2=18.28, P<0.01).
Figure 1.
Age-specific prevalence of diabetes mellitus and impaired glucose regulation among men and women. Prevalence of total type 2 diabetes (A), isolated impaired fasting glucose (B), isolated impaired glucose tolerance (C) and combined impaired fasting glucose and impaired glucose tolerance (D) in men and women (* P<0.05).
Prevalence of type 2 diabetes increased with age, from 0.9% (15–24 years age group) to 22.5% (65 years of age and older). The prevalence of type 2 diabetes was <10% before the age of 55 years. The prevalence of impaired glucose regulation increased with age.
The prevalence of isolated IFG, type 2 diabetes, and combined IFG and IGT in urban areas was significantly higher than in rural areas (13.8%, 12.8%, and 2.9%, respectively, in urban areas; and 5.2%, 5.2%, and 1.3%, respectively, in rural areas; all P<0.01). However, the prevalence of isolated IGT in rural areas was higher than that in urban areas (2.9% vs. 1.3%, P<0.01) (Table 1).
Table 1.
Comparison of abnormal glucose metabolisms prevalence and serum glucose concentrations between urban and rural residents in Shanghai.
| Characteristics | Rural areas | Urban areas | ||||
|---|---|---|---|---|---|---|
| Overall (n=5084) | Without hyperlipidemia (n=3413) | With hyperlipidemia (n=1671) | Overall (n=9301) | Without Hyperlipidemia (n=6389) | With Hyperlipidemia (n=2912) | |
| Male, % (95% CI) | 39.5 (38.2, 40.8) | 41.1 (39.4, 42.8) | 36.3 (34.0, 38.6)* | 44.5 (43.5, 45.5)*** | 41.5 (40.3, 42.7) | 51.1 (49.3, 52.9)* |
| Age (years), mean (SD) | 47.0 (14.0) | 45.0 (14.4) | 51.7 (11.4)* | 50.9 (14.7)*** | 48.9 (15.3) | 55.3 (12.3)* |
| BMI (kg/m2), mean (SD) | 23.6 (3.4) | 23.0 (3.2) | 24.9 (3.4)* | 24.1 (3.5)*** | 23.6 (3.4) | 25.4 (3.3)* |
| WC (cm), mean (SD) | 77.8 (9.4) | 75.6 (8.9) | 82.3 (9.0)* | 80.2 (10.2)*** | 78.2 (9.9) | 84.6 (9.3)* |
| FPG (mmol/L), mean (SD) | 4.7 (1.4) | 4.6 (1.1) | 4.9 (1.8)* | 5.5 (1.6)*** | 5.3 (1.3) | 6.0 (2.1)* |
| 2h-PG (mmol/L), mean (SD) | 5.7 (2.5) | 5.3 (2.0) | 6.5 (2.3)* | 6.0 (2.9)*** | 5.6 (2.4) | 7.0 (3.7)* |
| IFG, % (95% CI) | 5.2 (4.6, 5.8) | 5.4 (4.6, 6.2) | 4.8 (3.8, 5.8) | 13.8 (13.1, 14.5)*** | 13.0 (12.2, 13.8) | 15.4 (14.1, 16.7)** |
| IGT, % (95% CI) | 6.1 (5.4, 6.8) | 4.5 (3.8, 5.2) | 9.6 (8.2, 11.0)* | 3.0 (2.7, 3.4)*** | 2.3 (1.9, 2.7) | 4.5 (3.7, 5.3)* |
| IFG+IGT, % (95% CI) | 1.3(1.0, 1.6) | 1.1(0.8, 1.4) | 1.8 (1.2, 2.4)** | 2.9 (2.6, 3.2)*** | 2.0 (1.7, 2.3) | 5.1 (4.3, 5.9)* |
| Diabetes, % (95% CI) | 5.2 (4.6, 5.8) | 3.5 (2.9, 4.1) | 8.9 (7.5, 10.3)* | 12.8 (12.1, 13.5)*** | 8.8 (8.1, 9.5) | 21.5 (20.0, 23.0)* |
P-value <0.01 between with and without hyperlipidemia;
P-value <0.05 between with and without hyperlipidemia;
P-value <0.01 between rural and urban areas.
BMI – body mass index; WC – waist circumference; FPG – fasting plasma glucose; 2h-PG – 2-h postprandial plasma glucose; IFG – isolated impaired fasting glucose; IGT – isolated impaired glucose tolerance; IFG+IGT – combined impaired fasting glucose and impaired glucose tolerance.
The prevalence of type 2 diabetes was higher in subjects with hyperlipidemia than that in subjects without hyperlipidemia (16.9% vs. 7%, P<0.01) (Table 2). A similar higher prevalence was observed for isolated IGT (6.3% vs. 3.0%, P<0.01) and combined IFG and IGT (3.9% vs. 1.7%, P<0.01).
Table 2.
Comparison of abnormal glucose metabolisms prevalence between with hyperlipidemia and without hyperlipidemia.
| Characteristics | Without hyperlipidemia (n=9802) | With hyperlipidemia (n=4583) |
|---|---|---|
| Isolated Impaired Fasting Glucose, % (95% CI) | ||
| Men | 10.0 (9.1, 10.9) | 11.7 (10.3, 13.1)** |
| Women | 10.7 (9.9, 11.5) | 11.4 (10.2, 12.6) |
| Age ≤64 | 9.4 (8.8, 10.0) | 11.2 (10.1, 12.3)* |
| Age ≥65 | 15.7 (13.9, 17.5) | 12.5 (10.6, 14.4)** |
| Total | 10.4 (9.8, 11.0) | 11.5 (10.6, 12.4)** |
| Isolated impaired glucose tolerance, % (95% CI) | ||
| Men | 2.9 (2.4, 3.4) | 5.2 (4.2, 6.2)* |
| Women | 3.1 (2.7, 3.5) | 7.4 (6.4, 8.4)* |
| Age ≤64 | 2.6 (2.3, 2.9) | 6.2 (5.4, 7.0)* |
| Age ≥65 | 5.4 (4.3, 6.5) | 6.8 (5.3, 8.3) |
| Total | 3.0 (2.7, 3.3) | 6.3 (5.6, 7.0)* |
| Combined impaired fasting glucose and impaired glucose tolerance, % (95% CI) | ||
| Men | 1.5 (1.1, 1.9) | 4.7(3.8, 5.6)* |
| Women | 1.8 (1.5, 2.2) | 3.2 (2.5, 3.9)* |
| Age ≤64 | 1.3 (1.1, 1.5) | 3.3(2.7, 3.9)* |
| Age ≥65 | 3.6 (2.7, 4.5) | 5.6 (4.3, 6.9)** |
| Total | 1.7 (1.4, 2.0) | 3.9(3.3, 4.5)* |
| Diabetes, % (95% CI) | ||
| Men | 8.3 (7.5, 9.1) | 17.3 (15.7, 18.9)* |
| Women | 6.0 (5.4, 6.6) | 16.6 (15.1, 18.1)* |
| Age ≤64 | 4.6 (4.1, 5.1) | 13.7 (12.6, 14.8)* |
| Age ≥65 | 19.4 (17.4, 21.4) | 26.8 (24.2, 29.4)* |
| Total | 7.0 (6.5, 7.5) | 16.9 (15.8, 18.0)* |
P-value <0.01 between with and without hyperlipidemia;
P-value <0.05 between with and without hyperlipidemia.
Analysis of risk factors for type 2 diabetes
The 14 385 subjects were divided into diabetes group (1456) and non-diabetes group (12 929). Univariate analysis showed that sex, age, BMI, WHR, waist circumference, FPG, 2-h PG, triglyceride, TC, TC/HDL-C (total cholesterol/HDL cholesterol ratios), and non-HDL-C (non-HDL cholesterol) were associated with diabetes (Table 3).
Table 3.
Anthropometric data and serum glucose and lipid levels in 14,385 participants in Shanghai, China.
| Characteristics | All subjects (n=14,385) | With hyperlipidemia (n=4583) | Without hyperlipidemia (n=9802) | P-value | |||
|---|---|---|---|---|---|---|---|
| Isolated hypertriglyceridemia (n=3582) | Isolated hypercholesterolemia (n=457) | Mixed hyperlipidemia (n=544) | Overall | ||||
| Male, n (%) | 6150 (42.8) | 1736 (48.5) | 153 (33.5) | 206 (37.9) | 2095(34.1) | 4055 (65.9) | <0.01 |
| Age (years), mean (SD) | 49.5 (14.5) | 52.7 (12.4) | 58.5 (10.4) | 57.8 (10.4) | 54.0 (12.1) | 47.6 (15.1) | <0.01 |
| BMI (kg/m2), mean (SD) | 24.0 (3.5) | 25.3 (3.3) | 24.1 (3.4) | 25.8 (3.4) | 25.2 (3.3) | 23.4 (3.4) | <0.01 |
| WC (cm), mean (SD) | 79.4 (10.0) | 84.0 (9.1) | 80.3 (9.9) | 85.3 (9.2) | 83.8 (9.2) | 77.3 (9.7) | <0.01 |
| WHR, mean (SD) | 0.85 (0.07) | 0.88 (0.06) | 0.84 (0.07) | 0.88 (0.06) | 0.87 (0.07) | 0.83 (0.07) | <0.01 |
| TG (mmol/L), mean (SD) | 1.5 (1.2) | 2.7 (1.4) | 1.2 (0.3) | 3.4 (2.9) | 2.6 (1.7) | 1.0 (0.3) | <0.01 |
| TC (mmol/L), mean (SD) | 4.7 (1.1) | 4.8 (0.8) | 6.8 (1.0) | 6.9 (0.9) | 5.2 (1.2) | 4.4 (0.8) | <0.01 |
| HDL-C (mmol/L), mean (SD) | 1.4 (0.5) | 1.3 (0.5) | 1.8 (0.5) | 1.5 (0.4) | 1.3 (0.5) | 1.4 (0.5) | <0.01 |
| TC/HDL, mean (SD) | 3.5 (1.2) | 4.0 (1.2) | 4.1 (1.3) | 5.1 (1.7) | 4.1 (1.3) | 3.2 (1.0) | <0.01 |
| non-HDL (mmol/L), mean (SD) | 3.2 (1.0) | 3.5 (0.8) | 5.0 (1.1) | 5.5 (0.9) | 3.9 (1.2) | 2.9 (0.8) | <0.01 |
| FPG (mmol/L), mean (SD) | 5.2 (1.6) | 5.5 (2.0) | 5.7 (2.0) | 6.2 (2.5) | 5.6 (2.1) | 5.0 (1.3) | <0.01 |
| 2h-PG(mmol/L), mean (SD) | 5.9 (2.8) | 6.7 (3.4) | 6.3 (3.6) | 7.9 (4.4) | 6.8 (3.6) | 5.5 (2.0) | <0.01 |
| IFG, % (95% CI) | 10.8 (10.3,11.3) | 10.6 (9.6,11.6) | 14.9 (11.6,18.2) | 15.3 (12.3,18.3) | 11.5 (10.6,12.4) | 10.4 (9.8,11.0) | 0.037 |
| IGT, % (95% CI) | 4.1 (3.8,4.4) | 6.9 (6.1,7.7) | 3.7 (2.0,5.4) | 4.8 (3.0,6.6) | 6.3 (5.6,7.0) | 3.0 (2.7,3.3) | <0.01 |
| IFG+IGT, % (95% CI) | 2.4 (2.1,2.7) | 3.9 (3.3,4.5) | 2.2 (0.9,3.5) | 5.5 (3.6,7.4) | 3.9 (3.3,4.5) | 1.7 (1.4,2.0) | <0.01 |
| Diabetes, % (95% CI) | 10.1 (9.6,10.6) | 15.3 (14.1,16.5) | 16.4 (13.0,19.8) | 27.9 (24.1,31.7) | 16.9 (15.8,18.0) | 7.0 (6.5,7.5) | <0.01 |
P-value for comparison between subjects with normal glucose tolerance or not at α=0.01 level.
BMI – body mass index; WC – waist circumference; WHR – waist-to-hip ratio; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein cholesterol; TC/HDL – total cholesterol/HDL cholesterol ratios; non-HDL – non-HDL cholesterol (the difference between total cholesterol and high-density lipoprotein cholesterol); FPG – fasting plasma glucose; 2h-PG – 2-h postprandial plasma glucose; IFG – isolated impaired fasting glucose; IGT – isolated impaired glucose tolerance; IFG+IGT – combined impaired fasting glucose and impaired glucose tolerance.
Multivariable logistic regression revealed that urban residence, age, waist circumference, isolated hypertriglyceridemia, isolated hypercholesterolemia, and mixed hyperlipidemia were independently related to type 2 diabetes. The odds ratio (OR) for type 2 diabetes and the corresponding 95% confidence intervals (CI) were calculated using a dichotomous variable logistic regression model (Table 4).
Table 4.
Multivariable-adjusted odds ratios for type 2 diabetes.
| Variable | Coefficient of regression (b) | Standard error (SE) | Wald χ2 | P | OR* | 95%CI |
|---|---|---|---|---|---|---|
| Urban residence | 0.721 | 0.074 | 95.199 | 0.000 | 2.056 | 1.779–2.377 |
| Age, per 10-yr increment | 0.496 | 0.026 | 356.549 | 0.000 | 1.642 | 1.560–1.729 |
| Waist circumference | 0.045 | 0.003 | 198.138 | 0.000 | 1.046 | 1.039–1.052 |
| Isolated hypertriglyceridemia** | 0.557 | 0.066 | 71.175 | 0.000 | 1.745 | 1.533–1.986 |
| Isolated hypercholesterolemia*** | 0.428 | 0.138 | 9.627 | 0.002 | 1.534 | 1.171–2.009 |
| Mixed hyperlipidemia# | 1.074 | 0.109 | 97.065 | 0.000 | 2.928 | 2.365–3.626 |
Odds ratios were calculated with the use of dichotomous variable logistic regression model. All covariables listed were included in the model simultaneously. Status with respect to sex and BMI were not significantly associated with the risk of diabetes and were not included in the final model.
Isolated hypertriglyceridemia was defined as serum triglycerides ≥1.7 mmol/L and total cholesterol <6.2 mmol/L.
Isolated hypercholesterolemia was defined as total cholesterol ≥6.2 mmol/l or on medication and triglycerides <1.7 mmol/L.
Mixed hyperlipidemia was defined as triglycerides ≥1.7 mmol/L and total cholesterol ≥6.2 mmol/L.
Age- and sex-specific blood glucose categories in hyperlipidemia
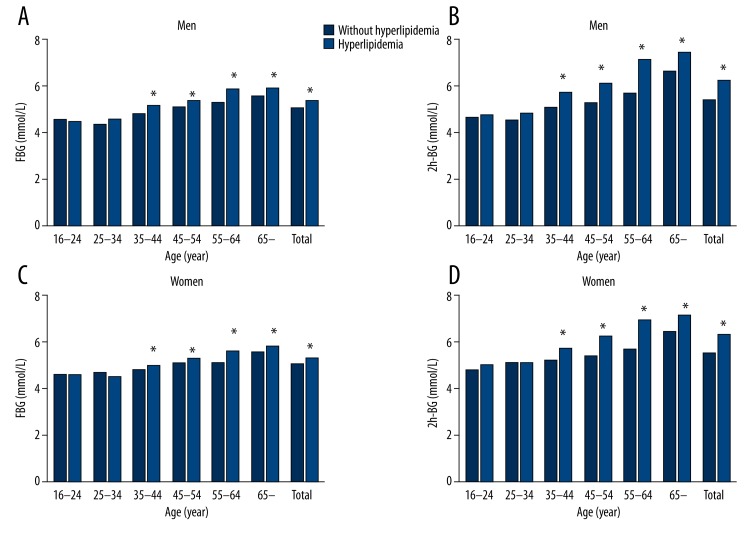
Compared with individuals without hyperlipidemia, levels of FBG and 2hPG increased in subjects with hyperlipidemia in most of the age groups both in men and women (Figure 2). No differences in average levels of FBG and 2hPG were seen between men and women. The levels of FBG and 2hPG increased with age.
Figure 2.
Age- and sex-specific serum glucose levels with hyperlipidemia. Fasting plasma glucose level in men (A), in women (C) and 2-h postprandial plasma glucose in men (B), in women (D) with and without hyperlipidemia (* P<0.05).
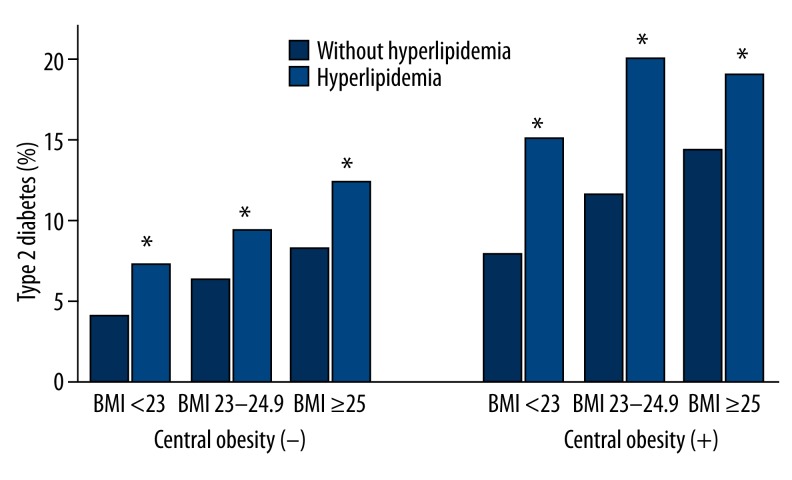
The prevalence of type 2 diabetes increased significantly with increasing BMI (P<0.05 for both hyperlipidemia and without hyperlipidemia) in normal waist circumference subgroups (Figure 3). In each BMI subgroup, the prevalence of type 2 diabetes was higher in central obesity than in normal waist circumference (P<0.05 for both hyperlipidemia and without hyperlipidemia).
Figure 3.
BMI- and waist circumference- specific prevalence of type 2 diabetes with hyperlipidemia. Prevalence of total type 2 diabetes in patients, based on BMI; Central obesity is defined as waist circumference greater than 90 cm in males and greater than 80 cm in females. * Statistically different from control group (P<0.05).
Discussion
The prevalence of type 2 diabetes was reported to be 6.87% in a sample of 5628 men and women aged 20–94 years, living in a Shanghai urban community in 2001 [5]. This study reports one of the largest population-based type 2 diabetes studies ever conducted in Shanghai. Our investigation demonstrated that the prevalence of type 2 diabetes among Shanghai adults was 10.1%, which is higher than the Chinese national average (9.7% in 2008) [2]. Furthermore, the prevalence rates of impaired fasting glucose and impaired glucose tolerance (17.3%) found in this study were significantly higher than average Chinese national standards (15.5%). The prevalence of diabetes was higher among urban than rural residents [2]. Thus, the high incidence of diabetes in Chinese adults prompted screening of the high-risk population for early detection of diabetes.
Patients with hyperlipidemia are at an increased risk of developing type 2 diabetes and abnormal glucose metabolism [4], as well as increased morbidity and mortality from type 2 diabetes and cardiovascular disease [9,10]. It is well established that serum lipid profiles are worse in diabetic than in non-diabetic individuals from different ethnic groups [3,4]. We found that subjects with hyperlipidemia showed higher FPG and 2h-PG levels than normolipidemic individuals. To the best of our knowledge, the prevalence of type 2 diabetes in the dyslipidemia population has been less studied in China. This might be the first large-scale epidemiologic study on the prevalence of and risk factors for type 2 diabetes in Chinese patients with dyslipidemia in China.
The most common pattern of dyslipidemia in Chinese with and without type 2 diabetes is represented by elevated serum triglyceride levels and hypertriglyceridemia [11]. Hypertriglyceridemia was an independent risk factor for type 2 diabetes in Qingdao, north China [12]. Hypertriglyceridemia (31.8%) was the most common phenotype of hyperlipidemia in this study, and therefore, a total of 547 diabetics (35.7%) were diagnosed with hypertriglyceridemia. However, the risk of isolated hypertriglyceridemia and isolated hypercholesterolemia for type 2 diabetes was almost similar (OR 1.7 vs. 1.5) in this study. Our study showed that subjects with mixed hyperlipidemia were more than 3 times higher at risk of developing type 2 diabetes compared with participants with normal lipidemia. Some studies supported the association of serum glucose with TC level, although not as clearly as compared with serum triglyceride level [3]. In addition, isolated low HDL-C showed no effect on the prevalence of type 2 diabetes in this study.
Type 2 diabetes and hyperlipidemia are diagnosed within the same individuals, due to common risk factors including age, sex, obesity, physical inactivity, and high fat diet. The prevalence of hyperlipidemia increased with age, which was reported in our previous study [6]. Among patients with hyperlipidemia aged above 35, prevalence of type 2 diabetes also increased, with a prevalence of 4.7% in ages 35 to 44 years, 8.1% in ages 45 to 54 years, 13.5% in those aged 55 to 64 years, and 22.5% in those aged 65 years or older. Although the prevalence of hyperlipidemia was higher in men than in women, we observed a similar risk of diabetes between men and women with hyperlipidemia, which was confirmed by multivariate analysis. Although these shared risk factors increased the risk of type 2 diabetes and dyslipidemia, these factors were interrelated and difficult to assess in isolation.
Type 2 diabetes is characterized by both insulin resistance and hyperinsulinemia. In hyperlipidemia patients, the free fatty acids act as precursors to gluconeogenesis in the liver, increase apoptosis in beta cells of the pancreas, and raise insulin resistance in muscles [13], resulting in impaired insulin secretion and persistent hyperglycemia. The biological links between diabetes and dyslipidemia are not clear [14].
Serum lipid values are the predictors of coronary heart disease among East Asians with diabetes. Aggressive interventions in dyslipidemia reduce the risk of cardiovascular event for patients with type 2 diabetes. Recent findings from the Cholesterol Treatment Trialists study using individual patient data in meta-analysis indicate that the benefits of primary prevention with statins include lower (<1% per year) risk of a major cardiovascular event [15]. On the other hand, improved glycemic control is very effective in reducing serum triglyceride levels. Insulin therapy (alone or with insulin sensitizers) may also be particularly effective in lowering serum triglyceride levels in diabetic patients with hyperlipidemia [16]. Endothelial function is associated with complex interaction between dyslipidemia and incident of type 2 diabetes and cardiovascular disease [17,18].
Our study has several limitations. First, previously diagnosed diabetes was defined as self-reported diabetes using a questionnaire, resulting in a potential bias. Second, the time point of diabetes diagnosis was not considered in the study. Finally, we failed to evaluate the role of pharmacological anamnesis and family history of diabetes in development of type 2 diabetes. In China, more than 40% of patients with type 2 diabetes remain undiagnosed and untreated.
Conclusions
Our study suggests that type 2 diabetes is very common in hyperlipidemia patients in Shanghai, and both serum levels of triglyceride and TC are associated with FPG and 2h-PG. Therefore, prompt intervention for prevention and treatment of diabetes in patients with hyperlipidemia, especially combined hypertriglyceridemia and hypercholesterolemia, are required. In 2009, the USPSTF concluded that the evidence was insufficient to recommend for or against routinely screening asymptomatic adults for type 2 diabetes or pre-diabetes, but it did recommend screening adults with hypertension or hyperlipidemia [19,20]. Screening adults for type 2 diabetes in Chinese patients with hyperlipidemia may be effective, especially in those with mixed hyperlipidemia in China.
Acknowledgments
GY.C. wrote the manuscript and researched data. X.X. researched data and contributed to discussion. JG.F. contributed to the discussion and reviewed/edited the manuscript. Many people helped with data collection and entry, including L.L., F.D., YY.L., and XJ.L.
Footnotes
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Source of support: This project was funded by the State Key Development Program for Basic Research of China (2012CB517501), Shanghai Municipal Health Bureau Foundation (01zD001), Shanghai Science and Technology Commission Foundation (10411956300), and the 100 Talents Program of the Shanghai Board of Health (XBR2011007)
References
- 1.Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2(12):980–91. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Qiao Q, Tuomilehto J, et al. Blood lipid levels in relation to glucose status in seven populations of Asian origin without a prior history of diabetes: the DECODA study. Diabetes Metab Res Rev. 2009;25(6):549–57. doi: 10.1002/dmrr.994. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Qiao Q, Tuomilehto J, et al. Blood lipid levels in relation to glucose status in European men and women without a prior history of diabetes: the DECODE Study. Diabetes Res Clin Pract. 2008;82(3):364–77. doi: 10.1016/j.diabres.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Jia WP, Pang C, Chen L, et al. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia. 2007;50(2):286–92. doi: 10.1007/s00125-006-0503-1. [DOI] [PubMed] [Google Scholar]
- 6.Wu JY, Duan XY, Li L, et al. Dyslipidemia in Shanghai, China. Prev Med. 2010;51(5):412–15. doi: 10.1016/j.ypmed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Forlani G, Cerrelli F, et al. WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with Type 2 diabetes. Diabet Med. 2004;21(4):383–87. doi: 10.1111/j.1464-5491.2004.01115.x. [DOI] [PubMed] [Google Scholar]
- 9.Cui HB, Wang SH, Wang DQ, et al. Modified classic risk factors for coronary artery disease in Chinese Han population. Chin Med Sci J. 2007;22(4):216–23. [PubMed] [Google Scholar]
- 10.Wang SH, Sun ZL, Ruan XZ, et al. Dyslipidaemia among diabetic patients with ischemic stroke in a Chinese hospital. Chin Med J. 2009;122(21):2567–72. [PubMed] [Google Scholar]
- 11.Xu H, Song Y, You NC, et al. Prevalence and clustering of metabolic risk factors for type 2 diabetes among Chinese adults in Shanghai, China. BMC Public Health. 2010;10:683. doi: 10.1186/1471-2458-10-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning F, Pang ZC, Dong YH, et al. Risk factors associated with the dramatic increase in the prevalence of diabetes in the adult Chinese population in Qingdao, China. Diabet Med. 2009;26(9):855–63. doi: 10.1111/j.1464-5491.2009.02791.x. [DOI] [PubMed] [Google Scholar]
- 13.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–43. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Yu M, Li M, et al. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol Cell Biochem. 2012;363(1–2):85–91. doi: 10.1007/s11010-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 15.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan I, Kucuksayan E, Aslan M. Effect of insulin analog initiation therapy on LDL/HDL subfraction profile and HDL associated enzymes in type 2 diabetic patients. Lipids Health Dis. 2013;12:54. doi: 10.1186/1476-511X-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pannacciulli N, De Pergola G, Ciccone M, et al. Effect of family history of type 2 diabetes on the intima-media thickness of the common carotid artery in normal-weight, overweight, and obese glucose-tolerant young adults. Diabetes Care. 2003;26(4):1230–34. doi: 10.2337/diacare.26.4.1230. [DOI] [PubMed] [Google Scholar]
- 18.Tavares AC, Bocchi EA, Guimaraes GV. Endothelial function in pre-pubertal children at risk of developing cardiomyopathy: a new frontier. Clinics. 2012;67(3):273–78. doi: 10.6061/clinics/2012(03)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin KW, Chang C. Screening for type 2 diabetes mellitus in adults. Am Fam Phys. 2009;80(10):1141. [PubMed] [Google Scholar]
- 20.Norris SL, Kansagara D, Bougatsos C, Fu R Force USPST. Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148(11):855–68. doi: 10.7326/0003-4819-148-11-200806030-00008. [DOI] [PubMed] [Google Scholar]