Abstract
Cadmium, a carcinogenic metal, is highly toxic to renal, skeletal, nervous, respiratory, and cardiovascular systems. Accurate and precise quantification of mainstream smoke cadmium levels in cigarette smoke is important because of exposure concerns.
The two most common trapping techniques for collecting mainstream tobacco smoke particulate for analysis are glass fiber filters and electrostatic precipitators. We observed that a significant portion of total cadmium passed through standard glass fiber filters that are used to trap particulate matter. We therefore developed platinum traps to collect the cadmium that passed through the filters and tested a variety of cigarettes with different physical parameters for quantities of cadmium that passed though the filters.
We found less than 1% cadmium passed through electrostatic precipitators. In contrast, cadmium that passed through 92 mm glass fiber filters on a rotary smoking machine was significantly higher, ranging from 3.5% to 22.9% of total smoke cadmium deliveries. Cadmium passed through 44 mm filters typically used on linear smoking machines to an even greater degree, ranging from 13.6% to 30.4% of the total smoke cadmium deliveries. Differences in the cadmium that passed through from the glass fiber filters and electrostatic precipitator could be explained in part if cadmium resides in the smaller mainstream smoke aerosol particle sizes. Differences in particle size distribution could have toxicological implications and could help explain the pulmonary and cardiovascular cadmium uptake in smokers.
1. Introduction
Cadmium, an IARC group 1 human carcinogen,1 is highly toxic to renal, skeletal, nervous, respiratory, and cardiovascular systems.2 Pulmonary exposure to nebulized cadmium compounds induces emphysema3 and pulmonary interstitial fibrosis.4,5 Elevated blood cadmium levels are strongly associated with increased prevalence of peripheral artery disease.6 Pancreatic cancer has been associated with smoking and elevated urine cadmium concentrations.7 Elevated cadmium/zinc ratios in serum of smokers has been suggested as a critical determinant for risk of prostate cancer.8 The pulmonary, cardiovascular, and carcinogenic toxicological consequences of acute and chronic exposure to cadmium have made its quantitation in clinical samples a necessary part of U.S. biomonitoring programs and a focus of emergency response preparedness for many years.9
Cadmium has a biological half-life of 13.6 to 23.5 years10 and it bioaccumulates in tissues as a result of smoking. Increases in cadmium levels in lung tissue have been correlated with smoking history.11 Elevated cadmium levels in blood,12,13 and in urine,12,14,15 as a consequence of smoking indicate systemic distribution via the lungs. Biomonitoring results show that cadmium concentrations in urine from smokers increase with age at a faster rate than from non-smokers.14
Concentrations of toxic metals such as barium and manganese are much higher than cadmium concentrations in tobacco.16,17 Cadmium, however, has a lower propensity to form nonvolatile oxides relative to many metals such as these. Therefore, cadmium, like its periodic homologue mercury, is a relatively volatile metal that is readily transported in tobacco smoke. As a consequence of its volatility, cadmium concentrations in the particulate phase (the total particulate matter or TPM) of the mainstream smoke are higher than most other toxic elements in domestic and counterfeit tobacco products.18–20
By definition, the particulate phase of cigarette smoke is the portion of mainstream smoke that is trapped by a glass fiber filter, commonly referred to as a Cambridge filter pad (CFP), which has a filtration efficiency of 99.9% for 0.1 μm or larger particles.21 The portion of the smoke that passes through the CFP is known as the gas or vapor phase portion of the mainstream smoke. Carbon monoxide, nitrogen oxides, and many volatile organic compounds are transported almost exclusively in the gas phase of cigarette smoke.22 Mercury is reduced to elemental vapor form during combustion,23 and is one of few metals that is found predominantly in the gas phase of cigarette smoke. Other toxic metals, including most of the cadmium, are transported largely in the particulate phase of smoke. Historically, cadmium in mainstream cigarette smoke has been exclusively analyzed in the TPM. The TPM is generally collected from mainstream tobacco smoke using a standard protocol specified by ISO 3402 (1999)24 and ISO 3308(2000)25 or by the Health Canada Intense smoking regimen.26 The most common means for trapping TPM use Cambridge glass fiber filters,27,28 quartz air sampling filters,19,29 or electrostatic precipitation 20,30 while the “gas phase” of the smoke (including mercury) passes through the filter or electrostatic precipitator and is collected separately. The electrostatic precipitators presently available on smoking machines impart higher TPM trapping efficiency than the traditional glass fiber filters.31
Cadmium (boiling point 765°C), though not as volatile as its periodic homologue, mercury (boiling point 357°C), is nevertheless one of the more volatile metals. We observed that platinum traps for trapping mercury from the “gas phase” of cigarette smoke (after passing through one or two Cambridge glass fiber filters) had significant amounts of cadmium along with mercury. Since metal volatility in the 900°C burning coal of a cigarette32 may determine whether the metal enters and remains in the vapor phase or condenses to form particulate, we specifically examined the efficiency of cadmium trapping by glass fiber filter pads compared with electrostatic precipitation in order to examine the “phase-partitioning” behavior and the physical state of cadmium in the vapor phase or particulate phase of mainstream cigarette smoke.
2. Experimental
2.1 Reagents and samples
An enriched 110Cd standard for isotope dilution (Isoflex, San Francisco, CA, USA) was dissolved in 50% v/v double-distilled nitric acid (GFS, Columbus, OH, USA) and diluted in 1% nitric acid. Dilutions which were calculated to produce concentrations that would result in 110Cd intensities that were within the same order of magnitude as the 111Cd intensities, so that relative isotopic intensities would be optimal for isotope dilution analysis. Argon (99.999% purity) was obtained from Airgas, Chamblee, GA, USA. Water was brought to ≥18 MΩ·cm with a combined reverse osmosis and deionized water system (Aqua Solutions, Jasper, GA, USA).
The 3R4F research reference cigarettes were obtained from University of Kentucky, Lexington, KY, USA. Coresta Monitor, CM6, cigarettes (Coresta, Paris, France) were obtained from Cerulean (Henrico, Virginia, USA). Commercial cigarette varieties were purchased in 2011 from retail outlets in the greater metropolitan Atlanta area in Georgia, USA. Sampling was intended to reflect the popular U.S. products from different U.S. cigarette manufacturers having significant market share. Products with a wide range of design features were included. These samples are a convenience sampling and are not intended as being nationally representative. The samples were assigned unique identification numbers and logged into a database. Samples were stored in their original packaging until needed. Only authorized personnel had access to the samples. Cigarettes were conditioned prior to smoking at 22 ± 1 °C and 60 ± 3 % relative humidity for a minimum of 48 hours, according to ISO method 3402.24
2.2 Smoke Collection
Rotary RM20H and Linear LX20 smoking machines (Borgwaldt KC, Hamburg, Germany) were used to collect mainstream smoke samples. Air flow, puff volume, and leak tests were performed daily to assure acceptable performance. A Borgwaldt KC V10 ventilation tester was used to verify that pressure drops remained within specifications when platinum traps were placed in tandem with filter pads. A Borgwaldt KC service representative examined puff profiles and verified that puff profiles and pressure drops remained within specifications with platinum traps in tandem with filter pads. Standard CFPs (44 mm, Borgwaldt KC) were used with the linear smoking machine. Either an electrostatic precipitator attachment or 92 mm CFPs (Borgwaldt KC) were used with the rotary smoking machine. When electrostatic precipitation was used, the tungsten probe potential was set to 24.0 kV. Smoking conditions (i.e., puff profile, volume, duration and frequency, air flow, etc.) were selected in the smoking machine software settings according to Health Canada Intense smoking regimen parameters.26 Ventilation-blocking cigarette holders were utilized for the Intense smoking regimen. One cigarette was smoked per trap for each of 10 replicate analyses.
2.3 Platinum traps for Cd
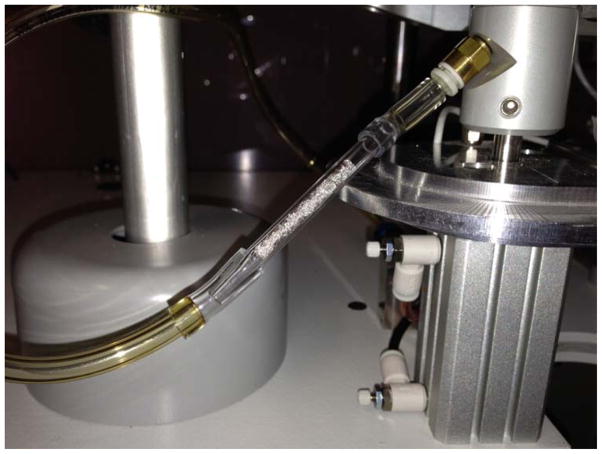
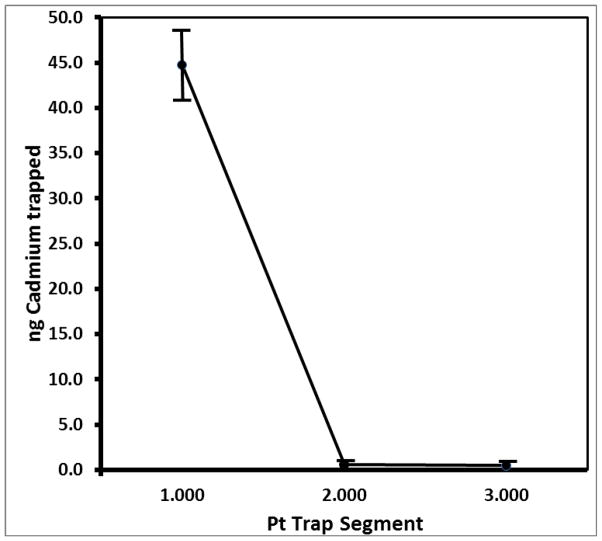
Traps for cadmium were prepared from 100 mesh Pt gauze segments (0.83 cm × 2.5 cm, 99.9% purity, Sigma, St. Louis, MO, USA) folded in half and rolled to fit tightly inside 2.0 mm Quartz injector tubes. The assembled traps were inserted in tandem as closely as possible to the primary traps (Figure 1). The rolled Pt trap segments were repeatedly heated to 1600°C in the electrothermal vaporization system until the backgrounds for cadmium were below the limit of detection (< LOD). In order to determine whether cadmium was quantitatively trapped, analyses were performed on one to five Pt segments in a single trap tube. After determination that cadmium was efficiently trapped (>99%) on the first three rolled squares, only three segments were subsequently used per trap (Figure 2).
Fig. 1.
A Pt trap used for quantitative determination of Cd breakthrough is shown placed in tandem with a glass fiber filter pad attachment on a rotary smoking machine.
Fig. 2.
Analyses of Cd trapped on individual Pt trap segments shows that trapping was quantitative with three Pt gauze segments per trap. Data is from mean of 10 individual segment analyses of cadmium from a commercial cigarette that freely passed through a 44mm glass fiber filter used with a linear smoking machine.
2.4 Isotope dilution of Pt traps with 110Cd for analysis
Glassy carbon ETV sample boats manufactured by HTW Hochtemperatur-Werkstoffe GmbH (Thierhaupten, Germany) and distributed in the U.S. by ESI-Meinhard (Golden, CO, USA) held the samples for the ETV. The boats were repeatedly heated to 2300°C in the ETV until the backgrounds for cadmium were < LOD. Following smoking, the Pt trap segments were placed in the glassy carbon boats, spiked with 100μL of 100 μg/L (10 ng) 110Cd in 1% HNO3, and dried prior to being placed in the ETV autosampler for analysis.
2.5 Analytical Instrumentation
The ETV 4000 system was obtained from Spectral Systems, Fürstenfeldbruck, Germany. The argon carrier and sheath gas flows were respectively 0.715 and 0.266 L/min with no reactive gas flow. The temperature program was initiated at 400°C for 15 seconds, followed by 5 second cooling to 50°C before stepping to 1,600°C for 15 seconds for desorption of cadmium.
The sample vapor in the argon from the ETV was introduced via 6 mm i.d. polypropylene tubing through a 1.8 mm o-ring-free sapphire injector and high performance torch (ESI, Omaha, NE, USA) directly into the ThermoFisher X2 Series (Orlando, FL, USA) Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) plasma. The ICP-MS was operated at 1400 watts RF power with 13.0 L/min cool gas, and 1.00 L/min auxiliary gas flows. Sampling position was optimized with a typical setting of 135 for optimum signal with low oxide formation. The nebulizer gas was set at 1.50 L/min so that the ETV controlled the sample argon flow to the plasma. The hexapole collision cell was operated with 5.50 mL/min cell gas (93% helium, 7% hydrogen) and Kinetic Energy Discrimination lens conditions (−17.0 V pole bias, −20.0 V hexapole bias). Other instrumental parameters were optimized for optimum signal while maintaining less than 0.1% oxide formation from Cerium using an ESI Apex desolvating introduction system (ESI, Omaha, NE, USA). Torch alignment was optimized using 132Xe from minor impurities in the argon. Detector dead time was updated as required.
2.6 Method Limits of Detection (LOD)
Standard deviations of ten procedural Pt trap blanks and ten replicate analyses of cadmium breakthrough on the electrostatic precipitator after smoking CM6 cigarettes on the 92 mm filters used on a rotary smoking machine, and the 44 mm filters used on the linear smoking machine were plotted versus the mean values according to Taylor.33 The method LOD was determined by adjusting the Taylor-derived LOD using a formula adapted from the National Committee for Clinical Laboratory Standards to take into account statistical probability for potential overlap between blank false positives and sample false negative values.34
2.7 Statistical examination of results
Results for quantitative determination of cadmium breakthrough from electrostatic precipitator, 92 mm CFP, and 44 mm CFP were compared using a t test in Microsoft Excel®.
3. Results and discussion
3.1 Isotope dilution used for quantitation
The 111Cd isotope was chosen for quantitation due to high isotopic abundance and the lack of elemental isobaric interferences from Pd, In, or Sn. 110Cd was chosen for the enriched isotope dilution spike because of high isotopic purity available and the low environmental presence of 110Pd, the only potential elemental isobaric interference. Isotope dilution analysis was used, because it provides a definitive means for obtaining analytical accuracy and has been used for establishment of standard reference material values.35,36
3.2 Platinum traps
Our goal was to bypass labor-intensive sample preparations such as these for cadmium breakthrough analysis. The Pt traps were initially prepared with quartz wool and coarse platinum powder. The quartz wool increased the pressure drop across the combined traps to exceed the ISO smoking parameter specifications25 when they were placed in tandem with Cambridge glass fiber filters in either the rotary or linear smoking machine. Five or more 1 cm platinum gauze segments caused the pressure drop across the combined traps to exceed the ISO smoking parameter specifications25 when they were placed in tandem with Cambridge glass fiber filters in the linear smoking machine. Four or fewer Pt gauze segments did not create pressure drops outside of ISO specifications when either smoking machine was used. Figure 2 shows a plot of the mean 111Cd ion counts (cps) trapped on Pt trap segments 1–3 from injector tubes used over 10 analytical runs. The Pt gauze trap segments were optimal for trapping cadmium without additional trapping, oxidation, reduction, or other additional sample preparation.
Having found that cadmium could be quantitatively trapped with platinum (Pt) traps inserted in tandem after either a CFP or electrostatic precipitation attachment, electrothermal vaporization-inductively coupled plasma-mass spectrometry (ETV-ICP-MS) was used to determine the quantity of cadmium that freely passed through the primary traps (electrothermal vaporization or glass fiber filter) in what would traditionally be described as the “gas phase” of the smoke. The cadmium that passed through the primary traps will subsequently be described as “cadmium breakthrough” for simplicity.
3.3 ETV-ICP-MS
The ETV-ICP-MS is sensitive, imparting high elemental or compound vaporization and transport efficiency with or without a reactive gas.37 The use of a reactive gas in the ETV would have further increased the sensitivity, but at the expense of consuming the Pt traps. Since cadmium is a volatile element and does not require the reactive gas, the ETV was used without it. An additional advantage of using the Pt traps with ETV-ICP-MS with argon carrier gas alone is that there is no aqueous solvent or reactive gas to contribute hydrogen, oxygen, or halogens to the plasma. Therefore, oxides were undetectably low, and cadmium signal peaks had very high count rates (Figure 3). The adjusted method detection limit determined for cadmium that passed through the primary traps to the Pt traps was 0.30 ng/cigarette.
Fig. 3.
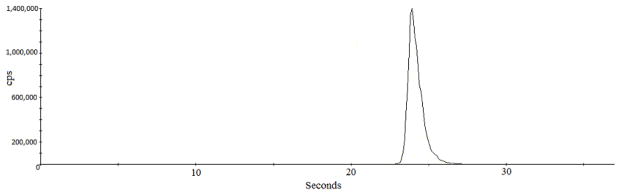
ETV-ICP-MS confers very high signal intensity on a few nanograms of 111Cd even without a reactive gas that would have further enhanced sensitivity.
3.4 Cadmium breakthrough from electrostatic precipitator
Cadmium breakthrough from electrostatic precipitators is reported in Table 1. The data is also reported as a percentage of the previously reported mean particulate phase cadmium concentrations determined using the Health Canada Intense smoking regimen.20 The results illustrate the very low (less than 1%) cadmium breakthrough from the electrostatic precipitator, as shown in the first two columns in Table 1, and confirms the high efficiency of the electrostatic precipitator for trapping particulate.31 Negligible cadmium passed through electrostatic precipitators to platinum traps regardless of variations in the ranges of paper porosities, filter lengths, rod lengths, or masses of tobacco in the respective cigarette products.
Table 1.
Cadmium in cigarette smoke that passed through electrostatic precipitator or Cambridge glass fiber filters, determined using Intense smoking regimen with platinum gauze secondary traps (n=10, one cigarette per trap). Cadmium that passed through primary traps is described as “cadmium breakthrough” for simplicity.
| Cadmium Breakthrough from Electrostatic Precipitator (Rotary) | Cadmium Breakthrough from 92 mm Glass Fiber Filter (Rotary) | Cadmium Breakthrough from 44 mm Glass Fiber Filter (Linear) | ||||
|---|---|---|---|---|---|---|
| Mass (ng/cigarette) | Percent of TPM Cd | Mass (ng/cigarette) | Percent of TPM Cd | Mass (ng/cigarette) | Percent of TPM Cd | |
| CM6 | 1.61 ± 0.36 | 0.91% | 34.5 ± 6.7 | 19.6% | 45.1 ± 7.2 | 25.6% |
| 3R4F | 0.44 ± 0.16 | 0.29% | 5.3 ± 0.9 | 3.5% | 20.6 ± 3.5 | 13.6% |
| American Spirit Nat. | 0.66 ± 0.26 | 0.33% | 45.8 ± 3.7 | 22.9% | 60.8 ± 5.4 | 30.4% |
| Camel Tur. Domestic | 0.33 ± 0.21 | 0.26% | 7.6 ± 1.6 | 6.0% | 19.5 ± 2.6 | 15.5% |
| Carlton White 100 | 0.32 ± 0.13 | 0.35% | 4.8 ± 0.7 | 5.2% | 14.9 ± 2.1 | 16.4% |
| Marlboro 100 (HP) | 0.34 ± 0.15 | 0.22% | 7.4 ± 1.0 | 4.8% | 23.1 ± 2.3 | 15.0% |
| Newport Green Men. | (< 0.30) | (<0.21%) | 6.3 ± 0.8 | 5.1% | 18.5 ± 2.7 | 14.8% |
American Spirit Natural is trademark of Santa Fe Natural Tobacco Company, a wholly owned independent subsidiary of Reynolds American. Camel Turkish Domestic Blend is trademark of R.J. Reynolds Tobacco. Carlton is trademark of American Tobacco Company. Marlboro 100 is trademark of Philip Morris USA. Newport menthol is trademark of Lorillard Tobacco Company. CM6 are test pieces produced to CORESTA specifications. 3R4F Research cigarettes are produced by University of Kentucky.
Since cadmium breakthrough from the electrostatic precipitator was less than 1% for all seven cigarettes, this suggests that our previously reported TPM cadmium data20 obtained using electrostatic precipitation is equivalent to total cadmium in cigarette smoke. The fact that negligible cadmium levels passed through the electrostatic precipitator to the Pt traps strongly suggests that cadmium is predominantly transported in cigarette smoke as particulate matter and not in the vapor or gas phase.
3.5 Cadmium breakthrough from glass fiber filters
Cadmium breakthrough from the 92 mm CFPs was significantly higher than from the electrostatic precipitator for all examined cigarettes (Table 1, p < 0.001 in every case). Cadmium breakthrough from the 44 mm CFPs was significantly higher than from the 92 mm CFPs for every cigarette that was examined (Table 1, p < 0.001 in every case except CM6, where p < 0.005). However, since a significantly greater fraction of cadmium broke through the CFPs relative to the electrostatic precipitator, we could conclude that measurable quantities of cadmium likely occur in a particle size range small enough to pass through the CFPs. Thus, one explanation of the higher breakthrough for the CFPs is that measurable quantities of cadmium are found in the ultrafine or nanoparticulate size range (< 0.1 μm). The breakthrough from CFPs varies from a mean of 3.5% to 22.9% of the total cadmium with the 92 mm filters, but from 13.6% to 30.4% with the 44 mm diameter filters. Cadmium breakthrough exhibited cigarette brand dependence. If the diameter of the opening into which the particles were being inspired were less than 92 mm or 44 mm (such as the diameter of a smoker’s oral cavity), it is possible that an even greater proportion of smoke cadmium mass might be found in the ultrafine particle size range.
Broday and Robinson have described fresh mainstream smoke particulate as 0.2 – 0.35 μm count mean diameter (CMD), 0.25 – 0.35 μm mass mean diameter, and 0.35 – 0.55 μm mass mean aerodynamic diameter.38 Becquemin et al. reported mean particle diameters for mainstream smoke from six cigarette varieties that ranged from 0.21 – 0.29 μm with 68 to 77% of the particles less than 0.3 μm diameter.39 Based on these data, a significant number of particles, though not the major portion of particulate mass, would be smaller than CFPs could efficiently trap. If the particle size in which cadmium precipitated were in the lower end of the particle size spectrum, this would explain the higher cadmium breakthrough from 92 mm and 44 mm CFPs versus electrostatic precipitation.
3.6 Discussion of cigarette physical parameters that affect cadmium breakthrough
Though the observed increase in cadmium breakthrough with smaller glass fiber filter diameter was significant for all cigarettes examined, the percent of total cadmium that broke through the filters was not the same for every cigarette, even though the Health Canada Intense smoking regimen was used because it eliminates the filter ventilation or air dilution as a factor. This raises the question of what design features or cigarette characteristics might influence the degree of cadmium breakthrough. In this regard, it is instructive to compare the American Spirit Natural with the CM6 cigarette’s physical characteristics. Both cigarettes are 84 mm length and approximately the same rod diameter. They have similar paper porosity and no filter ventilation holes. Of the 84 mm length, the American Spirit had a 23 mm filter vs. 21 mm for the CM6. Though the American Spirit Natural cigarette had slightly less tobacco volume than CM6 due to filter length, American Spirit Natural had the highest mass of filler tobacco of all of the cigarettes analyzed in this work (881 ± 44 mg tobacco, p = 0.011), and the highest of 50 cigarette varieties for which smoke cadmium delivery has been reported,20 whereas CM6 had 762 ± 26 mg tobacco. Higher tobacco mass not only burns to produce a greater total particulate mass, but cadmium concentration in the filler was also higher in the American Spirit Natural cigarettes than in other brands.17 Tobacco mass was significantly correlated with total cadmium delivery in both the ISO and Intense smoking regimens.20 The high American Spirit tobacco mass may be due to tighter packing of the tobacco in the cigarette rod, and possibly to the lack of puffed tobacco in the blend. Tighter packing of the tobacco would suggest consideration of the pressure drop as an important parameter. However, this parameter also depends on filter length, and was not significantly correlated with total cadmium delivery.20 An additional consideration in the design is that if the tobacco mass was higher in an 84 mm cigarette than in cigarettes with longer filters or 100 mm lengths, then the tobacco would have to occupy more space in the cigarette rod, leaving less void volume. Since the smoking machines used the same puff profile and mean air flow rate for the same smoking regimen for all cigarettes, the air would have to flow at a higher velocity through a rod with lower void volume. A faster flow rate through the rod could also contribute to smaller mean particle size. Thus, possible explanations for the highest percent cadmium breakthrough include some consideration of filter length, high cadmium concentration in the tobacco, higher tobacco mass and tobacco density in the rod, and high air velocity through a tightly packed rod with low void volume.
In support of the latter possibility, Dickens et al. reported data from human volunteer puff behaviors.40 They reported average puff flow rates that were equal to or greater than the average flow rate for the Health Canada Intense smoking machine regimen. They also reported smaller particle CMD (0.154–0.172 μm) than in the previous two studies cited. In addition, their data clearly showed a decrease in particle size with increasing average puff flow rate. Since cadmium is one of the more volatile metals, it would be expected to cool and precipitate later than less volatile metals. Perhaps for this reason, cadmium has less time to precipitate and agglomerate with other particles than less volatile metals and occurs in a smaller particle size range as a result. The particle size ranges discussed by Dickens et al.,40 are additional evidence that cadmium would precipitate in particles that approach the lower end of the particle size range. Based on this reasoning, factors which increase particle velocity through the cigarette rod may impact cadmium delivery more than other constituents of the particulate. One would therefore expect breakthrough of cadmium even when the majority of other particulate mass (which consists of the filtered particles) is retained by glass fiber filters.
Further support for dependence of particle size on air velocity comes from the fact that cadmium broke through from the 44 mm CFPs significantly more than through the 92 mm CFPs. Inside the filter holder, there are a few millimeters of space for the smoke to spread across the surface of the filters before passing through. This space is much larger for a 92 mm filter than for a 44 mm filter. The average velocity of the smoke through the larger volume in the 92 mm filter would be slower than through the space to the 44 mm filter at a fixed draw rate. Every cigarette examined had higher cadmium breakthrough when comparing CFP size. This behavior is consistent with the tendency toward smaller particulate with increasing puff flow rate.40
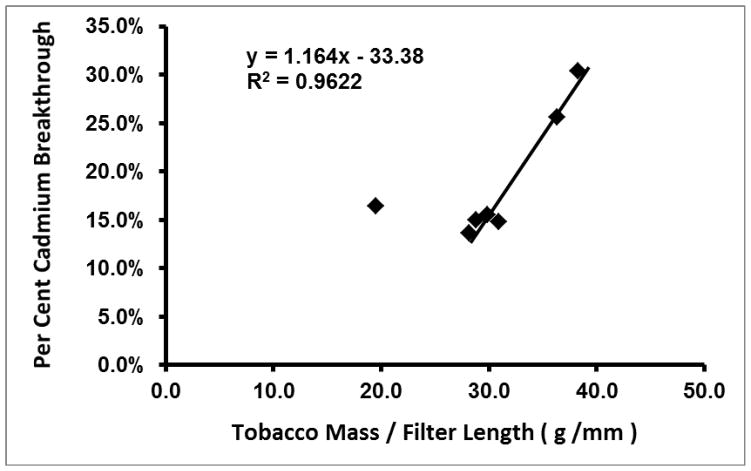
Another cigarette design factor that must be considered in relation to cadmium filter breakthrough and particle size implications is filter length. When tobacco mass (discussed above) is normalized to filter length in mm, a linear plot of the relationship between percent cadmium breakthrough and tobacco mass per mm cellulose acetate filter results (Figure 4). The two highest data points in the plot, American Spirit and CM6, have the two highest breakthrough percentages, high tobacco masses, and two of the three shortest filter lengths, which fits well with this explanation. Though Newport Green has a filter as short as CM6 (21 mm), it has less tobacco mass (649 ± 54 mg) than either American Spirit (881 ± 44 mg, 23 mm filter) or CM6 (762 ± 26 mg), therefore it falls in the cluster of cigarettes with lower percent cadmium breakthrough. The point that does not fall in the linear relationship is Carlton White 100. One possible reason that Carlton White 100 does not fall in this linear relationship with the other cigarettes is the utilization of a higher proportion of expanded tobacco in the blend, which would decrease rod void volume with a lower mass of tobacco. In this case, one would expect lower smoke cadmium delivery from lower tobacco mass but similar percent cadmium breakthrough in smaller particles due to higher air velocity through the tobacco portion of the rod. Carlton White 100 had the lowest tobacco mass of any of the seven cigarettes though it had the second highest rod tobacco volume. It had the sixth lowest Intense Regimen smoke cadmium delivery per cigarette;20 but it nevertheless had very similar percent cadmium breakthrough to four of the six other cigarettes studied here, consistent with this explanation. Though Carlton White 100 also had the highest mean paper porosity (59 ± 16 Coresta units), the porosity was not significantly different from Camel Turkish Domestic Blend, the second highest (56 ± 7 Coresta units), therefore paper porosity likely contributes to cadmium breakthrough to an insignificant degree, just as it was not significantly correlated with total cadmium delivery with the Health Canada Intense smoking regimen.20
Fig. 4.
Plot of percent cadmium breakthrough from 44 mm filters versus cigarette tobacco filler mass normalized to filter length.
3.7 Health implications of cadmium in ultra-fine particle diameters
If cadmium precipitates in a smaller mean particulate size range, it could have toxicological significance. As the mean particle size entering the lung falls, there is a greater likelihood of interstitialization or even complete passage across the air-blood barrier into circulation.41 If, as the data suggests, cadmium precipitates in a particle size range that is smaller than the CMD, then cadmium precipitates may preferentially be interstitialized as well as passed into the circulation. Consistent with this hypothesis, there is a correlation between cadmium exposure from cigarette smoke and smoking-related interstitial fibrosis,4,5 as well as significantly higher cadmium concentrations in the blood of smokers than in non-smokers.12,13 Cardiovascular disease is correlated with these elevated blood cadmium levels,6 whether the mechanism for entering the blood is crossing the interstitium due to small particle size or another mechanism.
As mean particle size falls, particles penetrate more deeply into the lung.42 As a consequence of multiple changes in cigarette design over the last 50 years,43 and consequent changes in smoking habits, an increasing trend toward adenocarcinoma in distal lung relative to the previously more common small cell carcinoma in proximal lung has been observed though small cell carcinoma risk from smoking is still high.44,45 If cadmium precipitates in particulate that consists of smaller particles than the CMDs discussed here, then cadmium would be expected to penetrate more deeply into the lung. Deep lung penetration by small particles enriched in cadmium and possibly other carcinogens may be a partial explanation for the increase in distal lung adenocarcinomas that have been correlated with changes in cigarette design and smoking habits.26,42–45
This work is also important because reports of Cd measured in mainstream smoke are highly variable.20,30,46 Often, differences in products being tested, or the technique used influence the results. However, if measurements and comparisons utilizing electrostatic precipitation and CFP are considered a significant source of difference, error could be related to the higher degree of cadmium breakthrough when using CFP. Evaluating error sources and providing more accurate quantification of cadmium is important to provide good estimates of exposure and potential public health impact.
4.1 Conclusions
The novel development of platinum traps to examine cadmium breakthrough when using either glass fiber filters or electrostatic precipitators to collect cigarette smoke TPM has a practical application in providing higher quality measurements that more accurately reflect smoke yields.
The work described here has interesting implications beyond improving quantification of Cd levels in mainstream smoke when using CFPs. The breakthrough observed for CFPs, but not electrostatic precipitators, has further potential consequences related to particle size. We found that when electrostatic precipitation was used, cadmium breakthrough for the seven cigarettes was minimal. The fact that cadmium was quantitatively trapped by electrostatic precipitation suggests that the most of cadmium is in particulate phase. Thus, any breakthrough observed with CFP likely result from cadmium being present in smaller or ultra-fine particle sizes. The fact that regardless of cigarette design, CFP cadmium breakthrough was significant for all cigarettes tested, suggests that cadmium precipitates in a smaller smoke particle size range than less volatile metals. The fact that percent breakthrough increased with smaller filter diameter supports cited work relating particle size dependency to air velocity and transport through the rod and beyond.
The probability that a significant portion of cadmium resides in a smaller particle size range could help explain correlations between cadmium exposure from smoking, high blood cadmium concentrations, interstitial lung disease, and may contribute to an explanation for the increasing trend toward adenocarcinoma in distal lung compared to smoking-related cancer 30 years ago.
Acknowledgments
This study was funded through an interagency agreement by the U.S. Food and Drug Administration Center for Tobacco Products.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References
- 1.IARC. Cadmium and cadmium compounds. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012;100C:121–45. [Google Scholar]
- 2.ATSDR. Toxicological profile for cadmium. Toxicological Profiles. 2012:45–259. http://www.atsdr.cdc.gov/ToxProfiles/tp5.pdf.
- 3.Snider GL, Hayes JA, Korthy AL, Lewis GP. Centrilobular emphysema experimentally induced by cadmium chloride aerosol. American Review of Respiratory Disease. 1973;108:40–8. doi: 10.1164/arrd.1973.108.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher P, Pacheco K, Newman LS. Inorganic dust pneumonias: The metal-related parenchymal disorders. Environmental Health Perspectives. 2000;108 (Supplement 4):685–96. doi: 10.1289/ehp.00108s4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snider GL, Lucey EC, Faris B, Jung-Legg Y, Stone PJ, Franzblau C. Cadmium-chloride-induced air-space enlargement with interstitial pulmonary fibrosis is not associated with destruction of lung elastin. American Review of Respiratory Disease. 1988;137:918–23. doi: 10.1164/ajrccm/137.4.918. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GG, Reis IM. Is cadmium a cause of human pancreatic cancer? Cancer, Epidemiology, Biomarkers & Prevention. 2000;9:139–45. [PubMed] [Google Scholar]
- 8.Anetor JI, Ajose F, Anetor GO, Ayanda AA, Babalola OO, Adeniyi FAA. High cadmium/zinc ratio in cigarette smokers: potential implications as a biomarker of risk of prostate cancer. Nigerian Journal of Physiological Sciences. 2008;23:41–9. doi: 10.4314/njps.v23i1-2.54921. [DOI] [PubMed] [Google Scholar]
- 9.McShane WJ, Pappas RS, Wilson-McElprang V, Paschal D. A rugged and transferable method for determining blood cadmium, mercury, and lead with inductively coupled plasma-mass spectrometry. Spectrochimica Acta B. 2008;63:638–44. [Google Scholar]
- 10.Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, Dochi M, Nogawa K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers. 2009;14:77–81. doi: 10.1080/13547500902730698. [DOI] [PubMed] [Google Scholar]
- 11.Pääkö P, Kokkonen P, Anttila S, Kalliomäki PL. Cadmium and chromium as markers of smoking in human lung tissue. Environmental Research. 1989;49:197–207. doi: 10.1016/s0013-9351(89)80065-9. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann K, Becker K, Friedrich C, Helm D, Krause C, Seifert B. The Gerrman environmental survey 1990/1992 (GerES II): Cadmium in blood, urine, and hair of adults and children. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10:126–35. doi: 10.1038/sj.jea.7500081. [DOI] [PubMed] [Google Scholar]
- 13.Shaham J, Meltzer A, Ashkenazi R, Ribak J. Biological monitoring of exposure to cadmium, a human carcinogen. Journal of Occupational and Environmental Medicine. 1996;38:1220–27. doi: 10.1097/00043764-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Paschal DC, Burt V, Gunter EW, Pirkle JL, Sampson EJ, Miller DT, Jackson RJ. Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Archives of Environmental Contamination and Toxicology. 2000;38:377–83. doi: 10.1007/s002449910050. [DOI] [PubMed] [Google Scholar]
- 15.Becker K, Schulz C, Kaus S, Seiwert M, Seifert B. German environmental survey 1998 (GerES III): environmental pollutants in the urine of the German population. International Journal of Hygiene and Environmental Health. 2003;206:15–24. doi: 10.1078/1438-4639-00188. [DOI] [PubMed] [Google Scholar]
- 16.Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. Journal of Analytical Toxicology. 2008;32(4):281–91. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 17.Fresquez MR, Pappas RS, Watson CH. Establishment of Toxic Metal Reference Range in Tobacco from US Cigarettes. Journal of Analytical Toxicology. 2013;37:298–304. doi: 10.1093/jat/bkt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas RS, Polzin GM, Zhang L, Watson CH, Paschal DC, Ashley DL. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food and Chemical Toxicology. 2006;44(5):714–23. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Pappas RS, Polzin GM, Watson CH, Ashley DL. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic US brands. Food and Chemical Toxicology. 2007;45(2):202–9. doi: 10.1016/j.fct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic Metal Concentrations in Mainstream Smoke from Cigarettes Available in the U.S. Journal of Analytical Toxicology. 2014;38:204–11. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cigarette Smoke. 1978 Oct 5;:32. (est.). Bates: 80419204–80419345. http://tobaccodocuments.org/product_design/80419204-9345.html.
- 22.Adams JD, O’Mara-Adams KJ, Hoffmann D. Toxic and carcinogenic agents in undiluted mainstream smoke and sidestream of different types of cigarettes. Carcinogenesis. 1987;8:729–31. doi: 10.1093/carcin/8.5.729. [DOI] [PubMed] [Google Scholar]
- 23.Obrist D, Moosmüller H, Schürmann R, Chen L-WA, Kreidenweis SM. Particulate-phase and gaseous elemental mercury emissions during biomass combustion: controlling factors and correlation with particulate matter emissions. Environmental Science and Technology. 2008;42:721–27. doi: 10.1021/es071279n. [DOI] [PubMed] [Google Scholar]
- 24.International Organization for Standardization. Tobacco and tobacco products - Atmosphere for conditioning and testing. ISO 3402, 1999, 1–4.
- 25.International Organization for Standardization. Routine analytical cigarette-smoking machine — Definitions and standard conditions. ISO 3308, 2000, 1–23.
- 26.Hammond D, Fong GT, Cummings KM, O’Connor RJ, Giovino GA, McNeill A. Cigarette Yields and Human Exposure: A Comparison of Alternative Testing Regimens. Cancer, Epidemiology, Biomarkers & Prevention. 2006;15:1495–501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 27.International Organization for Standardization. Cigarettes — Determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine. ISO 4387, 2000, 1–17.
- 28.Wartman WB, Cogbill EC, Harlow ES. Determination of particulate matter in concentrated aerosols. Application to analysis of cigarette smoke. Anaytical Chemistry. 1959;31:1705–09. [Google Scholar]
- 29.Mussalo-Rauhamaa H, Salmela SS, Lepänen A, Pyysalo H. Cigarettes as a source of some trace and heavy metals and pesticides in man. Archives of Environmental Health. 1986;41:49–55. doi: 10.1080/00039896.1986.9935765. [DOI] [PubMed] [Google Scholar]
- 30.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regulatory Toxicology and Pharmacology. 2004;39:111–34. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Bell JJ, Brunnemann KD, Campbell JS, Debardeleben MZ, Glock E, Hoffmann D, Kuhn WF, Mccarty SW, Robertson G, Sheets TJ, Sisson VA, Wickham JE. Topics of current scientific interest in tobacco research. Recent Advances in Tobacco Science. 1991;17:127–8. [Google Scholar]
- 32.Baker RR. Product formation mechanisms inside a burning cigarette. Progress in Energy and Combustion Science. 1981;7:135–53. [Google Scholar]
- 33.Taylor JK. Quality Assurance of Chemical Measurements. Boca Raton: CRC Press; 1987. p. 79. [Google Scholar]
- 34.Tholen DW, Linnet K, Kondratovich M, Armbruster DA, Garrett PE, Jones RL, Kroll MH, Lequin RM, Pankratz TJ, Scassellati GA, Schimmel H, Tsai J. Protocols for Determination of Limits of Detection and Limits of Quantitation. National Committee for Clinical Laboratory Standards. 2004;24(34):9–35. [Google Scholar]
- 35.Moore LJ, Kingston HM, Murphy TJ, Paulsen PJ. The use of isotope dilution mass spectrometry for the certification of standard reference materials. Environment International. 1984;10:169–73. [Google Scholar]
- 36.Fassett JD. Isotopic and nuclear analytical techniques in biological systems: A critical study-X. Elemental isotope dilution analysis with radioactive and stable isotopes. Pure and Applied Chemistry. 1995;67:1943–9. [Google Scholar]
- 37.Ertas G, Holcombe JA. Determination of absolute transport efficiencies of Be, Cd, In, Pb and Bi for electrothermal vaporization sample introduction into an inductively coupled plasma using an in-line electrostatic precipitator. Spectrochimica Acta B, 2003. 2003;58:1597–1612. [Google Scholar]
- 38.Broday DM, Robinson R. Application of cloud dynamics to dosimetry of cigarette smoke particles in the lungs. Aerosol Science and Technology. 2003;37:510–27. [Google Scholar]
- 39.Becquemin MH, Bertholon JF, Attoui M, Roy F, Roy M, Dautzenberg B. Particle size in the smoke produced by six different types of cigarette tobacco. Revue des Maladies Respiratoires. 2009;26:e12–e18. doi: 10.1016/s0761-8425(07)91386-8. [DOI] [PubMed] [Google Scholar]
- 40.Dickens C, McGrath C, Warren N, Biggs P, McAughey J. Puffing and inhalation behaviour in cigarette smoking: Implications for particle diameter and dose. Journal of Physics. 2009 doi: 10.1088/1742-6596/151/1/012019. Conference Series 151. [DOI] [Google Scholar]
- 41.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicologic Pathology. 2006;34:949–57. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 42.Gower S, Hammond D. CSP Deposition to the Alveolar Region of the Lung: Implications of Cigarette Design. Risk Analysis. 2007;6:1519–33. doi: 10.1111/j.1539-6924.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann D, Hoffmann I. The changing cigarette. Journal of Toxicology and Environmental Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 44.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. Journal of the National Cancer Institute. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 45.Jiang XH, de Groh M, Liu SL, Liang HB, Morrison H. Rising incidence of adenocarcinoma of the lung in Canada. Lung Cancer, 2012. 2012;78:16–22. doi: 10.1016/j.lungcan.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Kuroki Y, Yokohama S, Takahashi H, Fujiwara M. Development of an analytical method for the simultaneous determination of trace metals and mercury in mainstream cigarette smoke by ICP-MS. Proceedings of the 63rd Tobacco Science Research Conference; Amelia Island, FL, USA. 2009. [Google Scholar]