Abstract
Objective
To determine trends in the incidence and clinical presentation of ankylosing spondylitis (AS), the incidence of cardiovascular disease (CVD) and cardiovascular (CV) risk factors among patients with AS and compare the observed incidence of CVD with that predicted by the Framingham risk score (FRS).
Method
A population-based inception cohort of residents of Olmsted County, Minnesota ≥18 years who fulfilled modified New York criteria for AS in 1980-2009 was assembled. Clinical features at presentation were recorded. Age and sex adjusted incidence rates and survival were estimated. Incident CVD and CV risk factors were identified. The 10-year CVD risk was calculated using the FRS. Standardized incidence ratios (ratios of observed CVD in AS to that predicted by the FRS) were calculated.
Results
86 patients were diagnosed with AS over the study period with an age and sex-adjusted incidence of 3.1 per 100,000 (95% CI 2.5, 3.8). The mean age at diagnosis was 35 years (range: 19-69). Inflammatory back pain, seen in 90%, was the most common presenting manifestation. The 10-year cumulative incidence of CVD was 15.8% ± 6.1%, three times higher than the predicted events based on the FRS (SIR 3.01; 95% CI 1.35, 6.69; p=0.007). Overall survival was similar to the general population.
Conclusions
AS occurs in about 3 persons per 100,000 per year. Clinical features, extra-articular manifestations and interval from symptom onset to diagnosis have remained constant in this population over the study period. The CVD risk in these patients is higher than expected and underestimated by the FRS.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease which primarily affects the spine and may have associated peripheral arthritis, dactylitis and enthesitis. Estimates of prevalence have varied widely depending on the population studied. Recent estimates in the US population were 197/100,000 (1). The incidence of ankylosing spondylitis has been estimated in Rochester, Minnesota in two longitudinal, population-based studies. The first, published in 1979 estimated the incidence of AS between 1935 and 1973 to be 6.6/100,000 but did not utilize established classification criteria for inclusion of patients (2). The second study, published in 1992, re-examined the incidence of AS with application of the modified New York criteria and extended the original cohort by 16 years and estimated the age and sex-adjusted incidence to be 7.3/100,000 (3).
While disease characteristics did not change significantly during the period of the study there was a decline in the overall incidence rate with a decrease of 1.5% per year in men (p 0.034) and 4.1% per year in women (p 0.004) in the age group with the highest incidence (25-44 years) (3). The reason for the decline in incidence is unclear, and there have not been additional studies to assess this trend.
CVD has been identified as the leading cause of death in patients with AS and studies suggest that the prevalence of CVD is higher in subjects with AS compared to age and sex matched controls (4) (5) (6). The incidence of CVD and CV risk factors has been well described in patients with rheumatoid arthritis but similar estimates in patients with AS are lacking. Prior studies of CVD in AS have relied heavily on use of diagnostic codes without verification of the diagnosis.
The aims of our study include assessing the incidence of AS in a population-based cohort over a 30 year period utilizing the modified New York criteria to determine if there have been significant changes in the incidence, disease characteristics and survival of patients with AS compared with previous cohorts, and to examined for the first time in a population-based US cohort the risk for cardiovascular disease (CVD) in patients with AS compared with that predicted by the Framingham Risk Score (FRS) to determine its applicability in this patient population (7).
Patients and Methods
A population-based inception cohort of patients with AS was assembled using the resources of the Rochester Epidemiology project which is a records linkage system that facilitates ready access to the medical records from all health care providers from the Mayo Clinic, its affiliated hospitals, the Olmsted Medical Center, local nursing homes and private practitioners. The potential of this data system for use in population based studies has been described previously (8, 9). This system ensures near complete ascertainment of all clinically recognized cases of AS among the residents of Olmsted County, Minnesota.
Medical records of residents of Olmsted County with any diagnosis consistent with AS (ankylosing spondylitis, rheumatoid spondylitis, spondyloarthropathy, Marie Strumpell disease) were reviewed. We identified residents aged 18 years or older first diagnosed between January 1, 1980 and December 31, 2009. From 1980 to 1989, only Rochester, Minnesota residents (which are a subset of all Olmsted County residents) were included in the cohort. Cases were included if they fulfilled the modified New York criteria for AS and the date at which the criteria were fulfilled was considered the date of diagnosis. Radiographic or MRI evidence of sacroiliitis was documented based on the radiologist report and where films were available were reviewed for confirmation. Clinical features present at diagnosis were recorded. Only clinician verified cases of enthesitis, dactylitis, arthritis, uveitis and inflammatory bowel disease as recorded in the medical record were included. All identified cases were followed until death, migration or December 31, 2011.
To examine CVD, the subgroup of patients with AS aged ≥ 30 years with no prior history of CVD who first fulfilled modified New York Criteria for AS between 1980-2009 were assembled. Physician diagnosed incident CVD (ischemic heart disease, myocardial infarction, angina, cardiovascular death, heart failure, peripheral arterial disease) and cerebrovascular disease (transient ischemic attack, ischemic, hemorrhagic stroke) was identified. Data on traditional CV risk factors: age (years), HDL cholesterol (mg/dl), total cholesterol (mg/dl), systolic blood pressure (mmHg), antihypertensive treatment, cigarette smoking status, diabetes mellitus, body mass index (kg/m2) were collected
Statistical Methods
Incidence rates were estimated and age- and/or sex-adjusted to the 2000 US white population. Age- and sex-specific incidence rates were calculated by using the number of incident cases (defined as patients newly diagnosed with AS meeting the modified New York criteria during the study period) as the numerator, and population estimates based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years. In order to compute 95% confidence intervals (95% CI) for incidence rates, it was assumed that the number of incident cases follows a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods using smoothing splines for age and calendar year. Survival was estimated using the Kaplan-Meier method and compared to expected survival for persons of the same age, sex and calendar year estimated using US population life tables.
The cumulative incidence of CVD was estimated using product-limit life table methods, accounting for the competing risk of death. Patients with CVD prior to AS incidence date were excluded from the analysis of cumulative incidence. The 10 year CVD risk was calculated using the FRS. To compare the events predicted by FRS to the observed events, observed follow up was truncated at 10 years after AS incidence. For patients with < 10 years of follow up, the predicted risk of CVD was adjusted proportionately. Standardized incidence ratios (SIR), which are the ratios of observed CVD in AS to that predicted by the FRS, were calculated assuming that the predicted rates are fixed and observed CVD events follow a Poisson distribution.
RESULTS
Between 1980 and 2009, a total of 86 patients were newly diagnosed with AS by the New York Criteria for AS. The baseline demographics of 86 patients (19 women and 67 men) diagnosed with AS by the modified New York Criteria are shown in Table 1. The mean age at symptom onset was 28.7 yrs. (SD: 9.2). The mean age at diagnosis was 34.9 (SD: 9.9) years, reflecting a mean time from symptom onset to diagnosis of 6.0 (SD: 6.3) yrs. There was no significant change in time from symptom onset to diagnosis over the period of the study. Patients diagnosed in the 1990s were slightly older at diagnosis (39.1; SD 8.6) yrs. with a later age of symptom onset (32.6 (SD 8.4) yrs. than patients in any other decade. Information on race was available for 66 patients (77%). There was a trend towards more diversity noted in the cohort with 100% of cases being white in 1980-89, 95% white in 1990-99 and 80% white in 2000-09, but this did not reach statistical significance (p=0.10).
Table 1.
Demographics of 86 patients with AS by modified New York Criteria
| 1980-89 (N=24) | 1990-99 (N=23) | 2000-09 (N=39) | Total (N=86) | p value | |
|---|---|---|---|---|---|
| Age, years | 32.0 (11.3) | 39.1 (8.6) | 34.1 (9.1) | 34.9 (9.9) | 0.012 |
| Sex, male | 22 (92%) | 17 (74%) | 28 (72%) | 67 (78%) | 0.16 |
| Age at symptom onset, years | 25.8 (8.0) | 32.6 (8.4) | 28.3 (9.7) | 28.7 (9.2) | 0.021 |
| Time from symptom onset to diagnosis, years | 6.2 (8.1) | 6.2 (5.4) | 5.6 (5.7) | 6.0 (6.3) | 0.71 |
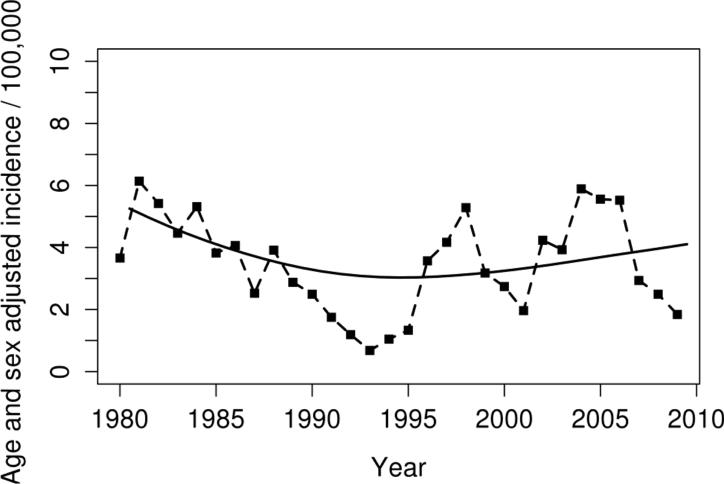
The overall age and sex-adjusted incidence of AS by the modified New York criteria was 3.1 per 100,000 (95% CI 2.5, 3.8). The decline in incidence previously reported in this population continued through the mid-1990s. After 1995 the incidence rate returned to previous estimates from the early 1980s. While there were year to year fluctuations in incidence noted there was no significant change in the overall incidence of AS by the modified New York criteria between 1980 and 2009 (Figure 1; p=0.73 for linear trend). Table 2 shows the annual incidence of AS by sex and age group. The age adjusted incidence in men was more than three times that in women: 4.9 (95% CI 3.7, 6.1) versus 1.3 (95 % CI 0.7, 1.9). The incidence of AS was highest in both men and women in the 25-34 year age group and lowest among those ≥55 years of age. The male: female ratio was 3.8:1 with no significant change over the period of the study.
Figure 1.
Age- and sex- adjusted (to US white 2000 population) incidence rates of ankylosing spondylitis in 1980-2009 using New York criteria according to calendar year. Points plotted represent 3 year moving averages. The solid line represents the non-linear trend, which was estimated using smoothing splines.
Table 2.
Annual incidence of ankylosing spondylitis in Olmsted County, Minnesota residents ≥ 18 years of age, 1980-2009 by sex and age group (Modified New York Criteria)
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Age group | No. of patients | Rate per 100,000 | No. of patients | Rate per 100,000 | No. of patients | Rate per 100,000 |
| 18-24 years | 10 | 6.4 | 3 | 1.8 | 13 | 4.0 |
| 25-34 years | 27 | 9.4 | 8 | 2.7 | 35 | 6.0 |
| 35-44 years | 19 | 7.2 | 5 | 1.9 | 24 | 4.5 |
| 45-54 years | 9 | 4.3 | 2 | 0.9 | 11 | 2.6 |
| 55-64 years | 1 | 0.7 | 1 | 0.7 | 2 | 0.7 |
| ≥65 years | 1 | 0.7 | 0 | 0.0 | 1 | 0.3 |
| Total (95% CI) | 67 | 4.9 (3.7, 6.1)† | 19 | 1.3 (0.7, 1.9)† | 86 | 3.1 (2.5, 3.8)‡ |
*Values are the annual incidence rate (95% confidence interval [95% CI]) per 100,000 population
Age-adjusted to the 2000 US white population.
Age- and sex-adjusted to the 2000 US white population.
We identified six patients (4 females), all of whom were diagnosed in the final decade of the study, who met Assessment of Spondyloarthritis International Society criteria for axial spondyloarthritis (SpA) during the period of the study who did not have radiographic evidence of sacroiliitis. Given this small number of patients, re-computing the incidence rates using these criteria would have little impact on our incidence rate estimates.
Clinical features at the time of diagnosis are shown in Table 3. Inflammatory back pain was the most common symptom at presentation, seen in 90% of patients. Thirty percent of patients had peripheral arthritis at the time of diagnosis, and was more common among patients diagnosed in the 1990s, seen in 11/23 (48%) patients. Twenty six percent of patients had a family history of a spondyloarthropathy at the time of diagnosis. Eighty seven percent of patients (60/69) who had HLA B27 testing at the time of diagnosis were positive. Uveitis was the most common extra-articular manifestation at diagnosis, reported in 1/3 of patients (28/86). A history of psoriasis was more common in subjects diagnosed between 1990 and 1999, seen in 6/23 patients (26%).
Table 3.
Clinical Features of ankylosing spondylitis present at time of diagnosis
| 1980-89 (N=24) | 1990-99 (N=23) | 2000-09 (N=39) | Total (N=86) | p value | |
|---|---|---|---|---|---|
| Radiographic evidence of sacroiliitis | 24 (100.0%) | 23 (100.0%) | 39 (100.0%) | 86 (100.0%) | 1.0 |
| Limitation of lumbar spine range of motion | 16 (67%) | 12 (52%) | 19 (497%) | 47 (55%) | 0.37 |
| Inflammatory back pain | 22 (92%) | 20 (87%) | 35 (90%) | 77 (90%) | 0.87 |
| Arthritis | 8 (33%) | 11 (48%) | 7 (18%) | 26 (30%) | 0.043 |
| Enthesitis | 1 (4%) | 2 (9%) | 4 (10%) | 7 (8%) | 0.69 |
| Uveitis | 6 (25%) | 6 (26%) | 16 (41%) | 28 (33%) | 0.31 |
| Dactylitis | 1 (4%) | 2 (9%) | 0 (0.0%) | 3 (4%) | 0.19 |
| Psoriasis | 2 (8%) | 6 (26%) | 2 (5%) | 10 (12%) | 0.038 |
| Crohn's disease/Ulcerative colitis | 2 (8%) | 2 (9%) | 5 (13%) | 9 (10%) | 0.81 |
| Good response to nonsteroidal antiinflammatory drugs* | 9 (69%) | 14 (88%) | 19 (66%) | 42 (72%) | 0.28 |
| Family history for spondyloarthropathy* | 3 (21%) | 7 (37%) | 8 (22%) | 18 (26%) | 0.43 |
| HLA-B27* | 16 (89%) | 13 (81%) | 31 (89%) | 60 (87%) | 0.74 |
Percentages calculated among patients with available data
With the exception of uveitis, which occurred twice as often in women as in men (53% compared with 27%, p 0.034), there was no significant difference in the age at symptom onset, clinical features at presentation or time to diagnosis between men and women. Women were more likely to report a family history of spondyloarthropathy but this did not achieve statistical significance (7/16 [44%] compared with 11/54 [20%]; p 0.06). Patients who were ≥45 years at the time of diagnosis were less likely to have inflammatory back pain at presentation and more likely to have decreased spinal mobility, abnormal sedimentation rate and higher BMI than younger patients (data not shown).
Survivorship after onset of AS related symptoms was as expected in the general population. There were 3 deaths during a median follow up duration of 8.7 yrs. (total 929 person-years), consistent with the 4.4 expected deaths (standardized mortality ratio: 0.72; 95% CI 0.15, 2.09).
The analysis of CV risk factors and the development of CVD included 51 patients (78% male) with a mean age of 39.1 (SD 7.6) at the time of diagnosis of AS who were age ≥ 30 years at AS diagnosis and who had no preceding history of CVD (two patients were excluded because of a history of pre-existing CVD). There was one death from CVD during the study period. During a median follow up duration of 10.7 years, 6 patients were diagnosed with ischemic heart disease, 4 of whom went on to have a revascularization procedure. Two patients had a myocardial infarction and 2 patients had angina. Seven patients developed heart failure, and 3 were diagnosed with valvular heart disease. Thirteen patients were newly diagnosed with hypertension after AS incidence, 11 of whom were started on anti-hypertensive treatment. Two patients had a cerebrovascular event (ischemic stroke or TIA). There were no patients with hemorrhagic stroke. One patient was diagnosed with diabetes mellitus after the diagnosis of AS was made. At diagnosis of AS, 37% (19 patients) were current smokers and the mean BMI of patients in the study population was 28.0 ± 5.8 kg/m2. Mean total cholesterol closest to diagnosis was 186 ± 33 mg/dl and mean HDL closest to diagnosis was 50 ± 32 mg/dl.
During the first 10 years of follow-up after AS incidence, 6 patients developed CVD (5 males and 1 female). The 10 year cumulative incidence of CVD was 15.8% (95% CI: 3.0%, 26.9%). This was almost 3 times higher than the predicted 2.0 events based on the FRS (mean 5.6%, SD 6.3%), SIR 3.01 (95% confidence interval 1.35, 6.69; p=0.007).
DISCUSSION
This study is the first to document the incidence of AS in a population based setting in the US since 1992. It is also among the first to assess the FRS in a population-based cohort of patients with AS.
The age and sex adjusted incidence of AS by modified New York criteria in the population of Olmsted County, Minnesota was estimated to be 3.1/100,000 between 1980 and 2009 with no linear trend in incidence observed during the study period, which agrees with prior estimates of incidence in Northern European countries. (3, 10, 11). However, we observed a temporary decrease in incidence of AS in the 1990s compared to older estimates of 7.3/100,000 (3). This may be influenced by changes in the diversity of the Rochester population since the publication of prior studies. AS is strongly associated with HLA-B27; and accounts for about 20% of the genetic risk based on twin studies with about 50% of the risk conferred by the entire major histocompatibility complex (MHC) and additional susceptibility loci identified outside of the MHC (12-14). The frequency of HLA B27 is highly variable among different ethnic groups. The prevalence of HLA B27 in adults aged 20-69 in the US is estimated to be 6.1% (95% CI 4.6-8.2), and is virtually absent in South America, sub-Saharan Africa and Australian aborigines (15, 16). Olmsted County census data from 2010 shows that while continuing to account for a minority of the population, the percentage of blacks and Hispanics has increased from 0.4 % to 4.8% and 0.6% to 4.2%, respectively, compared with census data from 1990 (17). There was a trend towards more diversity also noted among the patients with AS. Given the significant contribution of genetic factors to the development of AS in the population, the decrease in incidence noted may in part be attributed to this (12).
Patients diagnosed between 1990 and 1999 were more likely to have a history of psoriasis and peripheral arthritis at the time of diagnosis than in any other decade. This raises the possibility of inclusion of patients who may have had psoriatic spondylitis, but we think this is unlikely to be the case given that the same search strategies were applied across all decades. Because all patients included met modified New York criteria for AS, the likelihood that this would differentially impact the subset of patients diagnosed between 1990 and 1999 is low.
AS is a male predominant disease with widely variable sex ratios reported in the literature with estimates as high as 6:1 and 9:1 reported in older studies (18, 19). The ratio of 3.8:1 noted in our study is consistent with more recent estimates and similar to previous estimates noted in our population (2, 3, 10). The decline in sex ratio which has been noted has been ascribed to a delayed recognition of the disease in women rather than a true change in the rate of occurrence in females. In our study there was a trend towards an increase in the proportion of females diagnosed with AS which did not achieve statistical significance. Among patients tested, there was no significant difference in HLA B27 positivity between males and females. Uveitis was the most common extra-articular manifestation in our study reported in 1/3 of patients with AS. This is similar to the estimated prevalence in a recent systematic review with uveitis reported in mean 33.2% (SD 0.8%) of patients with AS (20). Sex differences in the clinical features and severity of AS have been reported among patients with long standing disease, with more peripheral arthritis noted in females (21, 22). With the exception of uveitis, seen more frequently in women in our study, we did not identify any significant difference in the clinical features at presentation between men and women. Older studies to address this question have had discordant results which are likely related to heterogeneity in disease duration in these studies and geographic variations in the prevalence of uveitis which have been noted in a recent systematic review (23-25) (26).
The median follow up duration in our study was 8.7 years, during which there was no observed excess mortality among subjects with AS. Excess mortality among subjects with AS in previous studies has been noted after longer duration of follow up with no difference in survival noted in one study in the first 10 years of follow up and increased mortality noted after 20 years (37 observed vs 46 expected, p=0.001) (4, 27, 28). It is possible that with longer followup in our patient population differences in survival may emerge.
CVD has been identified as the leading cause of death in patients with AS (4, 28, 29). Analysis of data obtained from ICD-9 diagnostic codes from an integrated US health plan revealed an increased prevalence of CVD (ischemic heart disease, atherosclerosis, peripheral arterial disease, congestive heart failure) and CV risk factors amongst patients with AS compared with age and sex matched controls (5). A recently published population-based study from Quebec compared 8616 patients with a diagnosis of AS to a random sample of residents of Quebec without a diagnosis of AS and found, after adjustment for age and sex, an increased risk of 25-60% compared with the general population depending on the CV or cerebrovascular condition. (6) Major limitations of these studies are the use of diagnostic codes for identification of patients with AS and CVD without verification of the diagnosis and the lack of data on risk factors. Our study has the advantage of physician verified diagnoses of CVD from chart review.
We examined the FRS as a predictor of CVD. The FRS significantly underestimated the CV risk of subjects with AS. This is similar to shortcomings of this tool noted in studies of patients with rheumatoid arthritis (RA) in this population. This shortcoming is likely, at least in part, explained by the underlying inflammatory milieu of systemic rheumatic disease and its contribution to CV risk as has been described in RA (30). A limitation of the retrospective nature of our study was the lack of disease activity scores to assess how much of this excess risk might be related to disease activity. Further studies are needed to determine the CV risk in this population and also to determine the role of traditional CV risk factors in development of CVD.
Limitations of our study include that the population of Olmsted County is predominantly white (87%) by most recent census estimates, which somewhat limits its generalizability. With the exception of a greater percentage of individuals employed in health care this population is otherwise similar to the US white population. In addition, our sample size was limited due to the size of the Olmsted County population and the rarity of AS. However, utilizing the Rochester Epidemiology Project allowed us several advantages. Access to the medical records of all patients within this defined geographic location reduces the likelihood of introduction of referral bias given that we are able to also capture patients with milder disease who may not have been referred for specialist care. The capacity for longitudinal follow up also allowed us to distinguish incident cases from prevalent cases of CVD in order to determine the utility of the FRS in this population.
Our findings suggest that the incidence of AS has remained stable. The decreased survivorship reported elsewhere was not evident over the time period of our study. Further research regarding disease characteristics associated with increased CV risk, development of tools that accurately predict CV risk in this population and the impact of treatment on CV risk is needed.
Significance and Innovations.
Epidemiologic data from the US population has relied heavily on studies of prevalence and with the exception of studies done in the Rochester population dating back more than 20 years the incidence of ankylosing spondylitis (AS) in the US population has not otherwise been described.
The incidence of AS is stable but there has been a trend towards an increased incidence in females. Clinical features at presentation and time to diagnosis are similar between men and women but uveitis occurs more commonly in women.
The increased risk of cardiovascular (CV) disease in patients with inflammatory arthritis is well described and the need for accurate scores for prediction of CV risk in this population remains a priority. Use of the Framingham risk score (FRS) as a predictor of cardiovascular (CV) risk has not been examined in patients with AS.
The FRS appears to underestimate CV risk in patients with AS.
Acknowledgments
Funding Source: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341(4):284–6. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter ET, McKenna CH, Brian DD, Kurland LT. Epidemiology of Ankylosing spondylitis in Rochester, Minnesota, 1935-1973. Arthritis Rheum. 1979;22(4):365–70. [Google Scholar]
- 3.Carbone LD, Cooper C, Michet CJ, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd. Ankylosing spondylitis in Rochester, Minnesota, 1935-1989. Is the epidemiology changing? Arthritis Rheum. 1992;35(12):1476–82. doi: 10.1002/art.1780351211. [DOI] [PubMed] [Google Scholar]
- 4.Khan MA, Khan MK, Kushner I. Survival among patients with ankylosing spondylitis: a life-table analysis. J Rheumatol. 1981;8(1):86–90. [PubMed] [Google Scholar]
- 5.Han C, Robinson DW, Jr., Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–72. [PubMed] [Google Scholar]
- 6.Szabo SM, Levy AR, Rao SR, Kirbach SE, Lacaille D, Cifaldi M, et al. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum. 2011;63(11):3294–304. doi: 10.1002/art.30581. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 8.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 9.Kremers HM, Myasoedova E, Crowson CS, Savova G, Gabriel SE, Matteson EL. The Rochester Epidemiology Project: exploiting the capabilities for population-based research in rheumatic diseases. Rheumatology (Oxford) 2011;50(1):6–15. doi: 10.1093/rheumatology/keq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaipiainen-Seppanen O, Aho K, Heliovaara M. Incidence and prevalence of ankylosing spondylitis in Finland. J Rheumatol. 1997;24(3):496–9. [PubMed] [Google Scholar]
- 11.Bakland G, Nossent HC, Gran JT. Incidence and prevalence of ankylosing spondylitis in Northern Norway. Arthritis Rheum. 2005;53(6):850–5. doi: 10.1002/art.21577. [DOI] [PubMed] [Google Scholar]
- 12.Brown MA, Kennedy LG, MacGregor AJ, Darke C, Duncan E, Shatford JL, et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 1997;40(10):1823–8. doi: 10.1002/art.1780401015. [DOI] [PubMed] [Google Scholar]
- 13.Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16(4):675–90. [PubMed] [Google Scholar]
- 14.Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42(2):123–7. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MA. HLA-B27 and its subtypes in world populations. Curr Opin Rheumatol. 1995;7(4):263–9. doi: 10.1097/00002281-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Belachew DA, Sandu N, Schaller B, Guta Z. Ankylosing spondylitis in sub-Saharan Africa. Postgrad Med J. 2009;85(1005):353–7. doi: 10.1136/pgmj.2007.064717. [DOI] [PubMed] [Google Scholar]
- 17. [cited; Available from: http://censusviewer.com/city/MN/Rochester.
- 18.Stecher RM, Hersh AH. Familial occurrence of ankylosing spondylitis. Br J Phys Med. 1955;18(8):176–83. [PubMed] [Google Scholar]
- 19.Polley HF. The diagnosis and treatment of rheumatoid spondylitis. Med Clin North Am. 1955;12:509–28. doi: 10.1016/s0025-7125(16)34704-6. [DOI] [PubMed] [Google Scholar]
- 20.Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67(7):955–9. doi: 10.1136/ard.2007.075754. [DOI] [PubMed] [Google Scholar]
- 21.Lee W, Reveille JD, Davis JC, Jr., Learch TJ, Ward MM, Weisman MH. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann Rheum Dis. 2007;66(5):633–8. doi: 10.1136/ard.2006.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibn Yacoub Y, Amine B, Laatiris A, Hajjaj-Hassouni N. Gender and disease features in Moroccan patients with ankylosing spondylitis. Clin Rheumatol. 2012;31(2):293–7. doi: 10.1007/s10067-011-1819-x. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez-Balderas FJ, Mintz G. Ankylosing spondylitis: clinical course in women and men. J Rheumatol. 1993;20(12):2069–72. [PubMed] [Google Scholar]
- 24.Will R, Edmunds L, Elswood J, Calin A. Is there sexual inequality in ankylosing spondylitis? A study of 498 women and 1202 men. J Rheumatol. 1990;17(12):1649–52. [PubMed] [Google Scholar]
- 25.Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203582. [DOI] [PubMed] [Google Scholar]
- 26.Marks SH, Barnett M, Calin A. Ankylosing spondylitis in women and men: a case-control study. J Rheumatol. 1983;10(4):624–8. [PubMed] [Google Scholar]
- 27.Lehtinen K. Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis. 1993;52(3):174–6. doi: 10.1136/ard.52.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prati C, Claudepierre P, Pham T, Wendling D. Mortality in spondylarthritis. Joint Bone Spine. 2011;78(5):466–70. doi: 10.1016/j.jbspin.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–5. doi: 10.1136/ard.2011.151191. [DOI] [PubMed] [Google Scholar]
- 30.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]