Summary
The lipopolysaccharide (LPS) of H. influenzae is highly variable. Much of the structural diversity is derived from phase variation, or high frequency on-off switching, of molecules attached during LPS biosynthesis. In this study, we examined the dynamics of LPS phase variation following exposure to human serum as a source of antibody and complement in multiple H. influenzae isolates. We show that lic2A, lgtC and lex2A switch from phase-off to phase-on following serial passage in human serum. These genes, which control attachment of a galα1–4gal di-galactoside structure (lic2A and lgtC phase-on) or an alternative glucose extension (lex2A phase-on) from the same hexose moiety, reduce binding of bactericidal antibody to conserved inner core LPS structures. The effects of the di-galactoside and alternative glucose extension were also examined in the context of the additional LPS phase variable structures phosphorylcholine (ChoP) and sialic acid. We found that di-galactoside, the alternative glucose extension, ChoP, and sialic acid each contribute independently to bacterial survival in the presence of human complement, and have an additive effect in combination. We propose that LPS phase variable extensions serve to shield conserved inner core structures from recognition by host immune components encountered during infection.
Introduction
The Gram-negative bacterium Haemophilus influenzae has established the human nasopharynx as its niche. Between 20–60% of the population is colonized asymptomatically by H. influenzae, and colonization is thought to be a pre-requisite for respiratory tract diseases including pneumonia, otitis media and exacerbations of chronic obstructive pulmonary disease (COPD), (Murphy and Sethi, 1992; Harabuchi et al., 1994; Murphy et al., 2009; Mackenzie et al., 2010). While the Hib vaccine protects against type b H. influenzae, there is still a significant burden of disease caused by non-typeable H. influenzae (NTHi) strains (Ulanova and Tsang, 2009; Agrawal and Murphy, 2011). Evasion of the host immune system is critical to the persistence of H. influenzae in the nasopharynx. H. influenzae is susceptible to classical pathway complement-mediated lysis, and components of this pathway including complement and antibody are present on the mucosal surface (Zola et al., 2009). The lipopolysaccharide, or LPS, is a major source of both intra- and interstrain surface variation in H. influenzae (Campagnari et al., 1987; Swords et al., 2003).
Several of the genes required for the expression of LPS structures in H. influenzae are phase variable due to the presence of tetranucleotide repeats (High et al., 1993; Hood et al., 1996b; Griffin et al., 2003; Fox et al., 2005). Slipped-strand mispairing, which occurs with these tandem repeats, creates stochastic on-off switching of gene expression. The result is high frequency (10−2–10−3 times per generation) phase variation of LPS modifications, as changes in the number of tetranucleotide repeats shifts the reading frame in and out of frame (translational switch) (Weiser et al., 1989). As there are several phase variable genes controlling attachment of LPS structures, the population of a given H. influenzae strain contains many different phase variants with distinct LPS structural configurations. In addition, the distribution of these genes varies among isolates. The selective pressure of host immune components can enrich for phase variants that are resistant to recognition and clearance. This has been demonstrated previously in the case of the phase variable structure ChoP, where attachment to the LPS is dependent on the lic1 locus, of which lic1A contains the tetranucleotide repeats determining phase variant status (Weiser et al., 1997). Bacteria with ChoP-modified LPS (lic1A phase-on variants) are targets of C-reactive protein (CRP), which initiates complement-mediated killing of ChoP expressing bacteria (Weiser et al., 1998). In environments with high CRP levels, such as in the blood, there is selection against bacteria with ChoP-modified LPS. However, lic1A phase-on variants are enriched during colonization, as ChoP expression reduces antibody binding and complement-mediated killing (Tong et al., 2000; Clark et al., 2012). ChoP and several other LPS phase variable structures increase bacterial survival in the presence of human antibody and complement, although the dynamics of these effects and how they relate to each other has not previously been explored. We sought to conduct a comprehensive analysis of the LPS phase variable structures that contribute to the evasion of complement-mediated killing in H. influenzae. Our findings highlight the independent and additive effects of several phase variable LPS structures on bacterial evasion of recognition by host immune components present at the mucosal surface.
Results
LPS structural requirements for evasion of complement-mediated lysis
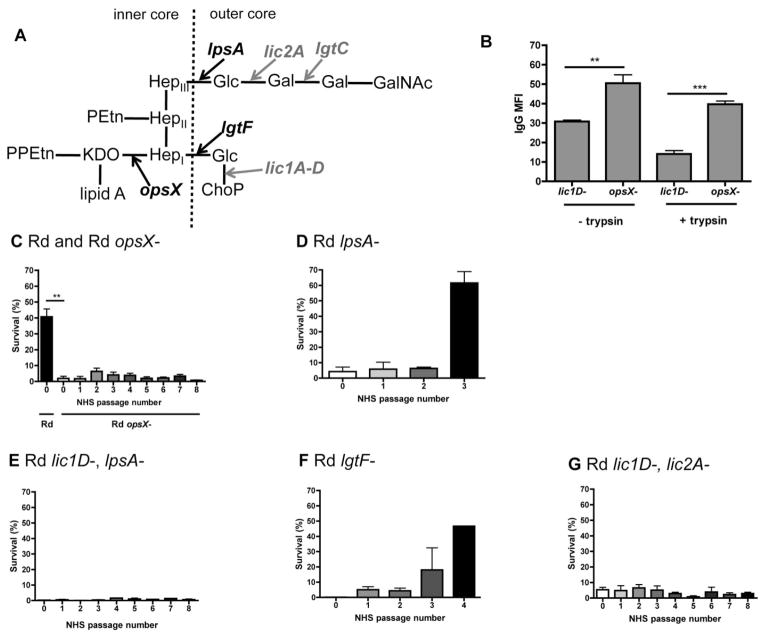
We sought to determine the minimal LPS structural components required for bacterial resistance to human antibody and complement-mediated lysis. Initial analysis was conducted with mutants in the H. influenzae strain Rd, which is a type d, unencapsulated strain with a minimally complex LPS structure compared with most NTHi isolates (see Table 1 for a full list of strains and mutants used in this study). We first examined the targets of human antibody binding by flow cytometry. We found that an opsX–mutant in Rd, which has a severely truncated inner core oligosaccharide, has increased binding of naturally acquired human IgG compared with bacteria with outer core oligosaccharides (Fig. 1A and B). This was demonstrated in a lic1D– background, as the lic1D-dependent attachment of ChoP to the LPS has previously been shown to reduce antibody binding (Clark et al., 2012), and we sought to determine whether additional LPS structures play a similar role. The phenotype of the lic1D– mutant is similar to that of a wild-type, ChoP phase-off variant. The difference in antibody binding between opsX– and lic1D–strains was maintained in the presence or absence of digestion with trypsin, suggesting that antibody binds to non-proteinaceous structures, and this is blocked by the presence of LPS outer core extensions (aside from ChoP).
Table 1.
H. influenzae strains used in this study.
| Strain | Mutant (−) or phase-on variant | Reference |
|---|---|---|
| Rda | opsX– | Hood et al. (1996a) |
| lpsA– | Hood et al. (1996a) | |
| lgtF– | Nakamura et al. (2011) | |
| lic2A– | Nakamura et al. (2011) | |
| lgtC– | Nakamura et al. (2011) | |
| lic1D– | Lysenko et al. (2000) | |
| lic1A-ON | Clark et al. (2012) | |
| lic1D–, siaP– | Severi et al. (2005) | |
| lic1D–, lpsA– | This study | |
| lic1D–, lic2A– | This study | |
| lic1D–, lic2A-ON, lgtC– | This study | |
| lic1D–, lic2A-ON, lgtC-ON | This study | |
| lic1A-ON, lic2A-ON, lgtC-ON | This study | |
| R2846b | lic1D– | This study |
| lic1A–ON | This study | |
| lic1D–, lpsA– | This study | |
| lic1D–, lgtF– | This study | |
| lic1D–, lic2A–, lex2A– | This study | |
| lic1D–, lic2A–, lex2A-ON | This study | |
| lic1D–, lex2A-ON, lgtC-ON | This study | |
| lic1D–, lic2A-ON, lgtC–, lex2A– | This study | |
| lic1D–, lic2A-ON, lgtC-ON, lex2A– | This study | |
| lic1A-ON, lex2A-ON | This study | |
| 2019c | lic1D– | This study |
| lic1D–, lic2A– | This study | |
| lic1D–, lic2A-ON, lgtC– | This study | |
| lic1D–, lic2A-ON, lgtC-ON | This study | |
| Eagan cap-d | lic1D– | This study |
| lic1D–, lic2A– | This study | |
| lic1D–, lic2A-ON, lgtC-ON | This study |
Type d, unencapsulated variant, avirulent strain (Hood et al., 1996a).
NTHi clinical isolate from patient with otitis media (Lundstrom et al., 2008).
NTHi clinical isolate from patient with chronic obstructive pulmonary disease (Tong et al., 2000).
Type b, spontaneous capsule– derivative of the clinical isolate Eagan (Deadman et al., 2009).
Fig. 1.
LPS structural requirements for resistance to human serum in the strain Rd. LPS structure proposed for Rd (Hood et al., 2001b), with arrows indicating extensions dependent on LPS biosynthesis genes (black, bold), or phase variable genes (grey, bold), and dashed line indicating the border between inner and outer core LPS structures (A). Mean fluorescence intensity (MFI) for binding of purified human IgG to the surface of the Rd lic1D– strain compared with the opsX– mutant, with or without trypsin digestion (B). Bactericidal assays, where survival in human serum is determined relative to controls with the same serum heat-inactivated to eliminate complement activity. Survival following serial passage in human serum is indicated for Rd and Rd mutants (C–G) in 3% normal human serum (NHS). Data shown are means and SEM (representative experiment in triplicate shown for D–G). Statistical analysis (n ≥ 3) was performed by an unpaired t-test; **P < 0.01, ***P < 0.001.
Next, human serum was used as a source of antibody and complement for bactericidal assays, and survival was determined relative to complement-inactivated controls. Serum was depleted of CRP to prevent killing of ChoP phase-on bacteria. The Rd opsX– mutant was sensitive to human serum compared with the lic1D– strain. Surviving colonies from these bactericidal assays were then grown and re-exposed to human serum, under the same conditions as in the original bactericidal assays. This was repeated several times, to determine if the level of serum resistance changed following serial passage in human serum. The opsX– mutant remained constitutively serum sensitive (Fig. 1C). Selective truncations of Rd oligosaccharide structures were used to establish the requirements for evasion of complement-mediated lysis. Serial passage of bacteria in human serum results in selection for increased resistance in an lpsA– mutant, which lacks hexose extensions from the third inner core heptose (HepIII) of Rd (Fig. 1A and D). In Rd, ChoP is attached to the hexose extension from HepI, leading us to hypothesize that increased bacterial survival was due to the enrichment of lic1A phase-on variants in the resistant population, compared with the original population. To determine phase variant status, the 5′ end of the lic1A gene was sequenced from genomic DNA isolated from the original and resistant bacterial populations, and the tetranucleotide repeats within these sequences were enumerated. We found that while the original population was lic1A phase-off, the more resistant population was predominantly lic1A phase-on (Table 2). This result was corroborated by colony immunoblotting using the ChoP-specific antibody TEPC-15 to distinguish between lic1A phase-on and lic1A phase-off colonies. By colony immunoblotting, the original population was phenotypically <2% lic1A phase-on, while the serum resistant population was >94% lic1A phase-on (data not shown). Previous work has documented the selection for lic1A phase-on variants in vivo following colonization in animal models and humans, validating this in vitro approach for phase variant analysis (Weiser et al., 1998; Tong et al., 2000). The Rd lpsA–, lic1D– mutant, in contrast, remained constitutively sensitive to human serum (Fig. 1E).
Table 2.
Exposure to NHS drives selection for LPS phase variants in H. influenzae.
| Strain | Serial passage | Gene | No. repeats (reading frame)a | Times observed |
|---|---|---|---|---|
| Rd | ||||
| lpsA– | NHS | lic1A | 17 (OFF) → 18 (ON) | 3/3 |
| lgtF– | NHS | lic2A | 23 (OFF) → 22 (ON) | 3/3 |
| lic1D– | NHS | lgtC | 21 (OFF) → 22 (ON) | 3/3 |
| lic1D– | Neu5Ac + NHS | lgtC | 21 (OFF) → 22 (ON) | 4/4 |
| lic1A-ONb | NHS | lic2A | 21 (OFF) → 22 (ON) | 3/3 |
| R2846 | ||||
| lpsA– | NHS | lic2A | 24 (OFF) → 25 (ON) | 3/3 |
| lic1D– | NHS | lic2A | 24 (OFF) → 25 (ON) | 6/7 |
| lex2A | 16 (OFF) → 14 (ON) | 1/7 | ||
| lic1D– | mAb 6E4 + BRS | lic2A | 24 (OFF) → 25 (ON) | 3/4 |
| lex2A | 16 (OFF) → 14 (ON) | 1/4 | ||
| lic1A-ONb | NHS | lic2A | 24 (OFF) → 25 (ON) | 1/2 |
| lex2A | 16 (OFF) → 14 (ON) | 1/2 | ||
| 2019 | ||||
| lic1D– | NHS | lic2A | 17 (OFF) → 16 (ON) | 3/3 |
| Eagan (cap–) | ||||
| lic1D– | NHS | lic2A | 17 (OFF) → 16 (ON) | 4/4 |
| lgtC | 23 (OFF) → 24 (ON) | 4/4 | ||
Refers to the reading frame of the indicated gene as in frame (ON) or out of frame (OFF) based on the number of tetranucleotide repeats.
Bactericidal assays with lic1A-ON variants were conducted with CRP-deleted NHS.
NHS, normal human serum; BRS, baby rabbit serum.
Serial passage in human serum results in selection for increased serum resistance in the lgtF– mutant, which lacks the hexose extensions from the first inner core heptose (HepI) of Rd (Fig. 1A and F). As there is no possibility for ChoP attachment in the absence of lgtF, this resistant population potentially contains changes in phase variable genes other than lic1A that affect resistance to antibody and complement. A screen of all ten known genes with tetranucleotide repeats (not including lic1A) in Rd (Power et al., 2009) was performed to compare phase variants in the original and serum resistant bacterial populations of the lgtF– mutant (see Table 3 for all primers used for tetranucleotide repeat sequence analysis). The only gene with altered phase variant status was lic2A, which was phase-off in the original population, and phase-on in the serum resistant population (Table 2). The expression of lic2A results in the attachment of a galactose residue to the LPS, which enables further hexose extensions in the presence of other phase-on LPS biosynthesis genes (Fig. 1A). Therefore, the lic1D–, lic2A–mutant contained the minimal LPS truncations required for constitutive sensitivity to human serum in Rd (Fig. 1G). These data established that selection for ChoP or galactose phase-on variants is necessary for evasion of complement-mediated killing in this strain.
Table 3.
Primers used in this study.
| Target gene (repeat)a | Direction | Sequence | Reference |
|---|---|---|---|
| lic1A (CAAT) | F | 5′-AGCTAACCGAGCTTGGGTAAAA-3′ | This study |
| R | 5′-AAATCATTGTGGCACGGACG-3′ | ||
| lic2A (CAAT) | F | 5′-CAAGTGATTTATCCCCACGCGCCA-3′ | Weiser and Pan (1998) |
| R | 5′-CGTTCTTTTTCCAATCCGCTTGTT-3′ | ||
| lgtC (GACA) | F | 5′-TTTCATATCAAGAATATAAAAATT-3′ | Weiser and Pan (1998) |
| R | 5′-GGTTTTGAAGAAAAAGGCGAA-3′ | ||
| lex2A (GCAA) | F | 5′-GGCGGAATTATGTTAATCAC-3′ | Erwin et al. (2006a) |
| R | 5′-GCTTGCATATAAGCTTTTCG-3′ | ||
| oafA (GCAA) | F | 5′-TTCCAGAATTACTTGTAGGATCTTTG-3′ | Erwin et al. (2006a) |
| R | 5′-CATTAAAAACAAGCAGGAAAATAATAG-3′ | ||
| lic3A (CAAT) | F | 5′-CTCAGCCTTTCGGCACCCCG-3′ | This study |
| R | 5′-GGCATCAAAGGCGGGTAGCTTGT-3′ | ||
| losA1 (CGAGCATA) | F | 5′-TCGAGCATCCATTTTCCCACT-3′ | This study |
| R | 5′-TGCCCTCAAAGAGATCCAACG-3′ | ||
| hgp haemoglobin and haemoglobin-haptoglobin binding protein, HI_0635 (CCAA) | F | 5′-TCATCAACCCCTCGAACTGC-3′ | This study |
| R | 5′-TCGTCAAGATCCTGTTGCCC-3′ | ||
| hgp haemoglobin and haemoglobin-haptoglobin binding protein, HI_0661 (CCAA) | F | 5′-CTTTGCCCAAAACGTCCAGC-3′ | This study |
| R | 5′-ACGTGCTTGCCTATTCCGTT-3′ | ||
| hgp haemoglobin and haemoglobin-haptoglobin binding protein, HI_1565 (CCAA) | F | 5′-TTATGCTTGGGCTAACGGCA-3′ | This study |
| R | 5′-CCGGTTTCATAGCGCACAAG-3′ | ||
| hgp haemoglobin and haemoglobin-haptoglobin binding protein, HI_0712 (CCAA) | F | 5′-TTCAGCTTGACGAAGCCCAT-3′ | This study |
| R | 5′-TCCGCTGGGAAAGTCACATC-3′ | ||
| Drug/metabolite exporter, HI_0687 (TTTA) | F | 5′-GCAGTTATTGGTTGGGCTGC-3′ | This study |
| R | 5′-GCATCCCATAAAAGCCAGCG-3′ | ||
| mod type III restriction/modification system methylase, HI_1058 (TGAC) | F | 5′-TTTTGCGTCAAAAAGCCGGT-3′ | This study |
| R | 5′-TGTGTATTGAATGGCGGGCA-3′ | ||
| Putative glycosyltransferase, HI_1386 (CCAA) | F | 5′-TTGGAGAAGATGGCAAAGGCT-3′ | This study |
| R | 5′-TGAAGTCACTACCGCAACGG-3′ |
Sequence of the tandem repeat present within the sequence amplified for each target gene.
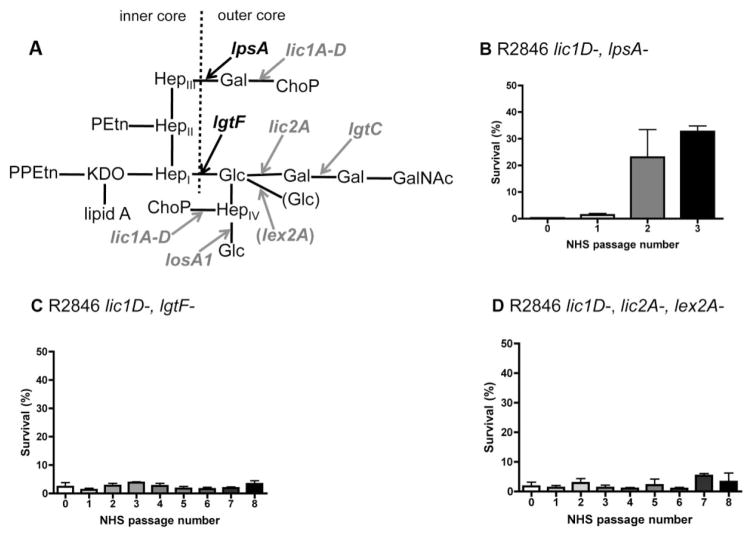
In order to extend these observations to a H. influenzae strain with a more complex LPS structure, survival in human serum was examined for selective LPS truncation mutants of the NTHi clinical isolate R2846 (Fig. 2A). Serial passage of the R2846 lic1D–, lpsA– mutant in human serum results in selection for a population with increased resistance, while the lic1D–, lgtF– mutant is constitutively serum sensitive (Fig. 2B and C). Sequence analysis of the LPS phase variable genes present in the lic1D–, lpsA–mutant determined that the serum resistant population of this strain was lic2A phase-on, compared with lic2A phase-off in the original population, as with Rd (Table 2). However, unlike in the Rd strain, both the original and serum resistant populations of R2846 were also lgtC phase-on. The expression of lic2A and lgtC results in attachment of a di-galactoside structure, galα1–4gal. The proximal galactose of this di-galactoside is added in lic2A phase-on variants, and the distal galactose is added in lic2A and lgtC phase-on variants. The galα1–4gal structure is also present in humans in the form of P blood group antigens and, therefore, is not a target of human antibody (Virji et al., 1990; Bitzan et al., 1994). Interestingly, selection for lic2A phase-on variants was observed even though in R2846 galactose is attached to a glucose extension from HepI, rather than HepIII as in Rd (Fig. 2A). Therefore, the R2846 lic1D–, lic2A– mutant (also lex2A-; see below) contains the minimal LPS truncations required for constitutive sensitivity to human serum in this strain (Fig. 2D). In the absence of functional lic2A, there is no possibility for attachment of either galactose, regardless of lgtC phase variant status. Our analysis of LPS structures contributing to evasion of complement-mediated killing in two separate H. influenzae strains revealed that selection for either ChoP or galactose phase-on variants aids bacterial survival in the presence of human serum.
Fig. 2.
LPS structural requirements for resistance to human serum in the strain R2846. LPS structure proposed for NTHi clinical isolate R2846 (Lundstrom et al., 2008), with arrows indicating extensions dependent on LPS biosynthesis genes (black, bold), or phase variable genes (grey, bold), and dashed line indicating the border between inner and outer core LPS structures (A). Also included in parentheses is the phase variable gene responsible for the proposed alternative glucose extension (grey, bold), which can be attached to the same hexose moiety as the di-galactoside. Bactericidal assays for serial passage in 5% normal human serum (NHS), with survival following serial passage in human serum, for R2846 mutants (B–D). Data shown are means and SEM (representative experiments in triplicate).
Variable LPS hexose extensions increase bacterial survival in human serum
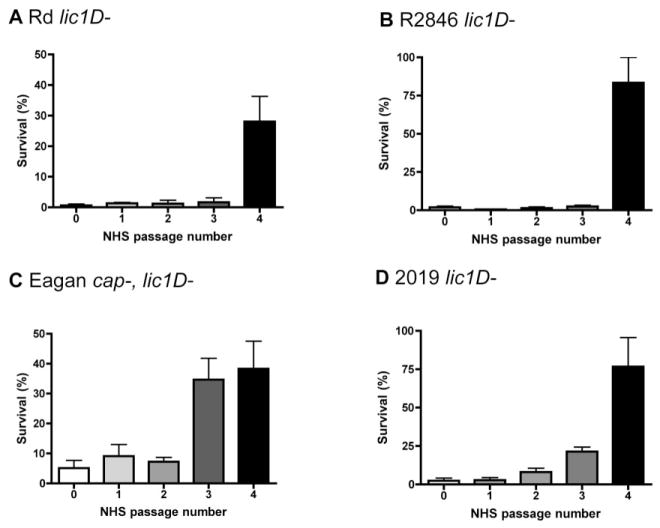
The contribution of hexose oligosaccharide extensions to serum resistance was examined in a lic1D– background of multiple strains, as the role of ChoP expression has previously been described for several H. influenzae isolates (Clark et al., 2012). Experiments with lic1D– mutants were conducted in serum without CRP depletion, as the presence of CRP does not affect survival of these strains. Serial passage in human serum results in selection for lgtC phase-on variants in the Rd lic1D– strain (Fig. 3A, Table 2). As the original and serum resistant populations in this strain background were also lic2A phase-on, the serum resistant population expressed the di-galactoside structure. Similarly, serial passage in human serum resulted in selection for variants expressing the di-galactoside structure in the R2846 lic1D– strain (Fig. 3B, Table 2). Of note, sequence analysis of the original and serum resistant populations of R2846 included all LPS phase variable genes used in the first Rd screen as well as three additional LPS phase variable genes found in R2846 that are not present in Rd; lex2A, oafA and losA1 (which has a eight base pair repeat). We also performed sequence analysis on survivors from each passage in between the original and serum resistant populations of Rd and R2846 lic1D– strains to determine the timing of the change in phase variant status. We confirmed that either lgtC (Rd) or lic2A (R2846) switched to phase-on in the round of passage in human serum corresponding to the shift in resistance (data not shown).
Fig. 3.
Exposure to human serum drives selection for resistant populations in multiple strains of H. influenzae. Bactericidal assays following serial passage of bacteria in human serum, with the round of serum exposure indicated, for Rd lic1D– (A, 3% normal human serum, NHS), R2846 lic1D– (B, 4% NHS), Eagan cap–, lic1D– (C, 2% NHS) and 2019 lic1D– (D, 4% NHS). Data shown are means and SEM (representative experiments in triplicate).
To extend these observations to other relevant H. influenzae strains, lic1D– mutants of the NTHi clinical isolate 2019 and a capsule– variant of the type b strain Eagan were also exposed to human serum. Without capsule, Eagan is significantly less resistant to complement-mediated lysis (Noel et al., 1996). In both strains, serial passage in human serum resulted in selection for a resistant population (Fig. 3C and D). The serum resistant population of 2019 was lic2A phase-on, compared with the lic2A phase-off original population (Table 2). In Eagan, the serum resistant population was both lic2A and lgtC phase-on, compared with the lic2A and lgtC phase-off original population (Table 2). Thus, passage in human serum results in selection for expression of the components of the same di-galactoside structure in four distinct H. influenzae strains.
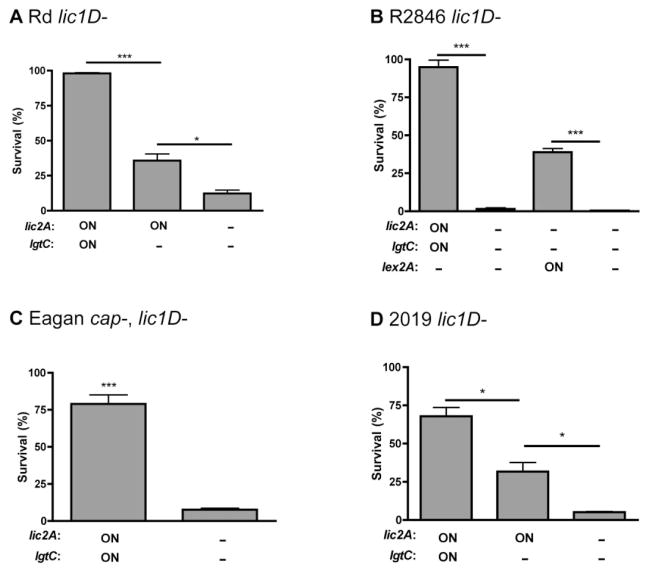
LPS mutants in lic2A and lgtC were used to confirm the contribution of the di-galactoside to survival in the presence of human serum. The mAb 4C4 specifically recognizes galα1–4gal, and was used for phenotypic confirmation of lic2A– mutants by Western blotting (data not shown). In the Rd lic1D– background, the lic2A and lgtC phase-on strain was the most serum resistant, the lic2A phase-on, lgtC– mutant had an intermediate level of serum resistance, and the lic2A– mutant was the least serum resistant (Fig. 4A). These data show that both lic2A and lgtC contribute individually to bacterial survival. In the R2846 lic1D– background, the lic2A and lgtC phase-on strain was also more serum resistant than the lic2A–mutant (Fig. 4B). We constructed the Rd and R2846 lic1D–, lic2A– mutants using two separate background strains; the serum resistant population (lic2A and lgtC phase-on) and the original population (lic2A and lgtC phase-off). The lic2A– mutants had the same phenotype in both background strains, supporting the proposition that phase variation of lic2A and lgtC accounts for the only difference between the original and serum resistant populations. Similar results were also observed for Eagan and 2019 (Fig. 4C and D). In 2019, the lic2A and lgtC phase-on variant population was isolated using the mAb 4C4, as the resistant population isolated after passage in human serum was lic2A phase-on, lgtC phase-off. Similar to Rd, in 2019 both lic2A and lgtC contribute individually to bacterial survival in the presence of human serum. These results demonstrate that each galactose extension on LPS in lic2A and lgtC phase-on variants contributes independently to the evasion of complement-mediated killing in multiple H. influenzae strains.
Fig. 4.
Contribution of LPS hexose extensions to survival in human serum. Bactericidal assays in human serum for lic2A, lgtC and lex2A (R2846 only) phase-on variants (ON) and mutants (−) in Rd lic1D–(A, 2% normal human serum, NHS), R2846 lic1D– (B, 4% NHS), Eagan cap–, lic1D– (C, 1% NHS) and 2019 lic1D– (D, 4% NHS). Data shown are means and SEM. Statistical analysis (n ≥ 3) was performed by an unpaired t-test; *P < 0.05, ***P < 0.001.
In a minority of cases, serial passage of the R2846 lic1D– strain in human serum resulted in selection for a resistant population that was lex2A phase-on instead of lic2A phase-on (Table 2). The expression of lex2A in R2846 results in the attachment of glucose to the same hexose moiety as galactose is attached to when lic2A is phase-on (Fig. 2A). The R2846 lex2A phase-on strain was more serum resistant than a lex2A– mutant (Fig. 4B). In order to determine whether the presence of ChoP affects selection for di-galactoside or the alternative glucose extension controlled by lex2A expression, we also exposed lic1A phase-on H. influenzae strains to serial passage in human serum. In R2846, the lic1A phase-on serum resistant population was lic2A phase-on in one experiment, and lex2A phase-on in another (Table 2). In Rd, serial passage of the lic1A phase-on strain in human serum resulted in selection for a lic2A phase-on serum resistant population (Table 2). These results demonstrate that LPS hexose extensions contribute to evasion of complement-mediated killing independent of ChoP expression.
Di-galactoside expression reduces antibody binding and classical pathway complement-mediated killing
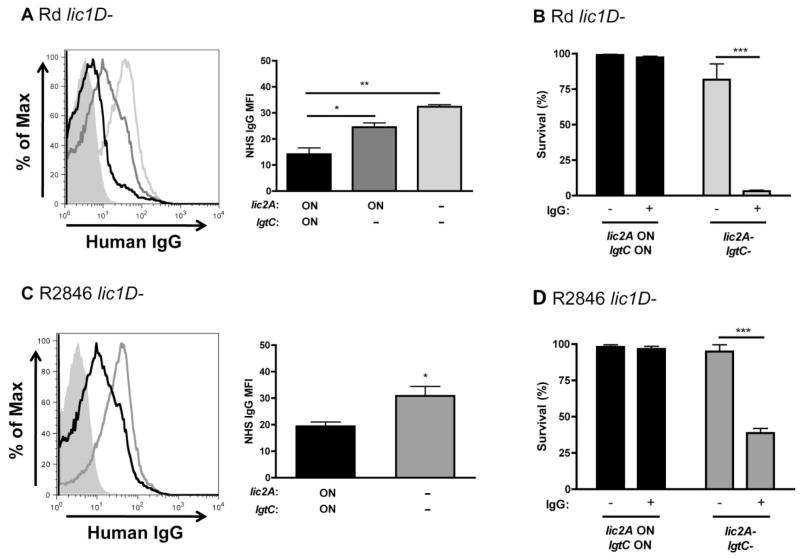
Haemophilus influenzae is susceptible to alternative and classical pathway complement-mediated lysis. We have previously shown that ChoP attachment to the LPS reduces antibody binding to the bacterial surface, increasing survival in the presence of complement (Clark et al., 2012). To determine whether modification of the LPS with di-galactoside or the alternative glucose extension affects antibody binding, flow cytometry was used to measure binding of human serum IgG to the bacterial surface. The Rd lic2A and lgtC phase-on variant population had the lowest amount of IgG binding, the lic2A phase-on, lgtC–strain had an intermediate amount of IgG binding, and the lic2A– mutant had the most IgG binding (Fig. 5A). Bactericidal assays in human serum with or without IgG depletion were used to determine whether the difference in antibody binding correlated with a difference in classical pathway complement-mediated killing. The Rd lic2A–mutant was killed in the presence, but not the absence, of human IgG, while the lic2A and lgtC phase-on variant was resistant in either condition (Fig. 5B). Similar results were observed for bactericidal assays conducted with purified human IgG either added or not added to a complement source that lacks antibody to H. influenzae, baby rabbit serum (BRS) (Fig. S1A). In contrast, there was no difference in IgM binding or bactericidal activity between lic2A and lgtC phase-on variants and lic2A– mutants (data not shown). A further comparison between alternative and classical pathway complement-mediated killing was performed using Mg-EGTA-treated human serum. Mg-EGTA treatment inhibits classical and lectin, but not alternative pathway complement-mediated lysis (Forsgren and Quie, 1974). The Rd lic2A– mutant was sensitive to normal human serum, but not Mg-EGTA-treated human serum, confirming that the classical pathway, rather than the alternative pathway, is responsible for the sensitivity of the lic2A– mutant (Fig. S1B). Similar results were observed for the R2846 lic2A and lgtC phase-on strain versus the lic2A– mutant (Fig. 5C and D; Fig. S1C and D). Collectively, these data show that di-galactoside expression reduces IgG binding and bactericidal activity in multiple strains of H. influenzae.
Fig. 5.
Di-galactoside expression protects against human IgG binding and bactericidal activity. Histogram of purified human IgG binding to lic2A and lgtC phase-on variants (black), a lic2A phase-on, lgtC– mutant (dark grey) and a lic2A– mutant (light grey) in Rd lic1D– (A). Graphical summary of IgG mean fluorescence intensity (MFI) is also shown. Bactericidal assays in IgG-depleted human serum with or without purified human IgG added back, as indicated, for lic2A and lgtC phase-on variants and lic2A– mutants in Rd lic1D– (B, 2% IgG-depleted normal human serum, NHS). Histogram of purified human IgG binding to lic2A and lgtC phase-on variants (black) and a lic2A– mutant (grey) of R2846 lic1D– (C), with a graphical summary of IgG MFI. Bactericidal assays in IgG-depleted human serum with or without purified IgG added back, as indicated, for lic2A and lgtC phase-on variants and lic2A– mutants in R2846 lic1D– (D, 4% IgG-depleted NHS). Data shown are means and SEM. Statistical analysis (n ≥ 3) was performed by an unpaired t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
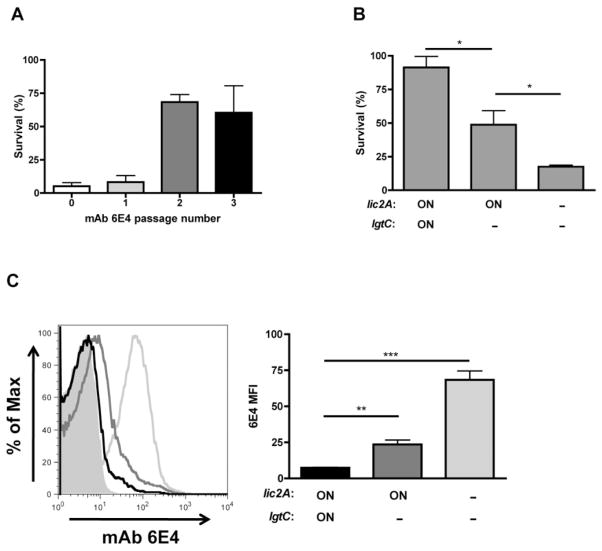
The LPS mAb 6E4, which binds to an inner core LPS epitope that includes the KDO structure (Spinola et al., 1990), was used to determine the effect of di-galactoside expression on antibody binding to LPS. Previously, we showed that ChoP expression reduces mAb 6E4 binding and killing in the presence of complement (Clark et al., 2012). Titration of mAb 6E4 was used to determine an antibody concentration where binding between modified and un-modified LPS could be differentiated by flow cytometry. R2846 retains mAb 6E4 binding in an lgtF–mutant (data not shown), which is missing all HepI extensions (Fig. 2A), suggesting that the di-galactoside itself is not required for mAb 6E4 recognition of LPS. Serial passage of the R2846 lic1D– strain in mAb 6E4 and a complement source (BRS) resulted in selection for a resistant population (Fig. 6A). Sequence analysis determined that the mAb 6E4 resistant population was lic2A phase-on (Table 2). These results are consistent with the changes observed following serial passage of R2846 in human serum. Bactericidal assays with LPS mutants were conducted to confirm the role of di-galactoside in evasion of mAb 6E4-mediated killing. The R2846 lic2A and lgtC phase-on strain is the most resistant to killing by mAb 6E4 and complement, the lic2A phase-on, lgtC–mutant has an intermediate level of resistance, and the lic2A– mutant is the least resistant (Fig. 6B). These data show that both lic2A and lgtC contribute to survival in the presence of the mAb 6E4 and complement. Next, flow cytometry was used to examine the amount of mAb 6E4 binding to the bacterial surface. Consistent with the bactericidal assay data, there was evidence for an individual contribution of both lic2A and lgtC in the reduction of mAb 6E4 binding (Fig. 6C). These results show that di-galactoside expression reduces the binding and bactericidal activity of an anti-LPS antibody that requires an inner core LPS structure for bacterial recognition.
Fig. 6.
Di-galactoside expression protects against binding and bactericidal activity of the anti-LPS mAb 6E4. Bactericidal assays for the serial passage of R2846 lic1D– in mAb 6E4 and baby rabbit serum (BRS), with the round of exposure indicated (A, 15% BRS and mAb 6E4; representative experiment in triplicate). Bactericidal assays in mAb 6E4 and BRS for lic2A and lgtC phase-on variants and mutants (B, 15% BRS and mAb 6E4). Histogram and graphical summary for binding of mAb 6E4 to lic2A and lgtC phase-on variants (black), a lic2A phase-on, lgtC– mutant (dark grey) and a lic2A– mutant (light grey) in R2846 lic1D–(C). Data shown are means and SEM. Statistical analysis (n ≥ 3) was performed by an unpaired t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
Serial passage in both human serum and mAb 6E4 with complement resulted in selection for di-galactoside expression. We next compared the lic2A and lgtC phase-on variants isolated following serial passage in human serum to those isolated after serial passage in mAb 6E4 with complement. First, the R2846 lic2A and lgtC phase-on population isolated after serial passage in human serum had reduced binding of mAb 6E4 compared with the lic2A– mutant (Fig. S2C). Similar results were also observed for Rd (Fig. S2D). Second, the R2846 lic2A and lgtC phase-on population isolated after serial passage in mAb 6E4 and complement had increased survival in the presence of human serum, compared with the lic2A–mutant (Fig. S2E). These results demonstrate that di-galactoside expressing variants isolated following exposure to different antibody sources recognizing inner core LPS epitopes have the same resistance profile in the presence of complement.
We also found that lex2A contributes to evasion of antibody binding to the bacterial surface. In the R2846 lic1D– strain, the lex2A phase-on population had reduced binding of human IgG compared with the lex2A– mutant (Fig. S2A). Upon repetition of the experiment where the R2846 lic1D– strain was serially passaged in mAb 6E4 and complement, one of the mAb 6E4 resistant populations was lex2A phase-on instead of lic2A phase-on (Table 2). Flow cytometry confirmed that the lex2A phase-on population had reduced mAb 6E4 binding compared with the lex2A– mutant (Fig. S2B). These data show that in addition to contributing to evasion of killing in human serum, both di-galactoside and the alternative glucose extension from the same hexose moiety reduce antibody binding to the bacterial surface. The expression of these phase variable hexose extensions protects H. influenzae against antibody binding conserved inner core LPS structures.
LPS phase variable structures have independent and additive effects on bacterial survival in the presence of complement
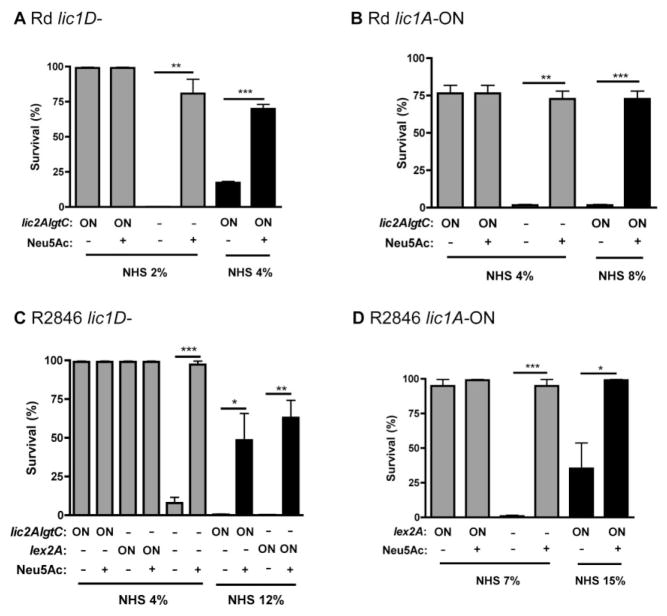
LPS phase variable structures including ChoP and di-galactoside contribute to evasion of antibody-dependent, complement-mediated lysis. We wanted to determine next whether the effects of these structures on bacterial survival are independent, additive, or both. Selection for di-galactoside expressing variants in the presence or absence of ChoP (Table 2) suggests that these two modifications contribute to survival independently, but may have an additive effect in combination. Included in the subsequent analysis was another phase variable LPS structure, sialic acid (Neu5Ac). The modification of LPS with sialic acid increases resistance to alternative pathway complement-mediated killing in H. influenzae (Figueira et al., 2007). H. influenzae must acquire sialic acid from the environment (Severi et al., 2005), so the presence or absence of sialic acid was controlled by supplementation of growth media with Neu5Ac. This approach was validated using a siaP– mutant, which inactivates the sialic acid transporter SiaPQM TRAP, preventing sialic acid uptake and LPS attachment (Severi et al., 2005). The Rd lic1D–, siaP– mutant remained constitutively sensitive to human serum, regardless of the presence of Neu5Ac, while the Rd lic1D– strain had increased survival in human serum with Neu5Ac added to growth media (data not shown). We found that in the presence of human serum and Neu5Ac, there was selection for increased serum resistance in the Rd lic1D– strain, and sequence analysis determined that this population was lgtC phase-on (Table 2). As lic2A was phase-on in the original and serum resistant populations, serial passage in human serum with sialic acid selected for di-galactoside expressing variants. These results suggest there is an independent, but additive effect of di-galactoside and sialic acid expression on bacterial resistance to human serum.
To examine the dynamics of ChoP, di-galactoside and sialic acid expression on bacterial survival, mutants were compared with phase variant populations following exposure to various concentrations of human serum. Human serum was used in increasing concentrations to resolve additive contributions of separate phase variable structures to bacterial survival. For the Rd lic1D– strain, there was an independent and additive effect of sialic acid and di-galactoside attachment to the LPS on serum resistance (Fig. 7A). Repetition of this experiment in Rd lic1A phase-on variants showed that there is also an independent and additive contribution of sialic acid and di-galactoside in the presence of ChoP (Fig. 7B). This experiment indicates that there is an additive effect for all three LPS modifications; ChoP, di-galactoside, and sialic acid each contribute to evasion of complement-mediated lysis, and can do so in combination for maximum serum resistance. In the R2846 lic1D– background, di-galactoside, the alternative glucose extension, and sialic acid each had an independent and additive effect on bacterial survival in human serum, similar to results observed for Rd (Fig. 7C). In addition to the di-galactoside, we tested the effect of a single galactose on bacterial survival in the presence or absence of sialic acid. We found that Rd and R2846 lic2A phase-on, lgtC– mutants also had an additive effect on bacterial survival in human serum in combination with sialic acid (Fig. S3A). In R2846 lic1A phase-on variants, an independent and additive contribution of sialic acid, the alternative glucose extension (lex2A phase-on) and ChoP was observed (Fig. 7D). We found that there was no difference in survival for lex2A and lgtC phase-on variants compared with a lex2A phase-on, lgtC–mutant, demonstrating there is no contribution of lgtC in combination with lex2A in R2846 (Fig. S3B). Evidence for an independent and additive effect of sialic acid and di-galactoside attachment to LPS was also observed in the lic1D– backgrounds of Eagan and 2019 (Fig. S3C and D). These experiments demonstrate, in four distinct H. influenzae isolates, that phase variable LPS structures can contribute individually to evasion of complement-mediated lysis, and have an additive effect on bacterial survival in combination.
Fig. 7.
Di-galactoside, sialic acid and ChoP have independent and additive effects on bacterial survival in human serum. Bactericidal assays in human serum for lic2A, lgtC and lex2A (R2846 only) phase-on variants (ON) or mutants (−), with or without sialic acid (Neu5Ac) added to growth medium (+). Results are shown for Rd lic1D– (A), a lic1A phase-on variant population of Rd (B), R2846 lic1D– (C) and a lic1A phase-on variant population of R2846 (D). Bactericidal assays were performed at the concentration of normal human serum (NHS) indicated and in the case of lic1A phase-on variants, serum was first depleted of C-reactive protein. Data shown are means and SEM. Statistical analysis (n ≥ 3) was performed by an unpaired t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
During colonization and disease, H. influenzae must evade killing by host immune components including antibody and complement (Zola et al., 2009). In this study, normal human serum was used to provide a source of antibody and complement for bactericidal assays, intended to test the effect of these factors at the mucosal surface. While several studies have highlighted individual LPS phase variable genes that contribute to bacterial survival following exposure to human serum (Weiser and Pan, 1998; Hood et al., 2001a; Griffin et al., 2005), a comprehensive analysis of their combined effects has not been described. We hypothesize that LPS phase variation allows bacteria to evade recognition and killing by host immune components in vivo through selective enrichment of resistant bacteria from a mixed population. A recent study conducted by M. Apicella and colleagues at the University of Iowa explored this hypothesis using experimental human colonization with the NTHi clinical isolate 2019, which was also tested in the current study (Poole et al., 2013). They found that the population of bacteria isolated from healthy volunteers following six days of colonization was enriched for lic1A and igaB (an IgA1 protease-encoding gene) phase-on variants. The selection for lic1A phase-on variants during human colonization confirms previous data in animal models and the observation that clinical samples of H. influenzae are enriched for ChoP-expressing variants (Weiser et al., 1998; Tong et al., 2000; Marti-Lliteras et al., 2011). IgA1 protease expression is phase variable due to the presence of a seven base pair repeat in igaB (Fernaays et al., 2006). Selection for bacteria expressing the IgA1 protease underlines the importance of antibody evasion at the mucosal surface.
In the human carriage study there was also an increase in the lex2A phase-on variant population, although this did not achieve statistical significance after 6 days of colonization. While the di-galactoside encoding genes lic2A and lgtC remained phase-off during the course of the carriage study, it may take longer periods of colonization to see enrichment in di-galactoside expressing bacteria. Alternatively, modification of LPS with di-galactoside or the alternative glucose extension may be more important during inflammation. In a study of phase variant populations from clinical samples, bacteria isolated from asymptomatic patients and patients with pneumonia were all lic1A phase-on variants (Weiser and Pan, 1998). Interestingly, while lic2A and lgtC expression in asymptomatic patients was variable, both genes were phase-on in every isolate obtained from patients with pneumonia. As we demonstrate in the current study that there is an additive effect for ChoP and di-galactoside expression, ChoP expression may be sufficient for successful colonization, while both ChoP and di-galactoside could be required for survival in a more inflammatory milieu. Human colonization studies are critical for the analysis of the in vivo contribution of LPS phase variable structures because the repertoire of antibodies differs between humans and animals used to model H. influenzae colonization and disease. For example, while the di-galactoside structure galα1–4βgal is found in human P blood group antigens (Naiki and Marcus, 1975), this structure is not present in rodents. As a result, rodents are capable of making antibody specific for this di-galactoside, and infection selects against lic2A and lgtC phase-on bacteria (Weiser and Pan, 1998; Erwin et al., 2006a).
We demonstrate in the current study that both components of the di-galactoside and the alternative glucose extension from the same hexose moiety contribute to bacterial survival in the presence of complement (Fig. 4). In some strains, lex2A expression controls attachment of a galactose rather than glucose, which allows for subsequent di-galactoside attachment (Deadman et al., 2009). In this way, all three phase variable structures controlled by lic2A, lgtC and lex2A expression could have an additive effect on evasion of antibody binding and complement-mediated lysis (Griffin et al., 2005). Of note, selection for lex2A phase-on variants was a more rare occurrence in R2846 than selection for lic2A phase-on variants (Table 2). Also, while lex2A is also present in Eagan, selection for lex2A phase-on variants was not observed. It is possible that due to the additive effect of lic2A and lgtC, which does not occur for lex2A and lgtC, selection for di-galactoside expression is more favourable for bacterial evasion of complement-mediated killing.
Serial passage of bacteria in human serum resulted in the identification of changes in lic2A, lex2A and lgtC tetra-nucleotide repeats in several H. influenzae strains (Table 2). Despite the LPS structural differences and varying levels of initial serum resistance of these strains, the same genes were found to contribute to bacterial resistance. However, there were no repeat shifts detected for lic3A (Rd or R2846), oafA (R2846) or losA1 (R2846). Expression of lic3A results in sialic acid attachment to the LPS. As all bacterial populations tested were lic3A phase-on, we controlled sialic acid expression by selective addition of Neu5Ac to growth media. We found that sialic acid increased bacterial survival in human serum for all H. influenzae strains included in the present study. While previous work has demonstrated that oafA and losA1 can contribute to bacterial survival in serum (Fox et al., 2005; Erwin et al., 2006b), in this study we did not isolate any oafA or losA1 phase-off variants, so these genes could not be fully accounted for in our analysis. In a previous study from this laboratory, a screen of genes contributing to serum resistance in the NTHi clinical isolate R2866 found that several LPS biosynthesis genes, including lic2A, lgtC and lex2A, are important for bacterial survival in human serum (Nakamura et al., 2011). In addition to these, a role for vacJ and its associated genes was described, where increased vacJ transcription reduced IgM binding. In contrast, we did not observe a change in vacJ expression between serum sensitive and resistant populations of Rd (data not shown). While other spontaneous mutations between the original and resistant populations isolated in this study cannot be ruled out without full genome sequence analysis, our data suggest the difference in survival is due solely to the change in repeat number of the identified LPS phase variable genes.
Previous work has shown that ChoP attachment to the LPS reduces antibody binding through an effect on the physical properties of the outer membrane, reducing membrane accessibility (Clark et al., 2012). For example, lic1A phase-on bacteria are more resistant to EDTA treatment, which is a measure of outer membrane stability. In contrast, di-galactoside expression does not affect EDTA sensitivity (Clark et al., 2012). Also, ChoP has a more global effect on antibody binding, while di-galactoside expression reduced binding of IgG, but not IgM, to the bacterial surface (Fig. 5), suggesting these two LPS modifications affect sensitivity to antibody binding through different mechanisms. The contribution of lgtC expression to bacterial survival has been previously explored in the NTHi strain R2866. It was found that lgtC phase-on bacteria have reduced deposition of the complement protein C4b, compared with lgtC– mutants (Ho et al., 2007). Reduction in C4b and IgG binding decreases sensitivity to classical pathway complement-mediated killing, and both could contribute to resistance. In the current study, we found that the di-galactoside and alternative glucose extension from the same hexose moiety each reduce binding of human IgG and the mAb 6E4, which binds an inner core LPS structure that includes KDO (Figs 5 and 6; Fig. S2). We also showed that human IgG recognizes conserved inner core LPS structures, as there was increased antibody binding to the truncated opsX– mutant compared with bacteria with more complete oligosaccharide extensions (Fig. 1B). These data suggest that LPS hexose structures affect antibody binding indirectly, possibly through steric hindrance. The attachment of sialic acid to the LPS, in contrast to ChoP and di-galactoside, affects the alternative pathway of complement-mediated lysis. It was shown previously that bacteria with sialylated LPS have delayed deposition of complement components on the outer membrane compared with those without sialic acid (Figueira et al., 2007). While ChoP, di-galactoside, the alternative glucose extension, and sialic acid attachment to LPS affect host recognition in different ways, all contribute to survival in the presence of complement (Fig. 7). The use of divergent mechanisms may contribute to the ability of these phase variable molecules to have an additive effect on resistance to complement-mediated killing.
The LPS structural variations introduced by the attachment of ChoP, di-galactoside, the alternative glucose extension and sialic acid may contribute to survival in other in vivo contexts that have yet to be explored. However, each of these structures is phase variable, which suggests there are also host environments for which expression is not favourable for survival. For ChoP, it has been shown that CRP can recognize ChoP-modified LPS and initiate classical pathway complement-mediated killing (Weiser et al., 1998). In the case of di-galactoside and sialic acid, however, the disadvantages during infection are less clear. Galectins, which bind several galactose-associated LPS residues, have the potential to recognize these structures (Sato et al., 2009; Vasta, 2009). In many H. influenzae strains, the residue GalNAc, which is recognized by some galectins, can be attached to the terminal end of hexose extensions including the galα1–4gal di-galactoside. Galectin binding can initiate immune responses resulting in bacterial clearance (Sato et al., 2009). Also, it has been shown that lic2A expression increases susceptibility of H. influenzae to infection with the bacteriophage HP1c1 (Zaleski et al., 2005). The galactose that is attached to the LPS in lic2A phase-on variants is likely part of the receptor required for phage predation. Host immune and microbial factors that target H. influenzae LPS modifications can thereby also provide a negative selective pressure for phase variants in some host environments.
In summary, we show that exposure to human antibody and complement drives selection for bacteria expressing phase variable LPS structures, including the galα1–4gal di-galactoside and the alternative glucose extension attached to the same hexose moiety. Selection for di-galactoside expression was observed in several distinct H. influenzae strains, and the attachment of di-galactoside or the alternative glucose extension reduces antibody binding to the LPS. Each of the phase variable structures including ChoP, sialic acid and di-galactoside are outer core modifications with reduced host recognition through molecular mimicry (Schauer, 1985; Virji et al., 1990; Fan et al., 2001). While LPS modifications provide a source of surface variation in H. influenzae, there is a limited repertoire of structural conformations and epitopes achieved by phase variation alone. Instead, phase variation of LPS outer core structures may serve to shield conserved inner core structures from antibody binding and complement deposition. LPS modifications including ChoP, sialic acid, di-galactoside and the alternative hexose extension each contribute to bacterial survival in the presence of antibody and complement, and have an additive effect in combination. The additive effect of each of these LPS phase variable structures is supportive of the hypothesis that in the presence of human antibody and complement, survival favours bacteria with the most highly decorated LPS. Phase variation ensures that H. influenzae populations are staged for rapid enrichment of the most-fit variants for successful colonization.
Experimental procedures
Bacterial strains and growth conditions
See Table 1 for a full list of all H. influenzae strains and mutants of these strains included in the present study. Bacteria were grown in brain heart infusion media (Becton Dickinson Biosciences, Franklin Lake, NJ) supplemented with 2% Fildes enrichment (Remel, Lenexa, KS) and 20 μg ml−1 β-Nicotinamide adenine dinucleotide hydrate (Sigma, St Louis, MO). Strains with multiple mutations (ex. lic1D–, lpsA–) were created through transformation of one mutant strain with genomic DNA of the second mutant strain. All mutants constructed for the present study were back-transformed and confirmed by PCR analysis.
For the lex2A mutant, a spectinomycin resistance cassette was inserted to disrupt gene function. The lex2A gene and flanking regions was amplified from R2846 with the primer pair lex2A amplify (Table S1) and cloned into the pCR™ 2.1-TOPO® vector, according to manufacturer’s instructions (Invitrogen, Grand Island, NY). A region of lex2A was deleted by inverse PCR with the primer pair lex2A XmaI (Table S1), introducing an XmaI site. The spectinomycin gene aad9 was inserted at the XmaI site following amplification with the primer pair aad9 (Table S1). The resulting plasmid used to transform R2846. The siaP– mutant was constructed as previously described (Severi et al., 2005). Briefly, the siaP gene (carried by the pUC19 plasmid) was disrupted by deletion of a BglII restriction fragment, and a kanamycin resistance cassette from the pUC4Kan plasmid was inserted. The resulting plasmid was used to transform the Rd lic1D– strain. In R2846, lic1A phase-on colonies were identified by colony immunoblotting with the mAb TEPC-15, as described previously (Weiser et al., 1997). The TEPC-15 enriched lic1A phase-on population is >98% lic1A phase-on. Similarly, the mAb 4C4 was used for detection and enrichment of lic2A and lgtC phase-on variants of 2019. Binding of mAb 4C4 was also used for phenotypic verification of lic2A– mutant strains, as was TEPC-15 binding for lic1D– strains.
Flow cytometric analysis
Flow cytometry was used to detect antibody binding to the bacterial surface as described previously (Nakamura et al., 2011). Briefly, 200 μl reactions were conducted in Hank’s buffer without Ca2+ or Mg2+ (Gibco, San Diego, CA) supplemented with 5% fetal calf serum (HyClone, Logan, UT) with a 20 μl of mid-logarithmic phase bacterial cells (OD620 0.5) diluted to 105 cfu ml−1. Reactions were incubated with primary antibody for 60 min at 37°C. Primary antibody sources consisted of purified IgG from NHS (4.8 μg) and mAb 6E4 (1:200 dilution). A Protein G column (GE Healthcare Bio-Sciences, Uppsala, Sweden) was used to purify IgG from NHS. An alternative source of purified IgG from a mixed NHS pool was obtained from Sigma. Reactions were washed and re-suspended in 1:200 dilutions of secondary antibody, followed by incubation at 4°C for 60 min. Secondary antibody sources included goat anti-human IgG-FITC and goat anti-mouse IgG-FITC (Sigma). After secondary incubation, reactions were washed and re-suspended in PBS with 1% bovine serum albumin and 0.5% paraformaldehyde prior to analysis on a BD FACS Calibur flow cytometer (Becton Dicksinson Biosciences). A total of 50 000 cells were collected, and mean fluorescence intensity (MFI) was determined using FlowJo software (Tree Star, Ashland, OR). Partial digestion of outer membrane proteins with trypsin was performed, where specified, as described previously (Clark et al., 2012). Briefly, 200 μl reactions containing 20 μl of mid-logarithmic phase bacterial cells (OD620 0.5) diluted to 105 cfu ml−1 in 10 mM Tris-HCL, pH 7.5 were washed and re-suspended in 1 mg ml−1 trypsin. Following incubation for 2 h at 37°C, cells were washed and re-suspended in Hank’s buffer for flow cytometry as described above.
Bactericidal assays
Bactericidal assays were conducted as described previously (Clark et al., 2012). Briefly, 200 μl reaction mixtures in Hank’s buffer were incubated for 45 min at 37°C following addition of serum. Serum sources included NHS, CRP-depleted NHS, and BRS to which the mAb 6E4 (1:200 dilution) or purified IgG from NHS (4.8 μg/200 μl reaction) was added. NHS was obtained from a single donor to provide a consistent repertoire of human antibody, although representative experiments were repeated with similar results from other NHS donors. Where specified, Neu5Ac (Sigma) was added to growth media prior to bactericidal assays to a final concentration of 0.1 mg ml−1. CRP was depleted from NHS for bactericidal assays conducted with lic1A phase-on variants with immobilized p-aminophenyl phosphoryl choline gel (Thermo Scientific, Rockford, IL). Percent survival was determined relative to complement-inactivated serum, which was obtained by incubation of NHS for 30 min at 56°C. For serial passage in NHS or mAb 6E4 and BRS, 5–25 surviving colonies were picked and grown for the subsequent bactericidal assay, performed under the same conditions. Inactivation of classical and lectin pathways of complement was accomplished by chelation of NHS with gelatin veronal buffer containing MgEGTA (50 μM final concentration in NHS), (Boston Bioproducts, Worcester, MA). EDTA sensitivity was compared by addition of EDTA (1–4 mM) to bacterial cells in Hank’s buffer followed by incubation for 4 h at 37°C. Percent survival was determined relative to no-EDTA controls.
Sequence analysis of bacterial populations
Genomic DNA was isolated from multiple colonies of each population (original and post exposure to NHS or mAb 6E4 with BRS) for sequence analysis. Primers listed in Table 3 were used to amplify a portion of the target gene and surrounding sequence that included the tetranucleotide (or in the case of losA1, octanucleotide) repeat. The number of repeats was counted from each sequence and used to determine reading frame status as in frame (ON) or out of frame (OFF), as listed in Table 2.
Statistical analysis
Differences between experimental groups were assessed for statistical significance with an unpaired Student’s two-tailed t-test (GraphPad Prism 4, GraphPad Software, La Jolla, CA).
Supplementary Material
Acknowledgments
We thank Derek W. Hood (University of Oxford) for providing LPS structural mutants noted in Table 1. Also, we thank Michael A. Apicella (University of Iowa) for generously providing mAb 6E4. This work was supported by training grants awarded to the University of Pennsylvania from the US Public Health Service (GM007229-35 and AI060516).
Footnotes
The authors have no conflict of interest to declare.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol. 2011;49:3728–3732. doi: 10.1128/JCM.05476-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzan M, Richardson S, Huang C, Boyd B, Petric M, Karmali MA. Evidence that verotoxins (Shiga-like toxins) from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect Immun. 1994;62:3337–3347. doi: 10.1128/iai.62.8.3337-3347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnari AA, Gupta MR, Dudas KC, Murphy TF, Apicella MA. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun. 1987;55:882–887. doi: 10.1128/iai.55.4.882-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Snow J, Li J, Zola TA, Weiser JN. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLoS Pathog. 2012;8:e1002521. doi: 10.1371/journal.ppat.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadman ME, Hermant P, Engskog M, Makepeace K, Moxon ER, Schweda EK, Hood DW. Lex2B, a phase-variable glycosyltransferase, adds either a glucose or a galactose to Haemophilus influenzae lipopolysaccharide. Infect Immun. 2009;77:2376–2384. doi: 10.1128/IAI.01446-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin AL, Allen S, Ho DK, Bonthuis PJ, Jarisch J, Nelson KL, et al. Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum. Infect Immun. 2006a;74:6226–6235. doi: 10.1128/IAI.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin AL, Bonthuis PJ, Geelhood JL, Nelson KL, McCrea KW, Gilsdorf JR, Smith AL. Heterogeneity in tandem octanucleotides within Haemophilus influenzae lipopolysaccharide biosynthetic gene losA affects serum resistance. Infect Immun. 2006b;74:3408–3414. doi: 10.1128/IAI.01540-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Goldfine H, Lysenko E, Weiser JN. The transfer of choline from the host to the bacterial cell surface requires glpQ in Haemophilus influenzae. Mol Microbiol. 2001;41:1029–1036. doi: 10.1046/j.1365-2958.2001.02571.x. [DOI] [PubMed] [Google Scholar]
- Fernaays MM, Lesse AJ, Cai X, Murphy TF. Characterization of igaB, a second immunoglobulin A1 pro-tease gene in nontypeable Haemophilus influenzae. Infect Immun. 2006;74:5860–5870. doi: 10.1128/IAI.00796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun. 2007;75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A, Quie PG. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974;10:402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KL, Yildirim HH, Deadman ME, Schweda EK, Moxon ER, Hood DW. Novel lipopolysaccharide biosynthetic genes containing tetranucleotide repeats in Haemophilus influenzae, identification of a gene for adding O-acetyl groups. Mol Microbiol. 2005;58:207–216. doi: 10.1111/j.1365-2958.2005.04814.x. [DOI] [PubMed] [Google Scholar]
- Griffin R, Cox AD, Makepeace K, Richards JC, Moxon ER, Hood DW. The role of lex2 in lipopolysaccharide biosynthesis in Haemophilus influenzae strains RM7004 and RM153. Microbiology. 2003;149:3165–3175. doi: 10.1099/mic.0.26387-0. [DOI] [PubMed] [Google Scholar]
- Griffin R, Bayliss CD, Herbert MA, Cox AD, Makepeace K, Richards JC, et al. Digalactoside expression in the lipopolysaccharide of Haemophilus influenzae and its role in intravascular survival. Infect Immun. 2005;73:7022–7026. doi: 10.1128/IAI.73.10.7022-7026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- High NJ, Deadman ME, Moxon ER. The role of a repetitive DNA motif (5′-CAAT-3′) in the variable expression of the Haemophilus influenzae lipopolysaccharide epitope alpha Gal(1-4)beta Gal. Mol Microbiol. 1993;9:1275–1282. doi: 10.1111/j.1365-2958.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- Ho DK, Ram S, Nelson KL, Bonthuis PJ, Smith AL. lgtC expression modulates resistance to C4b deposition on an invasive nontypeable Haemophilus influenzae. J Immunol. 2007;178:1002–1012. doi: 10.4049/jimmunol.178.2.1002. [DOI] [PubMed] [Google Scholar]
- Hood DW, Deadman ME, Allen T, Masoud H, Martin A, Brisson JR, et al. Use of the complete genome sequence information of Haemophilus influenzae strain Rd to investigate lipopolysaccharide biosynthesis. Mol Microbiol. 1996a;22:951–965. doi: 10.1046/j.1365-2958.1996.01545.x. [DOI] [PubMed] [Google Scholar]
- Hood DW, Deadman ME, Jennings M, Bisercic M, Fleischmann RD, Venter JC, Moxon ER. DNA repeats identify novel virulence genes in Haemophilus influenzae. Proc Natl Acad Sci USA. 1996b;93:11121–11125. doi: 10.1073/pnas.93.20.11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DW, Cox AD, Gilbert M, Makepeace K, Walsh S, Deadman ME, et al. Identification of a lipopolysaccharide alpha-2,3-sialyltransferase from Haemophilus influenzae. Mol Microbiol. 2001a;39:341–350. doi: 10.1046/j.1365-2958.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- Hood DW, Cox AD, Wakarchuk WW, Schur M, Schweda EK, Walsh SL, et al. Genetic basis for expression of the major globotetraose-containing lipopolysaccharide from H. influenzae strain Rd (RM118) Glycobiology. 2001b;11:957–967. doi: 10.1093/glycob/11.11.957. [DOI] [PubMed] [Google Scholar]
- Lundstrom SL, Li J, Deadman ME, Hood DW, Moxon ER, Schweda EK. Structural analysis of the lipopolysaccharide from nontypeable Haemophilus influenzae strain R2846. Biochemistry. 2008;47:6025–6038. doi: 10.1021/bi702510b. [DOI] [PubMed] [Google Scholar]
- Lysenko E, Richards JC, Cox AD, Stewart A, Martin A, Kapoor M, Weiser JN. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Lliteras P, Lopez-Gomez A, Mauro S, Hood DW, Viadas C, Calatayud L, et al. Nontypable Haemophilus influenzae displays a prevalent surface structure molecular pattern in clinical isolates. PLoS ONE. 2011;6:e21133. doi: 10.1371/journal.pone.0021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Faden H, Bakaletz LO, Kyd JM, Forsgren A, Campos J, et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J. 2009;28:43–48. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- Naiki M, Marcus DM. An immunochemical study of the human blood group P1, P, and PK glycosphingolipid antigens. Biochemistry. 1975;14:4837–4841. doi: 10.1021/bi00693a010. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, et al. Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog. 2011;7:e1001247. doi: 10.1371/journal.ppat.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel GJ, Brittingham A, Granato AA, Mosser DM. Effect of amplification of the Cap b locus on complement-mediated bacteriolysis and opsonization of type b Haemophilus influenzae. Infect Immun. 1996;64:4769–4775. doi: 10.1128/iai.64.11.4769-4775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JJ, Foster ED, Chaloner K, Hunt JR, Jennings MP, Bair T, et al. Analysis of nontypeable Haemophilus influenzae phase variable genes during experimental human nasopharyngeal colonization. J Infect Dis. 2013 doi: 10.1093/infdis/jit240. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power PM, Sweetman WA, Gallacher NJ, Woodhall MR, Kumar GA, Moxon ER, Hood DW. Simple sequence repeats in Haemophilus influenzae. Infect Genet Evol. 2009;9:216–228. doi: 10.1016/j.meegid.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids and their role as biological masks. Trends Biochem Sci. 1985;10:357–360. [Google Scholar]
- Severi E, Randle G, Kivlin P, Whitfield K, Young R, Moxon R, et al. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol. 2005;58:1173–1185. doi: 10.1111/j.1365-2958.2005.04901.x. [DOI] [PubMed] [Google Scholar]
- Spinola SM, Kwaik YA, Lesse AJ, Campagnari AA, Apicella MA. Cloning and expression in Escherichia coli of a Haemophilus influenzae type b lipooligosaccharide synthesis gene(s) that encodes a 2-keto-3-deoxyoctulosonic acid epitope. Infect Immun. 1990;58:1558–1564. doi: 10.1128/iai.58.6.1558-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE, Jones PA, Apicella MA. The lipooligosaccharides of Haemophilus influenzae: an interesting array of characters. J Endotoxin Res. 2003;9:131–144. doi: 10.1179/096805103125001531. [DOI] [PubMed] [Google Scholar]
- Tong HH, Blue LE, James MA, Chen Y, DeMaria TF. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun. 2000;68:4593–4597. doi: 10.1128/iai.68.8.4593-4597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanova M, Tsang RS. Invasive Haemophilus influenzae disease: changing epidemiology and host-parasite interactions in the 21st century. Infect Genet Evol. 2009;9:594–605. doi: 10.1016/j.meegid.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M, Weiser JN, Lindberg AA, Moxon ER. Antigenic similarities in lipopolysaccharides of Haemophilus and Neisseria and expression of a digalactoside structure also present on human cells. Microb Pathog. 1990;9:441–450. doi: 10.1016/0882-4010(90)90062-u. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Love JM, Moxon ER. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Shchepetov M, Chong ST. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JN, Pan N, McGowan KL, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski P, Wojciechowski M, Piekarowicz A. The role of Dam methylation in phase variation of Haemophilus influenzae genes involved in defence against phage infection. Microbiology. 2005;151:3361–3369. doi: 10.1099/mic.0.28184-0. [DOI] [PubMed] [Google Scholar]
- Zola TA, Lysenko ES, Weiser JN. Natural antibody to conserved targets of Haemophilus influenzae limits colonization of the murine nasopharynx. Infect Immun. 2009;77:3458–3465. doi: 10.1128/IAI.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.