Abstract
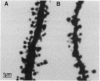
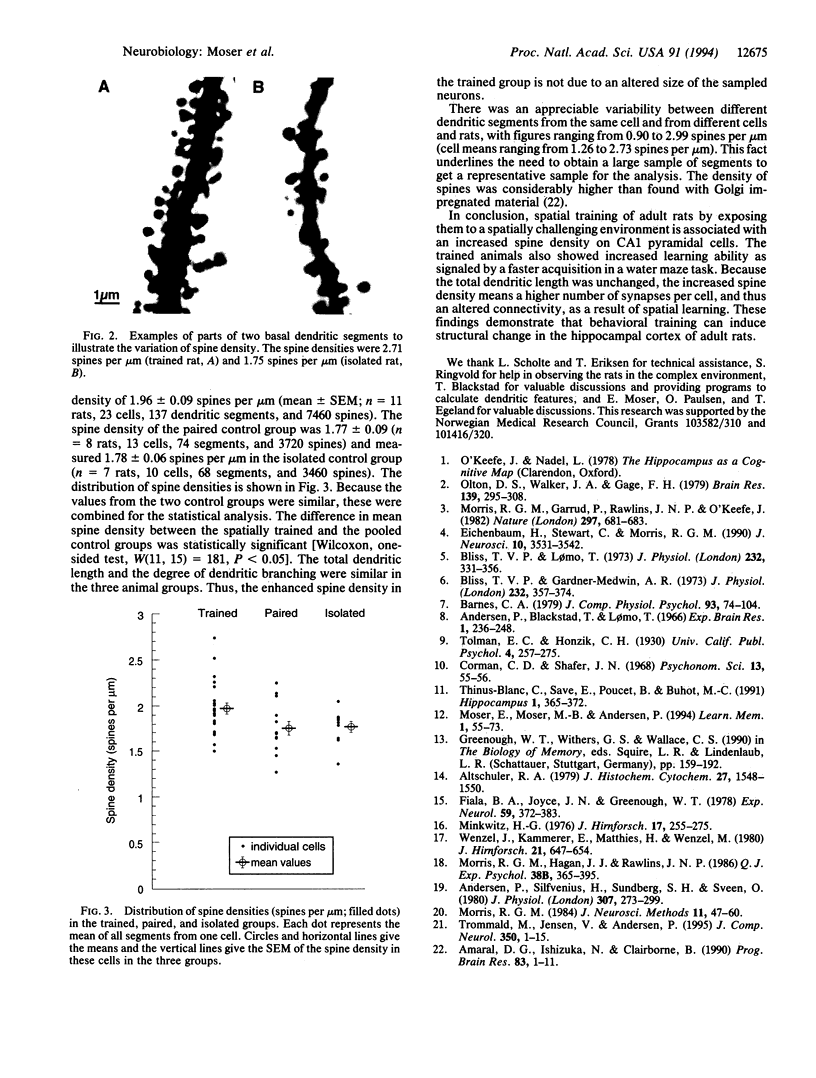
The search for cellular correlates of learning is a major challenge in neurobiology. The hippocampal formation is important for learning spatial relations. A possible long-lasting consequence of such spatial learning is alteration of the size, shape, or number of excitatory synapses. The dendritic spine density is a good index for the number of hippocampal excitatory synapses. By using laser-scanning confocal microscopy, we observed a significantly increased spine density in CA1 basal dendrites of spatially trained rats when compared to nontrained controls. With unchanged dendritic length, the higher spine density reflects an increased number of excitatory synapses per neuron associated with spatial learning.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuler R. A. Morphometry of the effect of increased experience and training on synaptic density in area CA3 of the rat hippocampus. J Histochem Cytochem. 1979 Nov;27(11):1548–1550. doi: 10.1177/27.11.512348. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Ishizuka N., Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Andersen P., Blackstad T. W., Lömo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1(3):236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Stewart C., Morris R. G. Hippocampal representation in place learning. J Neurosci. 1990 Nov;10(11):3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala B. A., Joyce J. N., Greenough W. T. Environmental complexity modulates growth of granule cell dendrites in developing but not adult hippocampus of rats. Exp Neurol. 1978 May 1;59(3):372–383. doi: 10.1016/0014-4886(78)90229-7. [DOI] [PubMed] [Google Scholar]
- Minkwitz H. G. Zur Entwicklung der Neuronenstruktur des Hippocampus während der prä- und postnatalen Ontogenese der Albinoratte. III. Mitteilung: Morphometrische Erfassung der ontogenetischen Veränderungen in Dendritenstruktur und Spinebesatz an Pyramidenneuronen (CA1) des Hippocampus. J Hirnforsch. 1976;17(3):255–275. [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Hagan J. J., Rawlins J. N. Allocentric spatial learning by hippocampectomised rats: a further test of the "spatial mapping" and "working memory" theories of hippocampal function. Q J Exp Psychol B. 1986 Nov;38(4):365–395. [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser E. I., Moser M. B., Andersen P. Potentiation of dentate synapses initiated by exploratory learning in rats: dissociation from brain temperature, motor activity, and arousal. Learn Mem. 1994 May-Jun;1(1):55–73. [PubMed] [Google Scholar]
- Olton D. S., Walker J. A., Gage F. H. Hippocampal connections and spatial discrimination. Brain Res. 1978 Jan 13;139(2):295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C., Save E., Poucet B., Buhot M. C. The effects of reversible inactivations of the hippocampus on exploratory activity and spatial memory. Hippocampus. 1991 Oct;1(4):365–371. doi: 10.1002/hipo.450010404. [DOI] [PubMed] [Google Scholar]
- Wenzel J., Kammerer E., Kirsche W., Matthies H., Wenzel M. Electron microscopic and morphometric studies on synaptic plasticity in the hippocampus of the rat following conditioning. J Hirnforsch. 1980;21(6):647–654. [PubMed] [Google Scholar]