Abstract
Purpose of Review
Fibroblast growth factor 23 (FGF23) regulates phosphate and vitamin D homeostasis and rises as kidney function declines. Animal studies have demonstrated direct and indirect effects of FGF23 that may promote heart disease. Herein we review the recent epidemiologic literature evaluating the relationship between FGF23 and cardiovascular disease.
Recent Findings
In observational prospective studies higher FGF23 associates with greater risk of incident cardiovascular disease including ischemic heart disease, stroke, heart failure, and atrial fibrillation. These studies establish a temporal sequence of events over long term-follow up that suggest a possible role of FGF23 in cardiovascular disease pathogenesis. In most studies risk is generally graded, however in the largest study to date, higher FGF23 within the low-normal range was not associated with higher risk. In several recent studies higher FGF23 associated more strongly with risk of congestive heart failure compared with atherosclerotic events, a finding consistent with surrogate endpoints and animal experiments. Currently the utility of FGF23 as a predictive biomarker of cardiovascular risk is not established, and interventions to reduce FGF23 need to be studied to confirm its possible pathophysiologic role.
Summary
Higher FGF23 is associated with the subsequent development of cardiovascular disease, and perhaps most notably heart failure, in a growing number of studies. These findings bolster ongoing efforts to lower FGF23 using strategies to reduce phosphate intake and absorption.
Keywords: Fibroblast growth factor 23, phosphate homeostasis, cardiovascular disease, congestive heart failure, chronic kidney disease
Introduction
Fibroblast growth factor 23 (FGF23) is a 32 kDa hormone that regulates phosphate and vitamin D homeostasis by augmenting urinary phosphate excretion and limiting conversion of 25-hydroxyvitamin D to its active form, 1,25-dihydroxyvitamin D. Lowered 1,25-dihydroxyvitamin D subsequently reduces absorption of phosphate in the gastrointestinal tract and promotes secondary hyperparathyroidism. Thus, FGF23 coordinates an integrated endocrine axis whose normal function maintains bone mineralization in healthy individuals. In the setting of chronic kidney disease (CKD), adaptations in this axis frequently occur to maintain phosphate excretion despite its reduced renal filtration (1).
Dysfunction of this phosphate/vitamin D axis was initially highlighted as a risk factor for accelerated cardiovascular disease in patients on dialysis, by observations that higher serum phosphate associated with increased mortality risk, whereas restoration of vitamin D activity with therapeutic administration of active analogues associated with lower risk (2, 3). Interest grew further with the discovery of a relationship between higher FGF23 and all-cause mortality in patients with end-stage renal disease on dialysis, with CKD stages 2–4 and in the general population (4–7). Cross-sectionally, FGF23 associated with cardiac remodeling suggesting that the increased mortality risk could be attributable to cardiovascular effects (8, 9).
Consistent with these observations, elegant experiments in animal models have documented direct effects of FGF23 to promote hypertrophy, contractility and arrhythmic potential of the myocardium (10–12). Additional studies have found indirect adverse cardiac effects of FGF23, such as activation of the renin-angiotensin system and promotion of sodium reabsorption in the distal tubule of the kidney (13–15). As a result of this initial body of epidemiologic and basic research, a compelling hypothesis emerged in which elevation of FGF23, while necessary to control phosphate and vitamin D homeostasis, may also contribute to the development of cardiovascular disease.
Bolstered by these exciting findings in controlled animal experiments, there has been an expanding body of epidemiologic literature evaluating FGF23 as a risk factor for clinical cardiovascular disease events. This review will focus on recent insights from such epidemiologic studies, with an emphasis on studies published in the last 12–18 months. The observational studies discussed here critically translate basic findings to diverse human populations and ultimately pave the way for interventional studies targeting FGF23 and the phosphate/vitamin D axis.
Epidemiology of Fibroblast Growth Factor 23 and Cardiovascular Disease
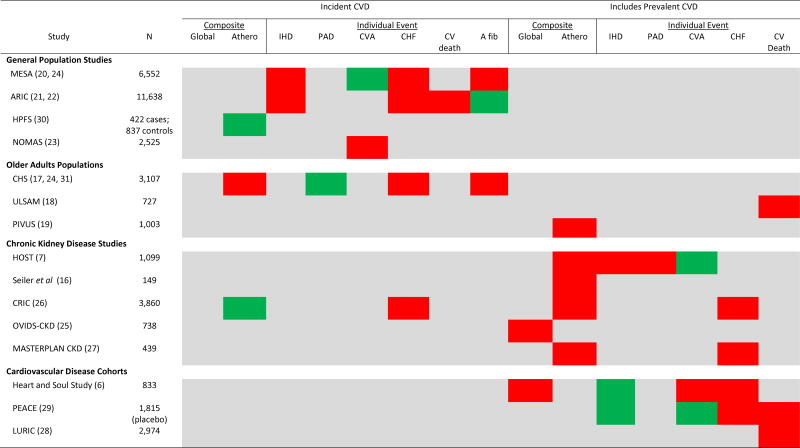
Higher FGF23 was initially described as a risk factor for clinical cardiovascular events in relatively small studies of adults with established coronary artery disease or moderate to advanced CKD (6, 7, 16). Similar results were subsequently reported in two studies of older adults in the general population (17, 18). Recently, there have been new reports from a variety of cohort studies in the general population (19–24); studies in CKD populations representing the full spectrum of kidney function (25–27); and additional studies in populations with established cardiovascular disease (28, 29). Figure 1 provides a qualitative summary and overview of populations and outcomes assessed in prospective studies that will be discussed in this review. These studies largely support a relationship between higher FGF23 and cardiovascular disease that is independent of kidney function, and, collectively, they address several key questions that advance the field.
Figure 1. Qualitative Summary of Major Prospective Studies Evaluating the Independent Relationship Between Fibroblast Growth Factor 23 and Clinical Cardiovascular Events Across Populations and Event Types.
Significant independent associations between FGF23 and events are highlighted in red with a report of no independent association highlighted in green. Reporting of precise effect sizes across studies are limited by differences in scaling of FGF23 (additive vs. multiplicative). Where possible, results from multivariable models including adjustment for kidney function are emphasized. Some endpoints that are listed as negative (green) had limited power to detect associations. Global composites include some atherosclerotic endpoints and congestive heart failure, but exact atherosclerotic endpoints included differ by study. Atherosclerotic composites include some combination of myocardial infarction, peripheral arterial disease, cerebrovascular accident/transient ischemic attack, or cardiovascular cause specific mortality, but exact composition differs by study. In the PIVUS Study this endpoint included all cause-mortality. Some of the results summarized here emanate from different publications involving the same cohort as indicated by citations. Abbreviations include: MESA, Multi-Ethnic Study of Atherosclerosis; ARIC, Atherosclerosis Risk in Communities Study; HPFS, Health Professionals Follow-Up Study; NOMAS, Northern Manhattan Study; CHS, Cardiovascular Health Study; ULSAM, Uppsala Longitudinal Study of Adult Men; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; HOST, Homocysteine in Kidney and End-Stage Renal Disease Study; CRIC, Chronic Renal Insufficiency Cohort Study; OVIDS-CKD, Osaka Vitamin D Study in Patients with CKD; PEACE, Prevention of Events with Angiotensin-Converting Enzyme Trial; LURIC, Ludwigshafen Risk and Cardiovascular Health Study; Athero, atherosclerotic; IHD, ischemic heart disease; PAD, peripheral arterial disease; CVA, cerebrovascular accident (may include transient ischemic attack); CHF, congestive heart failure; CV death, cardiovascular death; A fib, atrial fibrillation
Is there a dose-response relationship between FGF23 and cardiovascular events?
In most studies the relationships between higher FGF23 levels and cardiovascular disease is generally graded (6, 17, 20, 23, 26). For instance in the Chronic Renal Insufficiency Cohort (CRIC) Study, we found that the relative risk of atherosclerotic events and cardiovascular events increased by 24% and 45% respectively, with each 2-fold increase in FG23 after multivariable adjustment including adjustment for kidney function (26). This finding was remarkably similar to effect estimates observed within the CKD subgroup in the Cardiovascular Health Study (CHS)(17). We examined relative, or multiplicative, differences in FGF23 due to the exponential increase of FGF23 as kidney function declines (32); however, some studies in the general population have looked at risk associated with absolute, or additive, differences in FGF23 (18, 20, 21). These modeling differences make it difficult to directly compare shapes of associations across different populations. Despite the differences, some studies in the general population have also found a gradient of risk with a relatively linear association between higher absolute FGF23 levels and risk of cardiovascular disease (18, 20).
A notable exception is the Atherosclerotic Risk in Communities (ARIC) Study that measured FGF23 in over 11,000 individuals from 4 communities across the US (21). They reported a threshold level of FGF23, with a graded relationship between higher intact FGF23 and risk of incident coronary heart disease, heart failure or cardiovascular mortality at levels above, but not below, 40 pg/ml. In other general population studies confidence intervals were often wide in these lower ranges (18, 20), and, therefore, the power available in the ARIC Study may have allowed detection of this flat portion of the curve. Alternatively differences in modeling decisions, such as lack of adjustment for eGFR variation within the normal range (i.e. eGFR ≥ 60 ml/min/1.73m2) or use of intact as opposed to C-terminal assays may account for the slightly different shape of the association in this study.
How does FGF23 relate to different cardiovascular disease event subtypes?
Although many studies have found that higher FGF23 is independently associated with atherosclerotic endpoints, such as myocardial infarction, peripheral vascular disease and stroke (7, 16, 17, 20, 23, 26, 27), in many studies these associations are more modest than the comparable association with congestive heart failure (6, 17, 20, 26). In the CRIC Study, we found that this difference in effect size was statistically significant and that associations between FGF23 and heart failure were more robust to a variety of modeling strategies including analyses of events meeting a higher standard of adjudication as well as restriction to new-onset, or incident, disease (26). Furthermore, some studies have found no association between FGF23 and atherosclerotic event-types, such as incident coronary heart disease and peripheral vascular disease events (29–31). Recent results from ARIC revealed largely similar associations of FGF23 with atherosclerotic events and heart failure with an approximately 8% increased risk of each event type per each standard deviation (SD) increase in FGF23 (SD=16.4 pg/ml) after full adjustment (21). This difference could be due to ascertainment of heart failure events largely by billing codes in this study as opposed to medical record adjudication in many of the other reports (6, 17, 20, 26).
Over the last year, the role of FGF23 as a risk factor for atrial fibrillation has been investigated in three of the large general population cohorts. In each study atrial fibrillation was ascertained through billing claims and study related electrocardiograms. Higher FGF23 was associated with greater risk of incident atrial fibrillation in both the Multi-Ethnic Study Atherosclerosis (MESA) and the CHS over up to 10 years of follow up (24). The relative risk estimates were remarkably similar in the two studies with an approximately 30–40% increased risk for each 2-fold higher FGF23. These similarities were despite large differences in the underlying incident rate of atrial fibrillation in the two cohorts (6.2 and 25.6 cases per 1000 person years in MESA and CHS, respectively), differences in patient populations and ranges of FGF23. In the larger ARIC Study with up to 20 years of follow-up, FGF23 was not associated with greater risk of incident atrial fibrillation independent of kidney function (22), but meta-analysis of the three studies showed a significant association overall (22).
How do relationships between FGF23 and clinical cardiovascular disease align with subclinical cardiovascular disease outcomes?
The stronger and consistent relationships between FGF23, heart failure and atrial fibrillation is particularly intriguing in light of numerous cross-sectional studies linking higher FGF23 with abnormal left ventricular geometry including higher left ventricular mass, higher left ventricular mass-to-volume ratio and greater risk of hypertrophy and remodeling (8–10, 20, 24, 33, 34). In the last year, the relationship between higher FGF23 levels and increased left ventricular mass has also been observed among children with normal kidney function (35). Although children are less likely to have pre-existing heart disease that would confound these relationships, FGF23 was correlated with many other adverse metabolic risk factors such as insulin resistance, obesity and inflammation.
The observed differences in left ventricular geometry may be direct pathologic mechanisms leading heart failure and atrial fibrillation and are supported by induction of these changes by higher FGF23 in experimental animal models (10, 11, 15). To determine if changes in ventricular geometry may account for the relationships between FGF23 and cardiovascular disease, heart failure or atrial fibrillation, several of the studies have additionally adjusted for measures of left ventricular mass or left atrial diameter (17–19, 24, 26). In each case, adjustment for ventricular geometry did not fully attenuate the relationship of FGF23 with clinical events. However, these mediation analyses should be interpreted cautiously for a number of reasons: 1) generally FGF23 and left ventricular geometry have been measured concurrently and therefore the temporal sequence of events may be violated; 2) left ventricular geometry is measured imprecisely and at one timepoint, therefore, may not fully capture cumulative effects on the myocardium; and 3) other biases inherent to mediation analysis methodology may limit inferences (36, 37).
In contrast to relatively consistent associations between FGF23 and cardiac geometry, the associations between FGF23 and subclinical atherosclerotic disease are more variable. In CRIC we found no independent association between FGF23 and presence or severity of coronary artery calcification (38), although associations have been reported in other large studies (20). The associations between FGF23 and other measures of atherosclerosis or vascular elasticity, such as ankle brachial index, carotid intima-media thickness and arterial elasticity have been mixed with many (20, 31, 39), but not all (40, 41), studies showing no association. Some investigators have reported that FGF23 expression can be induced in atherosclerotic plaques; thus, some of these associations may be a result of this process (42).
Are the cardiovascular risks associated with FGF23 stronger in patients with pre-existing CKD?
In the CRIC study of patients with CKD and median levels of C-terminal FGF23 of 145 RU/ml (IQR 96 to 239 RU/ml), the highest quartile compared to the lowest was independently associated with a higher risk of cardiovascular events, including a 1.8 fold increased risk of atherosclerotic events, such as myocardial infarction, stroke and peripheral arterial disease events, and a 3 fold higher risk of congestive heart failure (26). In contrast, most estimates of the association between FGF23 and cardiovascular disease in the general adult population have been more modest with an approximately 1.3–1.7 fold higher risk among those with the highest compared to lowest levels (20, 21). As discussed above, differences in modeling strategies and follow up time make it difficult to directly compare these effects across studies. Consistent with potentially larger associations observed in the CRIC Study, several studies have found stronger relationships between FGF23 and both subclinical and clinical cardiovascular outcomes in patients with CKD compared to those with normal kidney function within their study populations (17, 18, 24, 33, 34). FGF23 rises as kidney disease progresses and GFR is one of the strongest correlates of FGF23 (7, 20, 21, 32). For this reason levels of FGF23 are substantially higher in CKD and may be more likely to influence risk of cardiovascular disease at this level. Alternatively, other features of CKD, such as deficiency of FGF23’s co-receptor, klotho, activation of the renin angiotensin system, or frank elevation of serum phosphate may interact with high FGF23 biologically to magnify risk (29, 38, 43, 44).
Perhaps most interestingly, FGF23 appears to be most tightly linked to subtypes of cardiovascular disease that are particularly related to CKD, such as heart failure, atrial fibrillation and intracranial hemorrhage (45–49). The overlap in these disease patterns could indicate that FGF23 is a major mediator of cardiovascular disease in patients with CKD, or that FGF23 levels are indicative of components of kidney dysfunction that are at least partially independent of GFR, such as tubular functions (18, 32). Where reported, adjustment for FGF23 may partially, but does not fully, attenuate the association between CKD and subclinical or clinical cardiovascular disease (24, 50); however, the formerly reviewed caveats of mediation studies also apply here (36, 37).
Translating Epidemiologic Findings and the Role of FGF23 in Cardiovascular Disease Prevention
The relatively consistent relationships observed between FGF23 and cardiovascular disease have several potential applications and implications that could be harnessed for patient care and prevention.
FGF23 as a biomarker of cardiovascular disease
One potential application of the observed epidemiologic associations between higher FGF23 and cardiovascular disease is use as a predictive biomarker. Independent effect sizes may not be large enough to add meaningfully to disease prediction models above established cardiovascular risk factors and kidney function, (i.e. GFR and albuminuria) in the general population or CKD (24, 25), although few of the studies have directly addressed this. While some studies have documented modest improvement in outcome prediction metrics among patients with pre-existing heart disease, it is not clear how these predictions would impact clinical decision-making in patients already at high cardiovascular risk (28, 29).
FGF23 as a targetable cause of cardiovascular disease
Despite a possibly limited role in disease prediction, the independent relationships between FGF23 and cardiovascular disease may still indicate a critical role in disease pathogenesis that could be targeted therapeutically. The attributable risk of elevated FGF23 could be even greater if FGF23 is a, or even the, biological factor mediating the relationship between CKD and CVD, in which case adjustment for GFR may underestimate the true impact of targeting FGF23 (24).
FGF23 as a correlate of a cause of cardiovascular disease
A third possibility is that FGF23 is correlated with other causes of cardiovascular disease, most notably other mineral metabolites which may also have adverse cardiovascular effects (6, 38, 44, 51–54). In particular, higher serum phosphate is associated with increased risk of cardiovascular disease and promotes vascular calcification and dysfunction experimentally (38, 52, 54–56), whereas deficiency of the FGF23 co-receptor, klotho, may also directly induce vascular calcification and cardiac hypertrophy (44, 57). Although associations between FGF23 and cardiovascular disease are almost uniformly independent of other measurable mineral metabolites, such as phosphate (16, 19–21, 23–26, 28), it is important to recognize that many of these exhibit substantial biological variability making them difficult to fully quantify and account for in analyses. In fact, several features of FGF23 measurement may make it the optimal biomarker for global dysfunction of the phosphate/vitamin D axis, including a favorable half-life with lower biological variability (27, 58), easy measurement in the circulation which is the biologically relevant site of action and a broad biological range allowing greater between individual discrimination. It is not known how much these measurement properties contribute to the “rank order” of associations observed between mineral metabolites and cardiovascular disease, but ultimately this distinction may not be critical if treatment strategies focus on normalizing this homeostatic axis globally.
Moving “Upstream”
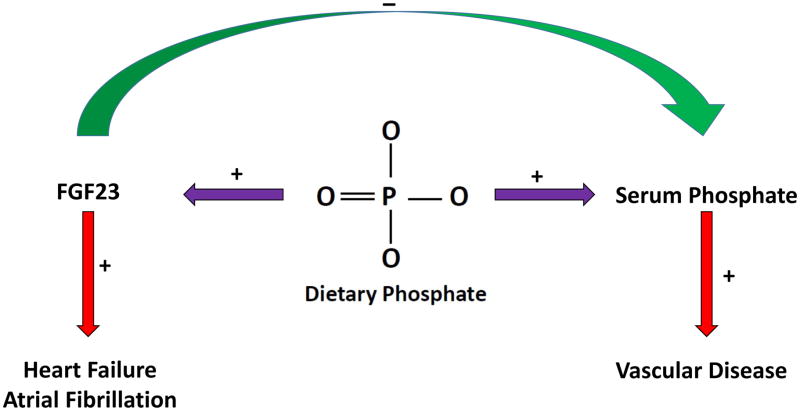
Recent preclinical animal studies that attempt to interrupt endocrine loops involving FGF23 dramatically raise serum phosphate and offer a warning for approaches to FGF23-lowering therapies (59). FGF23 has a critical role in homeostatic control of phosphate and vitamin D, each of which may have adverse effects if uncontrolled. The current body of evidence linking FGF23 with cardiovascular disease is compelling and warrants action, but more work is needed to understand how to modify FGF23 and, hence, the full phosphate/vitamin D axis using targets that are “upstream” of FGF23. Upstream targets, such as changes in dietary phosphate as well as existing or novel agents that limit phosphate absorption, hold potential to interrupt the root causes of phosphate/vitamin D axis dysfunction and therefore could lower FGF23 without adverse effects on phosphate or other mineral metabolites (Figure 2) (60–64). Few studies are available evaluating the relationship between phosphate intake and cardiovascular disease (65–69) and these efforts face enormous challenges including inaccurate dietary reporting, limitations of nutrition databases in terms of phosphate content and inaccuracies in 24 hour urine collections (70–72). However, cross-cultural comparisons suggest that reduction in dietary phosphate can be achieved with potentially large consequences for regulatory hormones, such as FGF23 (73). Despite the challenges, more work is needed in this area.
Figure 2. Moving “upstream” of fibroblast growth factor 23 (FGF23) to target dietary phosphate.
Therapies with a central focus on lowering FGF23 may fail to consider its important physiologic functions in maintaining phosphate (green arrow) and vitamin D homeostasis (not pictured). Although challenging, a central therapeutic focus on dietary phosphate, as a root cause of total phosphate axis dysfunction (purple arrows), may yield better results by mitigating the potential cardiovascular toxicities of both FGF23 and phosphate (red arrows).
Conclusions
Recent studies affirm the strong relationship between higher FGF23 and risk of cardiovascular events that is independent of kidney function and is observable among those without pre-existing heart disease, where FGF23 most clearly precedes disease development. It is currently not definitively known whether variation in FGF23 in the low-normal range, or only in the moderate to high range is associated with risk of cardiovascular disease. Clarifying this at-risk range will be critical to identifying groups in which therapies to reduce FGF23 should be targeted. To date, most evidence supports a stronger relationship between FGF23 and heart failure than atherosclerotic event subtypes, which may be consistent with its purported roles in promoting left ventricular hypertrophy, sodium retention and renin-angiotensin system activation, and modulating cardiac contractility. Ultimately, interventional trials aimed at the root cause of FGF23 elevation, such as excess dietary phosphate exposure, are required to understand whether FGF23 and the associated dysfunction of the phosphate/vitamin D axis is a modifiable cause of cardiovascular disease.
Key Points.
Higher levels of fibroblast growth factor 23 (FGF23) associate independently with greater risk of incident cardiovascular disease in prospective studies, including ischemic heart disease, stroke, congestive heart failure and atrial fibrillation.
The risk associated with higher FGF23 may be greater in patients with kidney disease where levels are generally higher and where it may be associated with other adverse factors such as deficiency of the FGF23 co-receptor, klotho, and higher phosphate.
Across studies, higher FGF23 is more consistently associated with increased risk of heart failure compared with atherosclerotic event types.
Interventional studies targeting dietary phosphate or phosphate absorption are needed to determine if FGF23 can be lowered and if this translates to prevention of cardiovascular disease.
Acknowledgments
Dr. Scialla is supported by NIH K23DK095949.
Financial Support and Sponsorship: Dr. Scialla is funded by K23DK095949 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflicts of Interest: None
The content of this article is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Martin A, David V, Quarles L. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92(1):131–55. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31(4):607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 3.Teng M, Wolf M, Ofsthun N, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N Engl J Med. 2008 Aug 7;359(6):584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011 Jun 15;305(23):2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010 May 18;152(10):640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011 Oct;22(10):1913–22. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009 May 19;119(19):2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirza M, Larsson A, Melhus H, Lind L, Larsson T. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touchberry C, Green T, Tchikrizov V, Mannix J, Mao T, Carney B, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304:E863–E73. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao Y, Chen Y, Lin Y, Shiu R, Chao T, Chen S, et al. FGF-23 dysregulates calcium homeostasis and electrophysiological properties in HL-1 atrial cells. Eur J Clin Invest. 2014;44(8):795–801. doi: 10.1111/eci.12296. [DOI] [PubMed] [Google Scholar]
- 13.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7(9):e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Borst M, Vervloet M, ter Wee P, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–9. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744–59. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine G. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;35:3983–9. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 17.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012 Jul 17;60(3):200–7. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnlov J, Carlsson A, Sundstrom J, Ingelsson E, Larsson A, Lind L, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83:160–6. doi: 10.1038/ki.2012.327. [DOI] [PubMed] [Google Scholar]
- 19.Arnlov J, Carlsson A, Sundstrom J, Ingelsson E, Larsson A, Lind L, et al. Serum FGF23 and Risk of Cardiovascular Events in Relation to Mineral Metabolism and Cardiovascular Pathology. Clin J Am Soc Nephrol. 2013;8(5):781–6. doi: 10.2215/CJN.09570912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Kestenbaum B, Sachs M, Hoofnagle A, Siscovick D, Ix J, Robinson-Cohen C, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409–17. doi: 10.1161/CIRCHEARTFAILURE.113.000952. In this study the authors examine the relationship between levels of intact FGF23 and risk of ischemic heart disease, stroke and congestive heart failure prospectively over long-term follow-up in over 6000 individuals in the general population without cardiovascular disease at baseline. This is one of the first studies to report associations between FGF23 within the “normal” range and risk of incident heart failure and ischemic heart disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Lutsey P, Alonso A, Selvin E, Pankow J, Michos E, Agarwal S, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2014 Jun 10;3(3):e000936. doi: 10.1161/JAHA.114.000936. In this study they report the association between higher baseline intact FGF23 and risk of incident atherosclerotic heart disease, congestive heart failure and cardiovascular mortality over 20 years of follow-up in over 11,000 individuals in the general population. This is among the largest studies and the first to demonstrate that variation in FGF23 within the low-normal range (<40 pg/ml) may not be associated with cardiovascular risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Alonso A, Misialek J, Eckfeldt J, Selvin E, Coresh J, Chen L, et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2014;3(5):e001082. doi: 10.1161/JAHA.114.001082. In this analysis from the ARIC Study they did not find a relationship between higher baseline intact FGF23 and risk of incident atrial fibrillation over long term follow-up. However this study included a meta-analysis with other general population studies that showed a relationship between higher FGF23 and incident atrial fibrillation in the pooled analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Wright C, Dong C, Stark M, Silverberg S, Rundek T, Elkind M, et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS) Neurology. 2014;82(19):1700–6. doi: 10.1212/WNL.0000000000000410. This study is the first to demonstrate increased risk of incident stroke associated with higher baseline levels of FGF23. The association appeared to be driven by increased risk of intracranial hemorrhage as compared to ischemic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Mathew J, Sachs M, Katz R, Patton K, Heckbert S, Hoofnagle A, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) Circulation. 2014;130(4):298–307. doi: 10.1161/CIRCULATIONAHA.113.005499. This study was one of the first to report higher risk of incident atrial fibrillation among individuals in the general population and older adults. The risk was independent of changes in left ventricular geometry measured through cardiac imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano C, Hamano T, Fujii N, Obi Y, Matsui I, Tomida K, et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50(6):1266–74. doi: 10.1016/j.bone.2012.02.634. [DOI] [PubMed] [Google Scholar]
- *26.Scialla J, Xie H, Rahman M, Anderson A, Isakova T, Ojo A, et al. Fibroblast Growth Factor 23 and Cardiovascular Events in Chronic Kidney Disease. J Am Soc Nephrol. 2014;25(2):349–60. doi: 10.1681/ASN.2013050465. In this analysis of the Chronic Renal Insufficiency Cohort Study, higher baseline C-terminal FGF23 was associated with a graded risk of atherosclerotic events and congestive heart failure hospitalization in patients with CKD, but the association between FGF23 and heart failure was stronger and more robust in sensitivity analyses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Bouma-de Krijger A, Bots M, Vervloet M, Blankestijn P, Ter Wee P, Van Zuilen A, et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):88–97. doi: 10.1093/ndt/gft456. In this study of approximately 400 patients with CKD the average of two measurements of FGF23 revealed similar risk estimates for a composite of myocardial infarction, stroke and cardiovascular mortality compared with use of a single measurement of FGF23; however, power may have been limited to detect important differences. [DOI] [PubMed] [Google Scholar]
- 28.Brandenburg V, Kleber M, Vervloet M, Tomaschitz A, Pilz S, Stojakovic T, et al. Fibroblast growth factor 23 (FGF23) and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. 2014;237(1):53–9. doi: 10.1016/j.atherosclerosis.2014.08.037. [DOI] [PubMed] [Google Scholar]
- **29.Udell J, Morrow D, Jarolim P, Sloan S, Hoffman E, O’Donnell T, et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Heart Assoc. 2014;63(22):2421–8. doi: 10.1016/j.jacc.2014.03.026. This study evaluated the relationship between baseline C-terminal FGF23 and risk of cardiovascular death and heart failure in the setting of a randomized controlled trial evaluating trandolapril versus placebo in patients with ischemic heart disease. Higher FGF23 was associated with cardiovascular mortality and heart failure in the trandolapril treated group but not the placebo group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor E, Rimm E, Stampfer M, Curhan G. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161(5):956–62. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garimella P, Ix J, Katz R, Chonchol M, Kestenbaum B, de Boer I, et al. Fibroblast growth factor 23, the ankle-brachial index, and incident peripheral artery disease in the Cardiovascular Health Study. Atherosclerosis. Atherosclerosis. 2014;233(1):91–6. doi: 10.1016/j.atherosclerosis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scialla J, Astor B, Isakova T, Xie H, Appel L, Wolf M. Mineral metabolites and chronic kidney disease progression in African Americans. J Am Soc Nephrol. 2012;24(1):125–35. doi: 10.1681/ASN.2012070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal I, Ide N, Ix J, Kestenbaum B, Lanske B, Schiller N, et al. Fibroblast growth factor-23 and cardiac structure and function. J Am Heart Assoc. 2014;3(1):e000584. doi: 10.1161/JAHA.113.000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jovanovich A, Ix J, Gottdiener J, McFann K, Katz R, Kestenbaum B, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231(1):114–9. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Ali F, Falkner B, Gidding S, Price H, Keith S, Langman C. Fibroblast growth factor-23 in obese, normotensive adolescents is associated with adverse cardiac structure. J Pediatr. 2014;165(4):738–43. doi: 10.1016/j.jpeds.2014.06.027. This is one of the first studies evaluating the relationship between higher FGF23 and cardiac structure in children without heart disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole S, Platt R, Schisterman E, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39(2):417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schisterman EF, Cole SR, Platt RW. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiology (Cambridge, Mass) 2009;20(4):488–95. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scialla J, Lau W, Reilly M, Isakova T, Yang H, Crouthamel M, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83(6):1159–68. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu J, Katz R, Ix J, de Boer I, Kestenbaum B, Shlipak M. Association of fibroblast growth factor-23 with arterial stiffness in the Multi-Ethnic Study of Atherosclerosis. Nephrol Dial Transplant. 2014;29(11):2099–105. doi: 10.1093/ndt/gfu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao Y, Peng C, Huang W, Zhang J, Xia M, Zhang Y, et al. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. Plos One. 2013;8(8):e72545. doi: 10.1371/journal.pone.0072545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24(10):3125–31. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 42.Voigt M, Fischer D-C, Rimpau M, Schareck W, Haffner D. Fibroblast growth factor (FGF)-23 and fetuin-A in calcified carotid atheroma. Histopathology. 2010;56(6):775–88. doi: 10.1111/j.1365-2559.2010.03547.x. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez J, Shlipak M, Whooley M, Ix J. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol. 2013;24(4):647–54. doi: 10.1681/ASN.2012090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, et al. Klotho and Phosphate Are Modulators of Pathologic Uremic Cardiac Remodeling. Journal of the American Society of Nephrology. 2014 doi: 10.1681/ASN.2014050465. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bos MJ, Koudstaal PJ, Hofman A, Breteler MMB. Decreased Glomerular Filtration Rate Is a Risk Factor for Hemorrhagic But Not for Ischemic Stroke: The Rotterdam Study. Stroke. 2007;38(12):3127–32. doi: 10.1161/STROKEAHA.107.489807. [DOI] [PubMed] [Google Scholar]
- 46.Dhingra R, Gaziano JM, Djousse L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4(2):138–44. doi: 10.1161/CIRCHEARTFAILURE.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–15. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 48.Nelson SE, Shroff GR, Li S, Herzog CA. Impact of Chronic Kidney Disease on Risk of Incident Atrial Fibrillation and Subsequent Survival in Medicare Patients. Journal of the American Heart Association. 2012;1(4):e002097. doi: 10.1161/JAHA.112.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal N, Fan D, Hsu Cy, Ordonez JD, Go AS. Incident Atrial Fibrillation and Risk of Death in Adults With Chronic Kidney Disease. Journal of the American Heart Association. 2014 Oct 24;3(5):e001303. doi: 10.1161/JAHA.114.001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park M, Hsu C-y, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. Journal of the American Society of Nephrology. 2012;23(10):1725–34. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wannamethee S, Welsh P, Papacosta O, Lennon L, Whincup P, Sattar N. Elevated parathyroid hormone, but not vitamin d deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ Heart Fail. 2014;7(5):732–9. doi: 10.1161/CIRCHEARTFAILURE.114.001272. [DOI] [PubMed] [Google Scholar]
- 52.Jono S, McKee M, Murry C, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:e10–e7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 53.Lutsey P, Alonso A, Michos E, Loehr L, Astor B, Coresh J, et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2014;100(3):756–64. doi: 10.3945/ajcn.114.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhingra R, Sullivan L, Fox C, Wang T, D’Agostino RS, Gaziano J, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 55.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, et al. for the C. Relation Between Serum Phosphate Level and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2005;112(17):2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 56.Six I, Maizel J, Barreto F, Rangrez A, Dupont S, Slama M, et al. Effects of phosphate on vascular function under normal conditions and influence of the uremic state. Cardiovasc Res. 2012;96:130–9. doi: 10.1093/cvr/cvs240. [DOI] [PubMed] [Google Scholar]
- 57.Lim K, Lu T, Molostvov G, Lee C, Lam F, Zehnder D, et al. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 58.Isakova T, Xie H, Barchi-Chung A, Smith K, Sowden N, Epstein M, et al. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol. 2012;7(5):820–8. doi: 10.2215/CJN.11721111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shalhoub V, Shatzen E, Ward S, Davis J, Stevens J, Bi V, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122(7):2543–53. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antoniucci DM, Yamashita T, Portale AA. Dietary Phosphorus Regulates Serum Fibroblast Growth Factor-23 Concentrations in Healthy Men. J Clin Endocrinol Metab. 2006;91(8):3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 61.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, et al. A 12-Week, Double-Blind, Placebo-Controlled Trial of Ferric Citrate for the Treatment of Iron Deficiency Anemia and Reduction of Serum Phosphate in Patients With CKD Stages 3–5. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.10.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, et al. Gastrointestinal Inhibition of Sodium-Hydrogen Exchanger 3 Reduces Phosphorus Absorption and Protects against Vascular Calcification in CKD. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014030317. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Block G, Wheeler D, Persky M, Kestenbaum B, Ketteler M, Spiegel D, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–15. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ix J, Ganjoo P, Tipping D, Tershakovec A, Bostom A. Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am J Kidney Dis. 2011;57(6):963–5. doi: 10.1053/j.ajkd.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Chang A, Lazo M, Appel L, Gutiérrez O, Grams M. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr. 2014;99(2):320–7. doi: 10.3945/ajcn.113.073148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dominguez J, Kestenbaum B, Chonchol M, Block G, Laughlin G, Lewis C, et al. Relationship between serum and urine phosphorus with all-cause and cardiovascular mortality: The osteoporotic fractures in men (MrOS) study. Am J Kidney Dis. 2013;61(4):555–63. doi: 10.1053/j.ajkd.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto K, Robinson-Cohen C, de Oliveira M, Kostina A, Nettleton J, Ix J, et al. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013;83(4):707–14. doi: 10.1038/ki.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwak S, Kim J, Choi Y, Chang Y, Kwon M, Jung J, et al. Dietary intake of calcium and phosphorus and serum concentration in relation to the risk of coronary artery calcification in asymptomatic adults. Arterioscler Thromb Vasc Biol. 2014;34(8):1763–9. doi: 10.1161/ATVBAHA.114.303440. [DOI] [PubMed] [Google Scholar]
- 69.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–12. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrett-Connor E. Nutrition epidemiology: how do we know what they ate? Am J Clin Nutr. 1991;54(1 Suppl):182S–7S. doi: 10.1093/ajcn/54.1.182S. [DOI] [PubMed] [Google Scholar]
- 71.Gutiérrez O. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Adv Chronic Kidney Dis. 2013;20(2):150–6. doi: 10.1053/j.ackd.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010:340. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
- *73.Eckberg K, Kramer H, Wolf M, Durazo-Arvizu R, Tayo B, Luke A, et al. Impact of westernization on fibroblast growth factor 23 levels among individuals of African ancestry. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu342. Epub ahead of print. In this study African-Americans residing in the Chicago area had levels of intact FGF23 that were 4 times higher and 24 hour urine phosphate that was 2 times higher than individuals residing in Igbo-Ora, Nigeria. [DOI] [PMC free article] [PubMed] [Google Scholar]